Abstract
AMPK (AMP-activated protein kinase) is activated allosterically by AMP and by phosphorylation of Thr172 within the catalytic α subunit. Here we show that mutations in the regulatory γ subunit reduce allosteric activation of the kinase by AMP. In addition to its allosteric effect, AMP significantly reduces the dephosphorylation of Thr172 by PP (protein phosphatase)2Cα. Moreover, a mutation in the γ subunit almost completely abolishes the inhibitory effect of AMP on dephosphorylation. We were unable to detect any effect of AMP on Thr172 phosphorylation by either LKB1 or CaMKKβ (Ca2+/calmodulin-dependent protein kinase kinase β) using recombinant preparations of the proteins. However, using partially purified AMPK from rat liver, there was an apparent AMP-stimulation of Thr172 phosphorylation by LKB1, but this was blocked by the addition of NaF, a PP inhibitor. Western blotting of partially purified rat liver AMPK and LKB1 revealed the presence of PP2Cα in the preparations. We suggest that previous studies reporting that AMP promotes phosphorylation of Thr172 were misinterpreted. A plausible explanation for this effect of AMP is inhibition of dephosphorylation by PP2Cα, present in the preparations of the kinases used in the earlier studies. Taken together, our results demonstrate that AMP activates AMPK via two mechanisms: by direct allosteric activation and by protecting Thr172 from dephosphorylation. On the basis of our new findings, we propose a simple model for the regulation of AMPK in mammalian cells by LKB1 and CaMKKβ. This model accounts for activation of AMPK by two distinct signals: a Ca2+-dependent pathway, mediated by CaMKKβ and an AMP-dependent pathway, mediated by LKB1.
Keywords: AMP-activated protein kinase (AMPK), Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), energy metabolism, LKB1, metabolic syndrome
Abbreviations: AMPK, AMP-activated protein kinase; AMPKK, AMPK kinase; CaMKK, Ca2+/calmodulin-dependent protein kinase kinase; CBS, cystathionine β-synthase; DTT, dithiothreitol; PP, protein phosphatase; Snf1, sucrose non-fermenting 1
INTRODUCTION
AMPK (AMP-activated protein kinase) is the downstream component of a protein kinase cascade that plays a key role in energy homoeostasis [1,2]. AMPK is a heterotrimeric complex of a catalytic subunit (α) and two regulatory subunits (β and γ). Isoforms of all three subunits have been identified in mammals that allow for the generation of 12 different heterotrimeric complexes [1]. The yeast counterpart of AMPK is the SNF1 (sucrose non-fermenting) complex, composed of Snf1 (α), Snf4 (γ) and either Sip1, Sip2 or Gal83 (β isoforms) [3]. Both AMPK and SNF1 are activated by phosphorylation by upstream kinases. In yeast, three upstream kinases have been identified corresponding to Sak1 (originally referred to as Pak1), Elm1 and Tos3 [4,5]. In mammals, LKB1 and CaMKKβ (Ca2+/calmodulin-dependent protein kinase kinase β) have been identified as physiological kinases upstream of AMPK [6–11]. More recently, evidence has been reported indicating that Tak1, a member of the MKK (mitogen-activated protein kinase kinase) family, is also capable of phosphorylating and activating AMPK [12].
In addition to activation by phosphorylation, AMPK is allosterically activated by AMP [13]. Several lines of evidence suggest that the γ subunit is involved in the allosteric activation. The γ subunit contains four CBS (cystathionine β-synthase) domains [14]. In human γ2, naturally occurring mutations in these domains cause cardiac hypertrophy due to massive glycogen accumulation, together with Wolff–Parkinson–White syndrome, a pre-excitation disorder [15–17]. Biochemical studies demonstrate that some of these mutations interfere with AMP-activation of AMPK [18,19]. Furthermore, the CBS domains have been shown to bind AMP using in vitro binding assays [19]. In contrast with AMPK, allosteric activation of SNF1 by AMP has not been demonstrated [20]. As well as allosteric activation, AMP has been proposed to play a role in the phosphorylation of AMPK. Originally, three separate mechanisms were proposed whereby AMP could promote phosphorylation of AMPK. The first mechanism was by direct activation of the upstream kinases by AMP [21]. However, the evidence supporting this mechanism was based on results obtained using partially purified preparations of the upstream kinase from rat liver, which was subsequently shown to be LKB1. Another previous study using highly purified recombinant preparations of LKB1 reveal that AMP does not directly activate LKB1 [8]. Similarly, CaMKKβ is not directly activated by AMP [7,9]. Two further mechanisms for promotion of phosphorylation by AMP were suggested to be substrate mediated. Binding of AMP to AMPK was proposed to render the kinase a better substrate for phosphorylation by upstream kinases [21], while making it a less attractive substrate for dephosphorylation by protein phosphatases [22]. Although these mechanisms provide an attractive model for AMP activation of AMPK, there is relatively little direct evidence supporting them, and for these studies partially purified preparations of AMPK and the upstream kinases were used. Moreover, recent studies have reported that AMP does not promote phosphorylation of AMPK by CaMKKβ [7,9]. Since both CaMKKβ and LKB1 activate AMPK by phosphorylating the same residue within AMPK, it is difficult to envisage a mechanism that would account for a substrate-mediated effect of AMP that is specific for LKB1. At the time that the present study was in preparation, Suter et al. [23] reported that AMP did not promote phosphorylation of AMPK by a recombinant preparation of LKB1. In view of the apparent discrepancy between earlier studies and more recent findings concerning the effect of AMP on phosphorylation, we decided to revisit the mechanisms for activation of AMPK by AMP using highly purified recombinant proteins. The results of the present study show that AMP allosterically activates AMPK and inhibits dephosphorylation of Thr172. Both these effects involve the γ subunit. However, AMP has no effect on phosphorylation of AMPK by either LKB1 or CaMKKβ. Instead, we present evidence suggesting that previous results indicating that AMP stimulates phosphorylation of AMPK by LKB1 may have been confounded by the presence of endogenous PP (protein phosphatase)2Cα in the preparations of the kinases used in the earlier studies.
MATERIALS AND METHODS
Materials
BL21-Codon-Plus (DE3)-RIL competent Escherichia coli cells were obtained from Novagen. Constructs for bacterial expression of the SNF1 complex (Snf1, Snf4 and Gal83) were a kind gift from Marian Carlson (Columbia University Medical Center, Columbia University, New York, NY, U.S.A.).
Preparation of recombinant proteins
Recombinant AMPK α1β1γ1 and α2β1γ1 complexes [24], CaMKKβ [9] and PP2Cα [25] were expressed in bacteria and purified as described in the respective references. cDNAs encoding Snf1 and Snf4 were cloned into multiple cloning sites 1 (SacI/HindIII) and 2 (KpnI/KpnI) respectively, of the pRSF DUET-1 vector (Invitrogen). This allowed for expression of Snf1 with an N-terminal His6-tag. cDNA coding for Gal83 was cloned into the pET DUET-1 vector (Invitrogen) between the Bg1II/KpnI sites. These constructs were co-transformed into E. coli [BL21-Codon-Plus (DE3)-RIL] and colonies were selected by growth on medium containing the appropriate antibiotic. The SNF1 complex was expressed in E. coli following induction with 1 mM isopropyl β-thiogalactopyranoside for 4 h at 25 °C. Cells were sonicated with 1 s pulses separated by 1 s intervals for 2 min at 8 W (Sonics Vibra cell) and insoluble material was removed by centrifugation at 16000 g for 15 min at 4 °C. The supernatant was sterile-filtered and SNF1 complex was purified on a HisTrap HP column using an ÄKTA™ purifier (Amersham Biosciences). Following nickel–Sepharose chromatography, the SNF1 complex was further purified by gel filtration chromatography on a HiLoad 16/60 Superdex 200 column (Amersham Biosciences). Fractions were analysed by SDS/PAGE and those containing the SNF1 complex were pooled and concentrated using a Vivaspin column (Vivascience). Authenticity of the SNF1 subunits was confirmed by MS of the proteins resolved by SDS/PAGE and subsequently digested with trypsin (results not shown).
Active LKB1 complex was obtained by co-expression of His–LKB1, STRADα and GST (glutathione S-transferase)–MO25α using a baculovirus-insect cell expression system (Bac-to-Bac® Baculovirus Expression System, Invitrogen). The LKB1 complex was expressed in Sf9 cells and was purified by affinity chromatography on glutathione–Sepharose. Recombinant protein was stored in 50 mM Hepes, pH 7.4, 200 mM NaCl, 10% (v/v) glycerol and 1 mM Tris(2-carboxyethyl)phosphine hydrochloride at −80 °C.
Site-directed mutagenesis
Point mutations (R69Q, H150R and R298G) were introduced into the γ1 subunit using the QuikChange® site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. The introduction of the mutations was verified by DNA sequencing.
AMPK/SNF1 assays
Recombinant AMPK or SNF1 was incubated in the presence or absence of CaMKKβ or LKB1 with 200 μM ATP, 2.5 mM MgCl2 and 1 mM DTT (dithiothreitol) for 20 min at 37 °C, and in the presence or absence of 150 μM AMP, as stated in the legends to the Figures. In some cases, an aliquot (5 μl) of this reaction was taken for Western blot analysis. A separate aliquot (5 μl) was removed and diluted in 50 mM Hepes (pH 7.4), and AMPK or SNF1 activity was determined by phosphorylation of the SAMS peptide (His-Met-Arg-Ser-Ala-Met-Ser-Gly-Leu-His-Leu-Val-Lys-Arg-Arg) [26] in the presence or absence of 150 μM AMP, as stated in the legends to the Figures. Results shown are presented as specific activity (nmol/min per mg of protein).
Western blot analysis
Thr172 (AMPK) and Thr210 (SNF1) phosphorylation was determined using rabbit anti-(phospho-Thr172) antibody (Cell Signaling). Total AMPK was detected using sheep anti-α1 or anti-α2 antibodies [27]. Total Snf1 was detected using mouse anti-His6 antibody (Abcam). PP2Cα was detected using mouse anti-PP2Cα antibody (Acris Antibodies). Primary antibodies were detected using LI-COR IRDye® IR Dye secondary antibodies and visualised using an Odyssey Infrared Imager (LI-COR Biotechnology). Quantification of results was performed using Odyssey software and expressed as a ratio of the signal obtained with the phospho-specific antibody relative to the appropriate total antibody.
Dephosphorylation of AMPK and SNF1
Recombinant AMPK or SNF1 was phosphorylated by CaMKKβ or LKB1, as described above. An aliquot (5 μl) of the phosphorylated AMPK or SNF1 was incubated in 50 mM Hepes, pH 7.4, and 2.5 mM MgCl2, in the presence or absence of recombinant PP2Cα (26 ng) and the presence or absence of 150 μM AMP for 20 min at 37 °C. The reaction was terminated by the addition of gel-loading buffer [50 mM Tris/HCl, pH 7.4, 2.5% (w/v) SDS, 1% (v/v) 2-mercaptoethanol, 10% (v/v) glycerol and 0.05% Bromophenol Blue]. Samples were resolved by SDS/PAGE and subjected to Western blot analysis.
Rat liver enzymes
Rat liver AMPK was purified up to the DEAE-Sepharose step as described previously [28], and rat liver LKB1 up to the Q-Sepharose step as described previously [29]. Rat liver AMPK was dialysed into 50 mM Hepes, 10% (v/v) glycerol, 1 mM EDTA, 1 mM DTT, 1 mM benzamidine, 0.1 mM PMSF and 4 μg/ml trypsin inhibitor in order to remove the PP inhibitors present in the purification buffer. Following dialysis, AMPK was incubated in the presence or absence of 2.5 mM MgCl2, and the presence or absence of 50 mM NaF, with or without 150 μM AMP for 10 min at 37 °C. Aliquots (5 μl) were removed for Western blot analysis or for AMPK activity determination using the SAMS peptide assay, measured in the presence of a final concentration of 150 μM AMP. In some cases, AMPK was incubated for 10 min at 37 °C in the presence of 200 μM ATP, 2.5 mM MgCl2 and in the presence or absence of LKB1 (16 ng), 50 mM NaF or 150 μM AMP (as stated in the legend to the Figure). Aliquots (5 μl) of these reactions were removed for AMPK activity determination (measured in the presence of a final concentration of 150 μM AMP).
Statistical analysis
Results are expressed as means±S.E.M. Statistical analysis was carried out using a two-tailed, unpaired Student's t test.
RESULTS
Effect of γ subunit mutations on allosteric activation by AMP
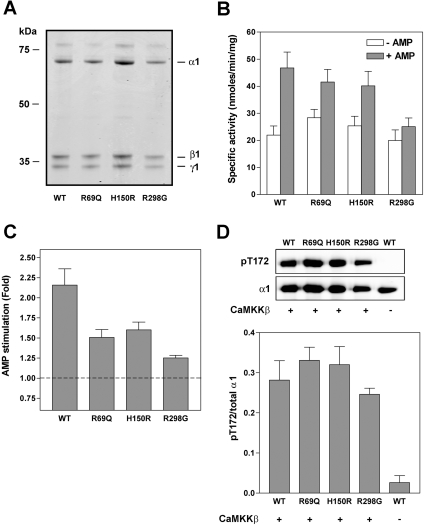
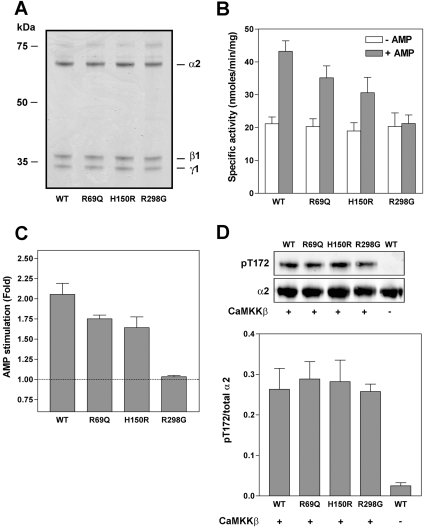
Two previous studies reported that naturally occurring mutations in the γ2-subunit of AMPK interfere with AMP-binding and allosteric activation of the kinase [18,19]. The residues mutated in γ2 are conserved in all mammalian γ isoforms, suggesting that they may have functionally important roles. In order to investigate more thoroughly the role of these residues in regulation of AMPK by AMP, we expressed AMPK complexes in E. coli using a tricistronic expression system [24]. In the present study, we have used γ1-containing complexes because, despite extensive efforts, we have been unable to obtain purified preparations of recombinant AMPK complexes containing the γ2 isoform (results not shown). Wild-type AMPK complexes (α1β1γ1 or α2β1γ1) or complexes harbouring mutations, R69Q (equivalent to R302Q in human γ2), H150R (H383R) and R298G (R531G) within the γ1 subunit, were expressed and purified by nickel–Sepharose chromatography. As shown in Figures 1(A) and 2(A), no difference in expression of the various complexes was detected. As reported previously [24], the recombinant AMPK was isolated in an inactive form, but could be activated by phosphorylation using recombinant preparations of the upstream kinases, CaMKKβ or LKB1. Following phosphorylation by CaMKKβ, AMP activated the wild-type AMPK complexes 2-fold and this activation was reduced in complexes harbouring mutations in the γ subunit (Figures 1B, 1C, 2B and 2C). The R298G mutation had the most significant effect on AMP activation, completely abolishing activation of the α2 complex. Mutations in the γ1 subunit did not have a significant effect on the degree of phosphorylation of Thr172 by CaMKKβ or LKB1, nor on the specific activity of AMPK measured in the absence of AMP (Figures 1 and 2, and results not shown).
Figure 1. Effect of mutations within γ subunit on allosteric activation of α1β1γ1 by AMP.
Wild-type (WT) AMPK complex (α1β1γ1) or complexes harbouring point mutations in the γ subunit (R69Q, H150R or R298G) were expressed in E. coli and purified by nickel–Sepharose chromatography. (A) Proteins (∼1 μg) were resolved by SDS/PAGE and detected by staining with Coomassie Blue. (B) and (C) Following phosphorylation by CaMKKβ, AMPK activity of the complexes was determined using the SAMS peptide assay in the presence or absence of 150 μM AMP. Results are the means±S.E.M. for four independent experiments and are shown as the specific activity of the kinase (nmol of phosphate incorporated/min per mg of complex) in (B) and the fold stimulation in (C). (D) Western blot analysis of AMPK complexes probed with an antibody against either phospho-Thr172 (pT172) or α1. A representative blot is shown in the upper panel and the bottom panel shows the relative quantitation of pT172:total α1 as means±S.E.M for four independent experiments. Wild-type AMPK that had not been incubated with CaMKKβ is included as a negative control.
Figure 2. Effect of mutations within γ subunit on the allosteric activation of α2β1γ1 by AMP.
The same set of experiments as described in the legend for Figure 1 were carried out using α2 complexes.
AMP does not stimulate phosphorylation of AMPK
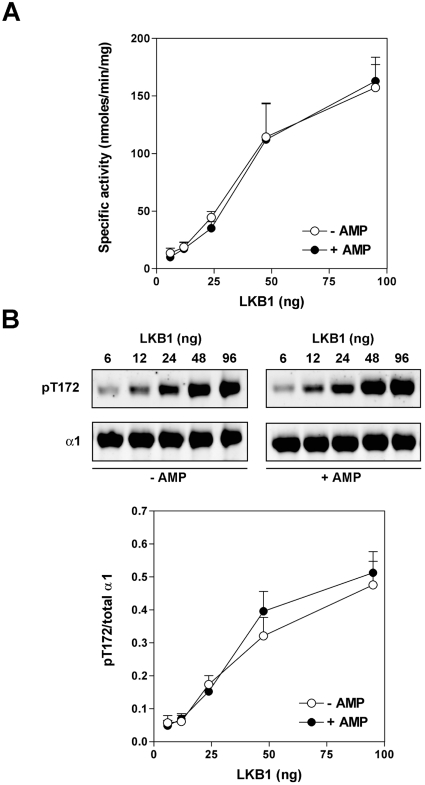
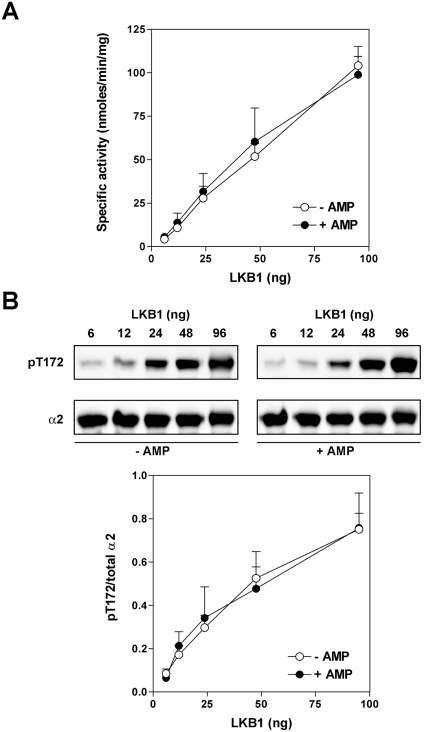
Previous studies reported that, in addition to allosteric activation, AMP also promotes the phosphorylation of AMPK by CaMKK and LKB1 [6,7,21,30]. In the CaMKK studies, partially purified preparations from pig brain were used [21,30]. In subsequent studies using recombinant CaMKKα and CaMKKβ, no effect of AMP was observed [7,9]. Taken together these results suggest that the effect of AMP on phosphorylation was mediated by a contaminating factor present in the pig brain preparations of CaMKK. In light of these findings we decided to reinvestigate the effect of AMP on phosphorylation of AMPK by LKB1 using recombinant proteins. Wild-type AMPK complexes were activated following phosphorylation by LKB1, and, under the conditions used, activation was almost linear with the amount of LKB1 added (Figures 3A and 4A). Note that, because AMP is present in one of the activation reactions with LKB1, we added AMP to the SAMS peptide phosphorylation so that the final concentration of AMP is 150 μM in this assay, irrespective of whether AMP was present or absent in the LKB1 incubation. Both α1- and α2-complexes were activated to similar degrees, and activation correlated closely with the extent of phosphorylation of Thr172 (Figures 3B and 4B). Using this highly purified reconstituted system, we did not detect any effect of AMP on phosphorylation of Thr172 or on activation of AMPK for either α1- or α2-complexes. It was reported recently [23] that AMP is generated in the AMPK assay using the SAMS peptide as a substrate. However, at the end of the phosphorylation reaction with LKB1 and AMPK we were unable to detect AMP in the assay mixture (in the absence of added AMP), as judged by ion-exchange chromatography (results not shown).
Figure 3. Effect of AMP on phosphorylation of α1β1γ1 by LKB1.
(A) Wild-type α1β1γ1 was incubated with MgATP and varying amounts of LKB1 in the presence (closed circles) or absence (open circles) of 150 μM AMP for 10 min at 37 °C. At the end of the incubation an aliquot (5 μl) was removed, diluted in buffer and the AMPK activity was determined using the SAMS peptide assay. Note that in the SAMS peptide assay, AMP (150 μM final concentration) is present irrespective of whether AMP was included in the initial incubation with LKB1. (B) The top panel shows a representative Western blot of phospho-Thr172 (pT172) and total α1 of aliquots (5 μl) of the reactions described above. The bottom panel shows the relative quantitation of pT172:total α1 as means±S.E.M for three independent experiments.
Figure 4. Effect of AMP on phosphorylation of α2β1γ1 by LKB1.
The same set of experiments as described in the legend for Figure 3 were carried out using α2 complexes.
Effect of γ subunit mutations on AMP inhibition of dephosphorylation of AMPK
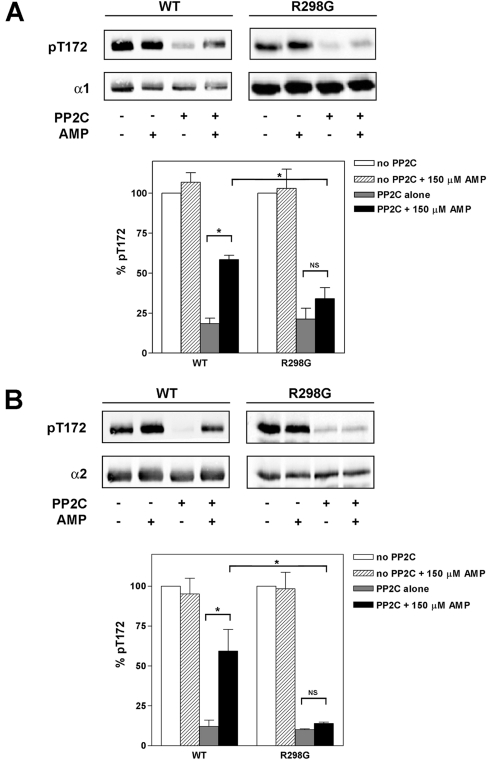
Wild-type AMPK or AMPK harbouring the R298G mutation in γ1 was phosphorylated by CaMKKβ and subsequently used as a substrate for dephosphorylation by recombinant PP2Cα. The phosphorylation state of Thr172 was assessed by Western blotting using anti-(phospho-Thr172) specific antibodies and quantified relative to the level of total α subunit expression using an IR scanner. As shown in Figure 5, incubation of AMPK with PP2Cα alone led to marked dephosphorylation of the α subunit. Inclusion of AMP in the incubation with PP2Cα significantly reduced the dephosphorylation of Thr172 for the wild-type α1- and α2-containing complexes (Figure 5). In contrast, AMP had no significant effect on the dephosphorylation of the R298G complexes. The inhibition of dephosphorylation by AMP for the R69Q and H150R complexes was significantly reduced, although to a lesser extent than seen with the R298G complex (results not shown). Similar results were obtained using AMPK phosphorylated by LKB1. These results are consistent with a previous study [22] which reported that AMP inhibits dephosphorylation of AMPK partially purified from rat liver. Our results extend these previous findings by demonstrating that the effect of AMP on dephosphorylation involves residues that are also involved in the allosteric regulation of AMPK.
Figure 5. Effect of AMP on dephosphorylation of recombinant AMPK by PP2C.
Following phosphorylation of recombinant AMPK complexes (containing either wild-type γ1 or the R298G mutant) by CaMKKβ, aliquots (5 μl) were removed and incubated in the presence or absence of PP2C (26 ng) and presence or absence of AMP (150 μM) for 20 min at 37 °C. At the end of the incubation the reaction mixture was resolved by SDS/PAGE and analysed by Western blotting using anti-(phospho-Thr172) (pT172) and anti-α1 (A) or anti-(phospho-Thr172) and anti-α2 (B) antibodies. In each case a representative blot is shown in the top panels. In the bottom panels, Thr172 phosphorylation relative to a control (absence of PP2C and AMP) is shown as the means±S.E.M. for four independent experiments. Significant differences in Thr172 phosphorylation are denoted by *P<0.05). NS, not significant.
AMP has no effect on yeast SNF1 kinase
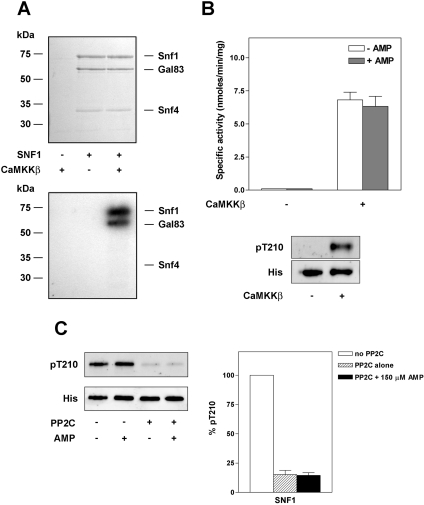
In notable contrast with the mammalian enzyme, the yeast counterpart of AMPK, SNF1, has been reported not to be allosterically activated by AMP [20,31], but the effect of AMP on dephosphorylation of SNF1 has not been reported. In order to investigate this, we co-expressed Snf1, Snf4 and Gal83 in E. coli. The SNF1 complex was purified by nickel–Sepharose chromatography followed by gel-filtration to near homogeneity (Figure 6A). SNF1 was virtually inactive following purification, but could be activated by phosphorylation with CaMKKβ in vitro. Using radiolabelled ATP, phosphorylation of both the Snf1 and Gal83 subunits was detected. The amino acid sequence surrounding Thr210 in Snf1, the equivalent site to Thr172 in AMPK, is highly conserved between yeast and mammals, and the anti-(phospho-Thr172) antibody cross-reacted with phosphorylated Snf1 on Western blots (Figure 6B). We were therefore able to measure the dephosphorylation of Thr210 by PP2Cα using the anti-(phospho-Thr172)-specific antibodies and quantify this relative to the level of total Snf1 expression (detected using an anti-His6 antibody). The results shown in Figure 6(C) demonstrate that AMP does not inhibit dephosphorylation of Thr210 in Snf1.
Figure 6. Effect of AMP on recombinant SNF1 complex.
SNF1 (Snf1, Snf4, Gal83) was expressed in E. coli and purified on nickel–Sepharose, followed by gel-filtration chromatography. (A) Purified SNF1 was incubated with [32P]-ATP in the presence or absence of CaMKKβ and resolved by SDS/PAGE. The proteins were visualized by staining with Coomassie Blue (upper panel) and radiolabelled products detected by autoradiography (lower panel). (B) SNF1 activity was determined by the SAMS peptide assay in the presence or absence of 150 μM AMP. Activities are plotted as nmol of phosphate incorporated/min per mg of SNF1, and are the means±S.E.M for three independent experiments. Also shown is a representative blot of the reaction mixture probed with anti-(phospho-Thr172) (to detect phospho-Thr210 in Snf1) or anti-His6 antibody (to detect total Snf1). (C) SNF1 phosphorylated by CaMKKβ was incubated in the presence or absence of PP2C (26 ng) and the presence or absence of 150 μM AMP for 20 min at 37 °C. At the end of the incubation proteins were analysed by Western blotting using either anti-(phospho-Thr172) or anti-His6 antibody as described in the legend for Figure 5. The results are shown as means±S.E.M. for four independent experiments.
Partially purified AMPK and LKB1 contain endogenous PP2Cα
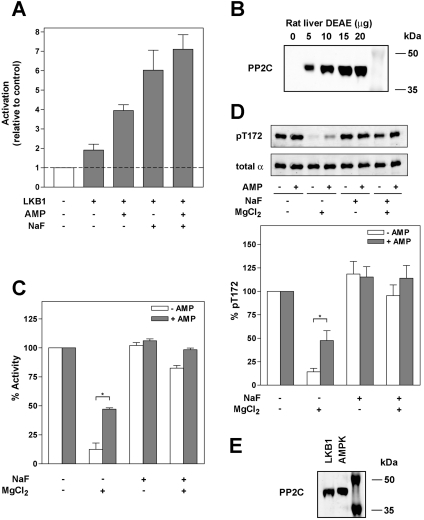
Our results using highly purified recombinant preparations of AMPK, CaMKKβ, LKB1 and PP2Cα reveal that AMP allosterically activates AMPK and inhibits the dephosphorylation of AMPK, but has no effect on phosphorylation by upstream kinases. The latter finding is at odds with several previous studies using partially purified preparations of AMPK and LKB1. One possibility that could explain this apparent discrepancy is that the preparations described in the other reports contain a factor that mediates the effect of AMP on phosphorylation. On the basis of our current results, we reasoned that this factor could in fact be a PP. To test this, we used a partially purified AMPK preparation in an activation assay with recombinant LKB1. AMPK was activated approximately 2-fold by LKB1, and AMP increased activation a further 2-fold (Figure 7A). However, if NaF, a PP inhibitor, was included in the reactions, AMPK activity was increased almost 6-fold by LKB1, and was not further activated by the addition of AMP, i.e. AMP and NaF have similar and non-additive effects on AMPK phosphorylation. Rather than showing an effect of AMP to promote phosphorylation, these results are most consistent with the hypothesis that AMP acts to inhibit the dephosphorylation of AMPK, with the overall effect being an increase in AMPK activity.
Figure 7. Inactivation of rat liver AMPK by endogenous PP activity.
(A) Following dialysis into buffer lacking PP inhibitors, rat liver AMPK was incubated for 10 min at 37 °C in the presence of MgATP and the presence or absence of LKB1 (16 ng), AMP (150 μM) and/or NaF (50 mM). Following incubation, AMPK activity was determined. Results shown are plotted relative to AMPK activity in the presence of MgATP alone and are the means±S.E.M. for three independent experiments. (B) Partially purified rat liver AMPK extract was immunoblotted with an antibody against PP2Cα. (C) Rat liver AMPK was incubated in the presence or absence of MgCl2 (2.5 mM) and in the presence or absence of either 50 mM NaF, 150 μM AMP or both for 10 min at 37 °C. Following incubation, aliquots (5 μl) were removed and AMPK activity was determined using the SAMS peptide assay in the presence of 150 μM AMP (final concentration). (D) In parallel, aliquots (5 μl) were blotted with anti-(phospho-Thr172) or a mixture of anti-α1 and -α2 antibodies. A representative blot is shown (upper panel) and the lower panel shows the quantification of the relative level of Thr172 phosphorylation for three independent experiments (means±S.E.M.). *Significant differences (P<0.05) in AMPK activity or Thr172 phosphorylation. (E) Rat liver AMPK and LKB1, purified up to the Q-Sepharose step (as described in [29]), were probed with an anti-PP2Cα antibody.
In order to determine directly the presence of a PP in a partially purified preparation of rat liver AMPK (purified up to the first ion-exchange step, as described in [28]) we adopted a shotgun MS approach. The results (not shown) revealed the presence of PP2Cα, and this was confirmed by Western blot analysis (Figure 7B). To investigate whether PP2Cα activity is present in the AMPK preparation, we incubated AMPK in the presence or absence of MgCl2, an essential co-factor for PP2Cα [32], and determined AMPK activity and Thr172 phosphorylation (Figures 7C and 7D). AMPK activity and Thr172 phosphorylation were significantly reduced only in the presence of MgCl2 and these decreases were blocked totally by the addition of 50 mM NaF. Consistent with our studies using recombinant AMPK and PP2Cα, AMP significantly inhibited, but did not abolish, these effects. PP2Cα was undetectable in more highly purified preparations of AMPK (up to the gel-filtration step, as described in [28]), or following immunoprecipitation. Using these preparations, AMPK activity was not affected following incubation with MgCl2. Furthermore, we did not detect an effect of AMP on activation of immunoprecipitated AMPK by recombinant LKB1 (results not shown). However, AMP stimulation of Thr172 phosphorylation has been reported using more highly purified preparations of AMPK [6,21]. In these studies, LKB1 was purified from rat liver up to the Q-Sepharose step (as described in [29]), raising the possibility that PP2Cα could be present in the preparations. We found that PP2Cα was readily detectable by Western blotting in rat liver LKB1 purified to the same stage (Figure 7E).
DISCUSSION
In the present study, we investigated the mechanisms by which AMP leads to activation of AMPK. We took advantage of the use of highly purified recombinant preparations of the components of the AMPK cascade. Wild-type AMPK complexes (α1β1γ1 and α2β1γ1) expressed in bacteria were activated by phosphorylation using either CaMKKβ or LKB1, consistent with results reported previously [8,9,24]. AMP allosterically activated both α1- and α2-containing complexes by approximately 2-fold. The degree of activation of recombinant α1β1γ1 is slightly greater than that reported previously for α1γ1 complexes isolated by sequential immunoprecipitation from rat brain (2.1-fold compared with 1.7-fold), whereas activation of recombinant α2β1γ1 is slightly lower than that reported for α2γ1 complexes from rat brain (2.1-fold compared with 2.8-fold) [33]. A complication with the measurements using native rat brain AMPK is that these complexes would contain a mixture of β1 and β2 isoforms, and this might account for the minor differences in AMP stimulation observed between the two studies.
The γ subunit isoforms contain four copies of a CBS domain and previous studies [18,19,34] have shown that these domains are involved in AMP binding and subsequent allosteric activation of AMPK. Part of the evidence implicating the CBS domains in regulation of AMPK comes from the identification of naturally occurring mutations in γ2 that cause severe cardiac defects in humans [15–17], and a naturally occurring mutation in γ3 in pig that leads to glycogen accumulation in skeletal muscle [35]. Most of these mutations lie within the CBS domains and interfere with the normal activation of AMPK by AMP [18,19]. For reasons that are unclear, we have been unable to express γ2- or γ3-containing complexes in bacteria and so we introduced mutations into the γ1 subunit to mimic three of the equivalent naturally occurring mutations found in γ2, including the equivalent mutation in pig γ3. Complexes containing the mutant γ1 subunits were expressed in bacteria and were activated by CaMKKβ or LKB1, similar to wild-type AMPK. Consistent with previous studies, AMP activation was reduced in the γ1-mutant complexes, with the R298G mutation having the greatest effect.
Davies et al. [22] reported previously that AMP inhibits dephosphorylation of AMPK. We confirmed that AMP has a significant inhibitory effect on dephosphorylation of recombinant AMPK by PP2Cα. While the present paper was in preparation, Suter et al. [23] published results using recombinant AMPK in which they also demonstrated AMP inhibition of dephosphorylation. Importantly, we now show that a mutation in CBS4 of AMPK (R298G) completely abolishes the effect of AMP on dephosphorylation. Furthermore, the R298G mutation almost completely abolishes allosteric activation of AMPK, suggesting that the same mechanism may account for both AMP effects.
SNF1, the yeast counterpart of AMPK, is activated by phosphorylation of Thr210, the equivalent residue to Thr172 in AMPKα. Although SNF1 does not appear to be directly activated by AMP [20,31], there are no reports as to whether AMP has an effect on its dephosphorylation. We were able to purify an SNF1 complex following co-expression of Snf1, Snf4 and Gal83 subunits in bacteria. As with AMPK, SNF1 was isolated in an inactive state, but could be activated by phosphorylation in vitro using CaMKKβ or LKB1 (results not shown). Consistent with previous studies, we did not detect any allosteric activation of recombinant SNF1 by AMP. In addition, we were unable to detect any effect of AMP on dephosphorylation of Thr210 by PP2Cα. In yeast, SNF1 is activated following growth under glucose-limiting conditions [36]. The signal transduction pathway linking glucose limitation to SNF1 activation is unclear, but our results suggest that AMP is unlikely to be directly involved. However, the availability of a reconstituted system for SNF1 activation would allow potential activators, such as glucose metabolites, to be investigated.
A previous study [21] reported that phosphorylation of AMPK by preparation of AMPKK (AMPK kinase) from rat liver was stimulated by AMP. Subsequent work has revealed that the identity of AMPKK in these preparations is LKB1 [6]. Using recombinant preparations of AMPK and LKB1, we have, however, been unable to detect any stimulation of Thr172 phosphorylation by AMP under any conditions. Recently, Suter et al. [23] published results using recombinant AMPK in which they also failed to detect an effect of AMP on T172 phosphorylation by LKB1 or CaMKKβ [23]. However, the authors were unable to account for the apparent discrepancy between their results and previous studies. In the present study, we provide a plausible explanation for this inconsistency. We reasoned that the partially purified preparations of AMPK and/or LKB1 used in previous studies examining AMP stimulation could contain an additional factor that mediates the effect of AMP on Thr172 phosphorylation.
In an effort to investigate this further, we began by adopting a shotgun MS approach to determine proteins present in a partially purified preparation of rat liver AMPK (purified up to the first ionexchange step, as described in [28]). The results (not shown) revealed the presence of PP2Cα, and this was confirmed by Western blot analysis. We present strong evidence that the AMP effect on Thr172 phosphorylation is due to the presence of PP2Cα, since we only observe AMP stimulation using preparations of AMPK that contain PP2Cα and the effect is abolished by NaF. Based on these findings, we suggest that AMP stimulation of Thr172 phosphorylation is due to inhibition of PP2Cα present in the preparations of AMPK and/or LKB1 and CaMKK. In support of this hypothesis, we showed that PP2Cα is present in a preparation of rat liver LKB1. Although we cannot rule out other possibilities, this explanation would account for the differences between our studies using defined purified preparations of recombinant proteins and those studies using less well-defined preparations. It is interesting to note that all the studies reporting AMP stimulation of Thr172 phosphorylation use okadaic acid to inhibit dephosphorylation, but do not include NaF. As PP2Cα is okadaic acid insensitive [37], it would remain active under the conditions of the assay (since PP2Cα would be activated by the Mg2+ present in the kinase reaction).
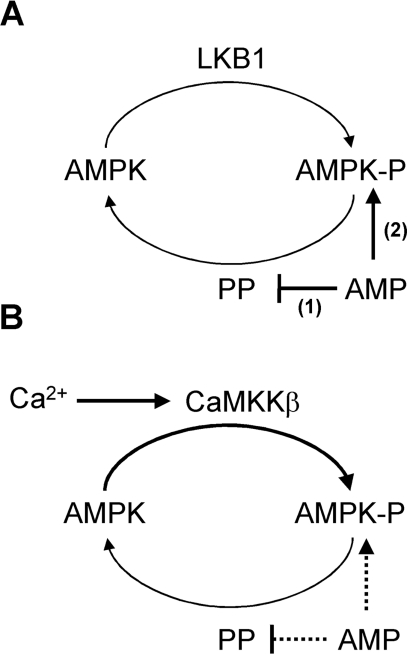
Overall, our results lead us to propose a revised model for activation of AMPK. The phosphorylation state of Thr172 depends on the relative rates of phosphorylation, catalysed by CaMKKβ and LKB1, and dephosphorylation, catalysed by protein phosphatases (Scheme 1). The identity of the protein phosphatase(s) acting on AMPK in vivo is unknown; however, the effect of AMP on dephosphorylation is substrate mediated [22] and so AMP would be predicted to inhibit all phosphatases acting on AMPK. Activation of AMPK occurs either by increased phosphorylation or by decreased dephosphorylation. LKB1 appears to be constitutively active and furthermore its activity is not changed by stimuli that activate AMPK [8,38]. According to our model, increased Thr172 phosphorylation by LKB1 would occur in response to decreased dephosphorylation following a rise in AMP. In parallel, AMP would allosterically activate AMPK (Scheme 1A). Dephosphorylation and inactivation of AMPK would occur when the concentration of AMP returned to basal levels. Unlike LKB1, the activity of CaMKKβ is subject to regulation within the cell, and is increased in response to signals that raise intracellular Ca2+ [39,40]. Therefore, signals that increase Ca2+ activate AMPK as a consequence of increased Thr172 phosphorylation via increased CaMKKβ activity. Under these conditions there is no requirement for increased AMP levels (Scheme 1B). Consistent with this model, recent studies have reported activation of AMPK by CaMKKβ-mediated signalling without detectable changes in AMP [7,41,42]. A further prediction of our model is that AMPK would be rapidly dephosphorylated following a fall in intracellular Ca2+, and this may account for the transient nature of AMPK activation observed in CaMKKβ-mediated responses [7,41,42]. It should be noted that the AMP- and Ca2+-signalling pathways for activation of AMPK predicted by our model are not mutually exclusive. It is possible that under some circumstances, where AMP and Ca2+ rise in concert, both pathways could operate. Under these conditions, both CaMKKβ and LKB1 would contribute to AMPK activation.
Scheme 1. Model for the regulation of AMPK.
(A) AMP-dependent activation: under conditions that lead to an increase in AMP, dephosphorylation of AMPK is inhibited (mechanism 1). The identity of the PP responsible for dephosphorylation of AMPK in vivo is unknown. Since LKB1 is constitutively active, inhibition of the dephosphorylation reaction leads to an increase in Thr172 phosphorylation and activation of AMPK. In addition to increasing Thr172 phosphorylation, AMP allosterically activates AMPK (mechanism 2). (B) Ca2+-dependent activation: signals that increase Ca2+ activate CaMKKβ, increasing Thr172 phosphorylation and activation of AMPK, and this can occur without an increase in AMP. However, it is possible that in some situations both Ca2+ and AMP may increase in parallel, and under these conditions AMP will allosterically activate AMPK and inhibit dephosphorylation (denoted by the dashed lines).
Although our results were obtained with γ1-containing AMPK complexes, we speculate that the same mechanisms will apply to γ2- and γ3-containing complexes. Previous studies have shown that mutations within γ2 and γ3 interfere with allosteric activation of AMPK measured in vitro [18,19,43], and so it seems likely that these mutations will also interfere with the effect of AMP on dephosphorylation. Two studies have reported that γ2 mutations decrease AMPK activity in transgenic animal models [44,45], whereas another study reported increased activity [46]. In a study in which γ3-containing AMPK complexes were overexpressed in COS cells, increased activity was observed in mutant γ3 relative to the wild-type protein [43]. Despite our understanding of the regulation of AMPK by AMP in vitro, elucidating the complex regulation of AMPK both in cells and in vivo, and determining the effect of mutations within the γ subunits, is far from complete.
In conclusion, our results demonstrate that AMP activates AMPK via a direct allosteric mechanism and indirectly by inhibiting dephosphorylation of Thr172. These effects may be mediated by binding of AMP to the same site within the γ subunit, since both effects are abolished by the same mutation within the γ subunit. We provide evidence that suggests that previous studies reporting activation of Thr172 phosphorylation by AMP may have been mediated by PP2Cα present in the partially purified preparations of the kinases. On the basis of our results, we propose a model for the regulation of AMPK that accounts for its activation in response to either Ca2+ signalling or energy depletion, mediated by an increase in AMP.
Acknowledgments
This work was supported by the Medical Research Council UK, an Integrated Project (LSHM-CT-2004-005272) from the European Commission and a BBSRC–CASE studentship award (to M.J.S.).
References
- 1.Carling D. The AMP-activated protein kinase cascade: a unifying system for energy control. Trends Biochem. Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Hardie D. G., Carling D., Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 4.Hong S. P., Leiper F. C., Woods A., Carling D., Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland C. M., Hawley S. A., McCartney R. R., Leech A., Stark M. J. R., Schmidt M. C., Hardie D. G. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr. Biol. 2003;13:1299–1305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 6.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 12.Momcilovic M., Hong S. P., Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 13.Hardie D. G., Salt I. P., Hawley S. A., Davies S. P. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem. J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- 14.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 15.Blair E., Redwood C., Ashrafian H., Ostman-Smith I., Watkins H. Mutations in the γ2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 16.Gollob M. H., Green M. S., Tang A. S. L., Gollob T., Karibe A., Hassan A., Ahmad F., Lozado R., Shah G., Fananapazir L., et al. Identification of a gene responsible for familial Wolff–Parkinson–White syndrome. New Eng. J. Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 17.Arad M., Benson D. W., Perez-Atayde A. R., McKenna W. J., Sparks E. A., Kanter R. J., McGarry K., Seidman J. G., Seidman C. E. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel T., Carling D. Functional analysis of mutations in the γ2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff–Parkinson–White syndrome. J. Biol. Chem. 2003;277:51017–51024. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- 19.Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., Hardie D. G. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods A., Munday M. R., Scott J., Yang X. L., Carlson M., Carling D. Yeast Snf1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J. Biol. Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- 21.Hawley S. A., Selbert M. A., Goldstein E. G., Edelman A. M., Carling D., Hardie D. G. 5′-AMP activates the AMP-activated protein kinase cascade and Ca2+/calmodulin activates the calmodulin-dependent protein kinase-I cascade, via three independent mechanisms. J. Biol. Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 22.Davies S. P., Helps N. R., Cohen P. T. W., Hardie D. G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase: studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2A(C) FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 23.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. Dissecting the role of AMP for allosteric stimulation, activation and deactivation of AMP-activated protein kinase. J. Biol. Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 24.Neumann D., Woods A., Carling D., Wallimann T., Schlattner U. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr. Purif. 2003;30:230–237. doi: 10.1016/s1046-5928(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 25.Marley A. E., Sullivan J. E., Carling D., Abbott W. M., Smith G. J., Taylor I. W. F., Carey F., Beri R. K. Biochemical characterization and deletion analysis of recombinant human protein phosphatase 2Cα. Biochem. J. 1996;320:801–806. doi: 10.1042/bj3200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies S. P., Carling D., Hardie D. G. Tissue distribution of AMP-activated protein kinase, and lack of activation by cyclic AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur. J. Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 27.Woods A., Salt I., Scott J., Hardie D. G., Carling D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- 28.Carling D., Clarke P. R., Zammit V. A., Hardie D. G. Purification and characterisation of the AMP-activated protein kinase. Eur. J. Biochem. 1989;186:129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 29.Hawley S. A., Davison M. D., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. Characterisation of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 30.Salt I. P., Celler J. W., Hawley S. A., Prescott A., Woods A., Carling D., Hardie D. G. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem. J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchelhill K. I., Stapleton D., Gao G., House C., Michell B., Kateis F., Witters L. A., Kemp B. E. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J. Biol. Chem. 1994;269:2361–2364. [PubMed] [Google Scholar]
- 32.McGowan C. H., Cohen P. Protein phosphatase-2C from rabbit skeletal muscle and liver: an Mg2+-dependent enzyme. Methods Enzymol. 1988;159:416–426. doi: 10.1016/0076-6879(88)59041-9. [DOI] [PubMed] [Google Scholar]
- 33.Cheung P. C. F., Salt I. P., Davies S. P., Hardie D. G., Carling D. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem. J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton S. R., Stapleton D., O'Donnell J. B., Kung J. T., Dalal S., Kemp B. E., Witters L. A. An activating mutation in the γ1 subunit of the AMP-activated protein kinase. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- 35.Milan D., Jeon J. T., Looft C., Amarger V., Robic A., Thelander M., Rogel-Gaillard C., Paul S., Iannuccelli N., Rask L., et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 36.Carlson M. Glucose repression in yeast. Curr. Opin. Microbiol. 1999;2:202–207. doi: 10.1016/S1369-5274(99)80035-6. [DOI] [PubMed] [Google Scholar]
- 37.Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases: specificity and kinetics. Biochem. J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakamoto K., Goransson O., Hardie D. G., Alessi D. R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am. J. Physiol. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 39.Tokumitsu H., Iwabu M., Ishikawa Y., Kobayashi R. Differential regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase isoforms. Biochemistry. 2001;40:13925–13932. doi: 10.1021/bi010863k. [DOI] [PubMed] [Google Scholar]
- 40.Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. Components of a calmodulin-dependent protein kinase cascade: molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- 41.Stahmann N., Woods A., Carling D., Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol. Cell. Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamas P., Hawley S. A., Clarke R. G., Mustard K. J., Green K., Hardie D. G., Cantrell D. A. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes B. R., Marklund S., Steiler T. L., Walter M., Hjalm G., Amarger V., Mahlapuu M., Leng Y., Johansson C., Galuska D., et al. The 5′-AMP-activated protein kinase γ3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J. Biol. Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- 44.Sidhu J. S., Rajawat Y. S., Rami T. G., Gollob M. H., Wang Z., Yuan R., Marian A. J., De Mayo F. J., Weilbacher D., Taffet G. E., et al. Transgenic mouse model of ventricular pre-excitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff–Parkinson–White syndrome. Circulation. 2005;111:21–29. doi: 10.1161/01.CIR.0000151291.32974.D5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies J. K., Wells D. J., Liu K., Whitrow H. R., Daniel T. D., Grignani R., Lygate C. A., Schneider J. E., Noel G., Watkins H., Carling D. Characterization of the role of γ2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff–Parkinson–White syndrome. Am. J. Physiol. 2006;290:H1942–H1951. doi: 10.1152/ajpheart.01020.2005. [DOI] [PubMed] [Google Scholar]
- 46.Arad M., Moskowitz I. P., Patel V. V., Ahmad F., Perez-Atayde A. R., Sawyer D. B., Walter M., Li G. H., Burgon P. G., Maguire C. T., et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff–Parkinson–White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]