Abstract
Plasmodium falciparum develops within the mature RBCs (red blood cells) of its human host in a PV (parasitophorous vacuole) that separates the host cell cytoplasm from the parasite surface. The pore-forming toxin, SLO (streptolysin O), binds to cholesterol-containing membranes and can be used to selectively permeabilize the host cell membrane while leaving the PV membrane intact. We found that in mixtures of infected and uninfected RBCs, SLO preferentially lyses uninfected RBCs rather than infected RBCs, presumably because of differences in cholesterol content of the limiting membrane. This provides a means of generating pure preparations of viable ring stage infected RBCs. As an alternative permeabilizing agent we have characterized EqtII (equinatoxin II), a eukaryotic pore-forming toxin that binds preferentially to sphingomyelin-containing membranes. EqtII lyses the limiting membrane of infected and uninfected RBCs with similar efficiency but does not disrupt the PV membrane. It generates pores of up to 100 nm, which allow entry of antibodies for immunofluorescence and immunogold labelling. The present study provides novel tools for the analysis of this important human pathogen and highlights differences between Plasmodium-infected and uninfected RBCs.
Keywords: cholesterol, equinatoxin II, malaria, Plasmodium falciparum, pore-forming toxin, streptolysin O
Abbreviations: DIC, differential interference contrast; EM, electron microscopy; EqtII, equinatoxin II; EXP, exported protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; HU, haemolytic unit; PC, phosphatidylcholine; PfEMP1, P. falciparum erythrocyte membrane protein 1; PV, parasitophorous vacuole; RBC, red blood cell; REX3, ring stage EXP3; SERP, serine-rich protein; SLO, streptolysin O; SM, sphingomyelin
INTRODUCTION
The pore-forming toxin, SLO (streptolysin O), is a 69 kDa protein from group A streptococci [1]. Water-soluble toxin molecules bind to cholesterol-containing target membranes and assemble into oligomeric curved rod structures (25–100 nm long by ∼7.5 nm wide) that penetrate into the hydrophobic domain of the bilayer [1–3]. SLO can be used to permeabilize a range of adherent and non-adherent cells, thereby allowing delivery of exogenous molecules to the cytoplasm and analysis of the components of this compartment [4].
The symptoms of the disease malaria are caused by the asexual development of the parasite Plasmodium falciparum inside the RBCs (red blood cells) of its human host. Separated from the host cell by a PV (parasitophorous vacuole) the parasite develops through three distinct stages referred to as ring (0–18 h), trophozoite (19–37 h) and schizont (38–48 h) stages. The mature human RBC has no protein synthesis or protein trafficking machinery. Nonetheless, the parasite achieves the export of a subset of its proteins beyond the confines of its own plasma membrane and the PV membrane, to extensively modify both the RBC cytoplasm and the RBC plasma membrane [5]. To probe the host cell compartment, SLO has been used to selectively permeabilize the host cell membrane of infected RBCs while leaving the PV membrane intact [6–9]. However, in mixtures of infected and uninfected RBCs, we have found that SLO preferentially permeabilizes uninfected RBCs and is much less effective against infected RBCs. This limits the usefulness of SLO for probing the host cytoplasm of P. falciparum-infected RBCs.
EqtII (equinatoxin II) is a eukaryotic pore-forming toxin that belongs to the family of actinoporins [10–12]. EqtII is a 19.8 kDa (179-amino-acid) protein from the venom of the sea anemone, Actinia equina. The soluble form is a 12-strand β-sandwich with a hydrophobic core and a pair of α-helices. The β-sheet is thought to bind to membrane surfaces and, following insertion of the N-terminal helix and tetramerization, to form a pore [11,13]. SM (sphingomyelin) has been shown to facilitate insertion of EqtII into the target bilayer [14,15]. In present study we have compared SLO and EqtII as tools for manipulating the permeability of the host cell membrane in P. falciparum-infected RBCs.
EXPERIMENTAL
Parasites
Parasites (3D7 strain) were continuously cultured using RBCs and pooled serum obtained from the Red Cross Transfusion Service (Melbourne, VIC, Australia). If required cultures with a parasitaemia of approx. 5% (mainly ring stage) were synchronized by treatment with 5% (w/v) sorbitol for 5 min at 37 °C [16,17]. For the purification of infected RBCs, the parasites were allowed to mature for 24 h and trophozoite stage infected RBCs were harvested by flotation on a cushion of Plasmagel™ {final concentration of 2% (w/v) gelatin in incomplete medium; [18]}, or on a Percoll/sorbitol gradient [16]. Transfectants expressing GFP (green fluorescent protein) fused to the signal sequence of EXP1 (exported protein-1) [19], REX3 (ring stage EXP3) [20] and a fragment of PfEMP1 (P. falciparum erythrocyte membrane protein 1; K119-PfEMP1–GFP) [21] have been described previously.
Preparing pore-forming toxins
SLO was obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.) and prepared by two methods that allow post- or pre-activation storage. Pre-activation storage involves resuspending SLO (25000 units) in 5 ml of PBS (10 mM sodium phosphate buffer and 145 mM NaCl, pH 7.4) containing 0.1% BSA and activating by incubation with 5–10 mM dithiothreitol at 37 °C for 2 h. Activated aliquots were stored at −20 °C. For post-activation storage the same quantity of SLO was resuspended in 1 ml of PBS and aliquots were stored at −20 °C. Prior to use, SLO aliquots were thawed and activated by incubation with 100 mM dithiothreitol at room temperature (22 °C) for 15 min. EqtII (donated by Dr Gregor Anderluh, University of Ljubljana, Ljubljana, Slovenia) was resuspended in PBS as a concentrated stock (150 μg/ml) and stored at 4 °C.
Determining haemolytic activity of SLO and EqtII
To assess toxin potency the haemolytic activities of both SLO and EqtII were determined by titration against RBCs suspended in 70 μl of PBS. Uninfected RBCs [(1–2)×108 cells] or mixed cultures [5–10% parasitaemia, (1–2)×108 cells] were incubated with 0–250 units of SLO or 0–9 μg of EqtII in a final volume of 70 μl for 6 min at room temperature. Cells were pelleted at 210 g for 4 min. Supernatants were diluted 1:100 with PBS and assayed spectrophotometrically in triplicate at 412 nm. The absorbance values were normalized against a sample subjected to hypo-osmotic lysis. One HU (haemolytic unit) is defined as the amount of dissolved SLO or EqtII necessary for the lysis of 50% of the RBCs during a defined incubation period (6 or 12 min) [8].
Competition of toxin-induced RBC lysis with lipid vesicles
L-α-Dimyristoyl PC (phosphatidylcholine), bovine brain SM and cholesterol were obtained from Avanti and prepared as 10 mg/ml stocks in chloroform. PC, PC/SM (2:1 molar ratio) and PC/cholesterol (1:1 molar ratio) were dried under N2 flow in glass vials. PBS was added before bath sonication for 1 h at 4 °C. Lipid vesicles and 108 RBCs were mixed with 4 HUs of post-activated SLO or 2 HUs of EqtII or PBS and incubated at 20 °C for 5 min in a final volume of 70 μl. Cells were pelleted at 210 g for 4 min. Supernatants were diluted 1:100 with PBS and assayed spectrophotometrically in triplicate at 412 nm. The absorbance values were normalized against a PBS and a no lipid control.
Western blot analysis of permeabilized cells
Aliquots of a culture of P. falciparum-infected RBCs were washed twice in PBS and 2×108 cells were treated with the indicated amounts of pre-activated SLO (0–26 HUs) or EqtII (0–7 HUs) in a total of 70 μl of PBS at 37 °C for 12 min, or with 1 HU of EqtII followed by 0.03% saponin in 70 μl of PBS on ice for 5 min. Cells were pelleted at 16000 g, washed once in PBS and resuspended in a volume equivalent to the supernatant fraction [6–8]. Equal amounts of pellet and supernatant were analysed by SDS/PAGE and Coomassie staining (haemoglobin) or immunoblot analysis [REX3, SERP (serine-rich protein) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase)]. Western blots were performed as described previously using 10 mM CAPS (pH 11.2), without methanol, as transfer buffer [22]. The rabbit anti-SERP serum was a gift from Professor K. Lingelbach (Philipps-University, Marburg, Germany). Monoclonal anti-P. falciparum GAPDH antibodies [23] were a gift from Dr C. Daubenberger (Swiss Tropical Institute, Basel, Switzerland). These antisera and the anti-REX3 serum [20] were used at 1:2000.
Parasite viability following treatment with SLO
Viability experiments were performed in triplicate. Infected RBCs were treated with SLO (under sterile conditions) or mock-treated and diluted into 6 ml dishes containing fresh uninfected RBCs and cultured under standard conditions.
Microscopy
For scanning EM (electron microscopy), the cells were layered on a glass coverslip, treated with EqtII as described above, fixed with 2% (v/v) glutaraldehyde for 1 h, rinsed, air-dried and metal-coated just before observation at 5 kV on a JEOL (Tokyo, Japan) field emission scanning electron microscope.
For immunogold EM, infected RBCs (108 cells, ∼5% parasitaemia) or isolated mature stage infected RBCs (108 cells) were fixed with 2% (w/v) paraformaldehyde in PBS for 10 min at 20 °C, washed twice with PBS and then treated with EqtII (2 HUs) for 6 min at 20 °C. The cells were re-fixed under the same conditions for a further 5 min, centrifuged at 1400 g for 2 min and incubated in PBS containing 2% (w/v) BSA for 15 min. The cells were resuspended in primary antibody at a suitable concentration (1:4 for anti-PfEMP1 [24] and 1:20 for rabbit anti-GFP) in PBS/BSA for 1.5 h at 20 °C. Cells were washed once with PBS/BSA and incubated with 6 nm gold-conjugated Protein A (Aurion). The cells were fixed in 2.5% glutaraldehyde for 1 h, rinsed, post-fixed with OsO4, en-block stained with uranyl acetate and then embedded in LR-White resin. The blocks were sectioned (70–80 nm thickness), stained with uranyl acetate and lead citrate, and observed at 80 kV with a JEOL 2010HC.
Fluorescence microscopy was performed using either an Olympus (Tokyo, Japan) BX50 epifluorescence microscope or an inverted Leica (Wetzlar, Germany) TCS-SP2 confocal microscope using ×100 oil immersion objectives (1.4 NA) [25]. Parasitized RBCs expressing GFP fusion proteins, with or without treatment with the pore-forming toxins, were mounted wet on a glass slide, covered by a glass coverslip, sealed and imaged within 20 min at ambient temperature (maintained at 20 °C). Serial optical sections (∼0.08 μm per section) were used to generate average of maximum projection three-dimensional reconstructions using either the Leica SP2 imaging software or NIH ImageJ (http://rsb.info.nih.gov/ij). Immunofluorescence labelling was performed as for immunogold labelling except that the anti-PfEMP1 was used at 1:50 dilution.
RESULTS
SLO lyses uninfected RBCs more readily than P. falciparum-infected RBCs
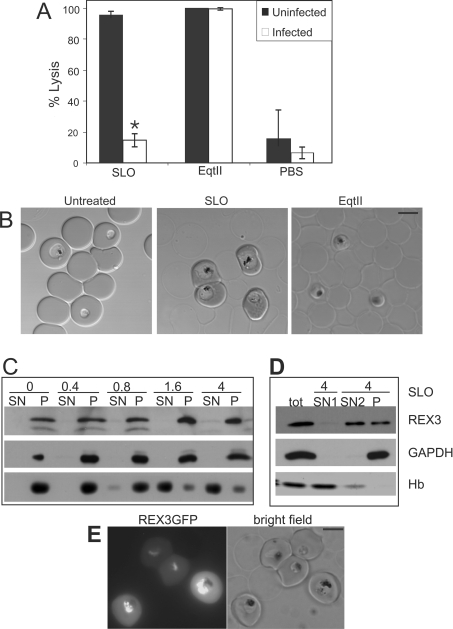
SLO is a bacterial toxin that forms pores in cholesterol-containing membranes, causing non-osmotic lysis of cells [1–3]. We found that approx. 25 units of post-activated SLO were needed to achieve 50% lysis of 108 RBCs (defined as 1 HU). The amount of SLO per HU was found to vary depending on the number of RBCs and the activation method (results not shown). Four HUs were found reproducibly to give close to 100% lysis of uninfected RBCs as evidenced by the release of haemoglobin (results not shown).
SLO has been used previously to lyse P. falciparum-infected RBC without disrupting the PV membrane that harbours the parasite [6]. However, we have found that SLO preferentially permeabilizes uninfected RBC in a mixed population (Figures 1A and 1B). When samples of infected RBCs (∼5% parasitaemia, 108 cells) were treated with 4 HUs of activated SLO, the population of uninfected RBCs were mostly lysed (Figure 1B, middle panel) as judged by the loss of diffraction in DIC (differential interference contrast) images of the cells. Conversely, the infected cell population remained largely intact (Figure 1B, middle panel). Analysis of DIC images of cell populations established that less than 20% of the infected RBCs were lysed under these conditions (Figure 1A).
Figure 1. Comparison of the lytic properties of SLO and EqtII against uninfected and infected RBCs.
(A, B) 3D7 infected RBCs (108 cells, ∼5% parasitaemia) were treated with 4 HUs of SLO or 2 HUs of EqtII. The percentage lysis of RBCs was determined by imaging samples by confocal microscopy and counting 2000 cells per sample. The results shown in (A) represent the means±S.D. for three separate experiments. The asterisk (*) indicates a P-value for the SLO pair of <0.0001. (C) An aliquot of a malaria culture (2×108 cells; ∼5% parasitaemia) was treated with pre-activated SLO (amounts indicated in HUs above each lane), separated into pellet (P) and supernatant (SN) and subjected to SDS/PAGE. The release of haemoglobin (Hb) was monitored by Coomassie Blue staining (bottom row). The release of P. falciparum GAPDH (soluble cytoplasmic parasite protein) and REX3 (exported into the host cell) was assessed by immunoblot analysis. (D) An aliquot of a malaria culture (2×108 cells; ∼5% parasitaemia) was treated with 4 HUs of pre-activated SLO and separated into pellet and supernatant 1 (SN1). The pellet was treated again with 4 HUs of SLO and separated into pellet (P) and supernatant 2 (SN2). More than 50% of REX3 was released by the second SLO treatment, while GAPDH remained in the pellet. (E) Pre-activated SLO treatment of a transfected parasite line expressing a REX3–GFP fusion protein in the cytoplasm of infected RBCs demonstrates that uninfected RBCs are lysed while infected RBCs remain intact. Scale bar, 5 μm.
To confirm that the cells that appeared intact by DIC did not release any protein, we used Western blot analysis to monitor the release of protein from uninfected and infected RBCs in SLO-treated mixed cultures. SLO (≥1.6 HUs) released most of the haemoglobin from 2×108 cells from a malaria culture, while REX3 (a parasite protein that is present in the RBC cytoplasm; [20]) was not released even with 4 HUs of SLO (Figure 1C). Indeed treatment with up to 26 HUs released only a small proportion of the REX3 (results not shown). Selective lysis of uninfected RBCs was also evident in cultures of transgenic parasites expressing a REX3–GFP fusion protein. The fusion protein is partly exported to the cytoplasm of the host cell [20]. After SLO treatment, the fluorescence signal remained in the cytoplasm of infected RBCs (Figure 1E). Bright-field images confirmed the selective lysis of uninfected cells in the population (Figure 1E, right panel). Similar results were obtained with a parasite line expressing REX4–GFP, another protein that is exported into the host cell (results not shown). These results confirm the preferential lysis of uninfected RBCs by SLO.
The fact that SLO preferentially lyses uninfected RBCs in a mixed population indicates that the uninfected cells act as a competitor for the toxin. In accordance with this, it was possible to release more than 50% of REX3 from infected RBCs if the uninfected RBCs were lysed with an initial SLO step (4 HUs, 2×108 cells) followed by a second SLO step (4 HUs) (Figure 1D). P. falciparum GAPDH, which is present as a soluble protein in the parasite cytoplasm, was not released (Figure 1D). This demonstrates that the second SLO treatment did not affect the integrity of the parasite and thus REX3 was released by selective lysis of the RBC membrane. Thus two sequential treatments with 4 HUs of SLO is more effective in lysing infected RBCs than a single treatment with 26 HUs.
SLO treatment can be used to purify viable ring stage parasitized RBCs
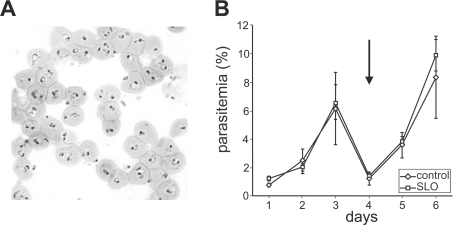
It is possible to take advantage of the preferential lysis of uninfected RBCs as a means of purifying intact infected RBCs. Both ring and mature stage infected RBCs appear to be protected from SLO-induced permeabilization in the presence of uninfected RBCs. Ring stage parasites cannot be isolated using commonly employed enrichment protocols that rely on changes in the density of infected cells (Percoll-density-gradient centrifugation) [16], the formation of knobs (gelatin flotation) [26] or the paramagnetic properties of haemozoin [27]. However, we found that SLO can be used to preferentially lyse the uninfected RBCs in a ring stage culture (containing 5–10% infected RBCs). Using SLO we enriched the ring stage parasites to >80% parasitaemia using conditions that release >90% of the haemoglobin (4 HUs of SLO per 2×108 cells) (Figure 2A). The method was suitable for both synchronous parasite cultures containing ring stages or cultures fractionated using Percoll to remove the later stage parasites.
Figure 2. Isolation of ring stage parasitized RBCs using selective lysis with SLO.
(A) Infected RBC (3D7 strain, ∼5% ring stage parasites) were pelleted and treated with 4 HUs of pre-activated SLO and then repelleted. Analysis of a Giemsa-stained thin smear indicates >80% parasitaemia. (B) Triplicate aliquots of a mixed culture were treated with SLO to reach a parasitaemia of >80% or mock-treated and brought back into culture. The SLO- and mock-treated cultures were followed by daily counting of the parasitaemia. The arrow indicates a 10-fold dilution of the cultures.
Parasites isolated by density centrifugation methods are viable and can be brought back into culture, which is useful for many experiments. We tested whether treatment with SLO to remove uninfected RBCs affected the viability of the purified ring stage infected RBCs. No difference in viability and growth rates was observed between mock-treated and SLO-enriched mixed stage infected RBCs reintroduced into culture (Figure 2B). Furthermore, no adverse effects were observed in long-term cultures derived from parasites reintroduced into culture after SLO treatment. Thus SLO treatment does not appear to affect the viability of the parasite.
EqtII is an alternative permeabilizing agent for infected RBCs
In view of the difficulty of lysing infected RBCs in mixed cultures, we sought a permeabilizing agent that could be used for non-selective lysis of the host cell membrane. EqtII is a pore-forming toxin that belongs to the family of actinoporins [10,11]. The mechanism of action of this protein toxin has been extensively studied but it has not been used previously as an agent for probing the cytoplasmic compartment of parasitized RBCs. We found that approx. 1 μg of EqtII is sufficient to cause 50% lysis of 108 RBCs. We have defined this amount of EqtII as 1 HU. Complete lysis of 108 uninfected RBC was achieved using approx. 2 μg of EqtII as evidenced by the quantitative release of haemoglobin (results not shown). This is equivalent to a ratio of approximately one EqtII per 400 phospholipid molecules in good agreement with previous estimates [15,28]. Uninfected RBCs and infected RBCs of all stages were lysed with equal efficiency in both mixed cultures and in isolated mature stage infected RBCs (Figure 1A and results not shown). The intracellular parasites were not lysed by EqtII treatment as evidenced by the maintenance of the refractive index shadows around the parasites in the DIC images (Figure 1B, right-hand panel).
The malaria parasite develops within a PV that extends membranous protrusions known as the tubulovesicular network into the RBC cytoplasm. SLO has been shown to release the soluble RBC components but to leave the PV intact [6–8]. By contrast, low concentrations of the detergent saponin lyse both the RBC and PV membranes but leave the parasite plasma membrane intact [29]. We were interested to determine which membranes are lysed by EqtII. To do this we made use of the fact that we and other colleagues have previously generated a series of transfected malaria parasites expressing GFP chimaeras of proteins that are trafficked to different compartments within the parasite and the host cell cytoplasm [19–21].
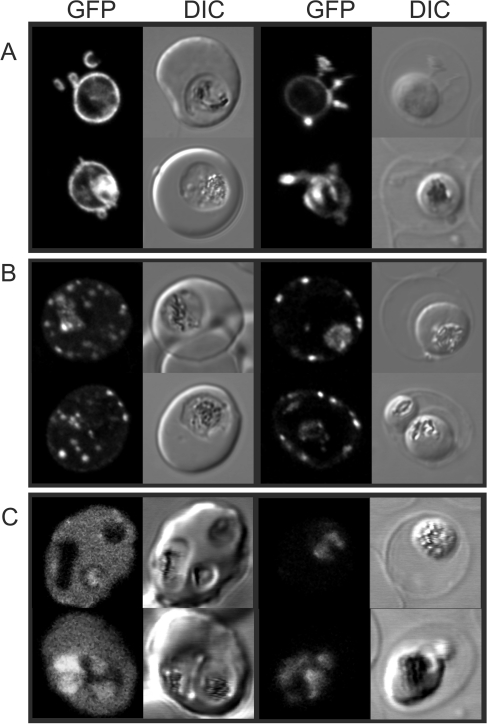
A GFP chimaera of the signal sequence of EXP1 is trafficked to the PV lumen where it is present as a soluble protein [19]. Upon lysis of the host cell membrane with EqtII, we found that EXP1–GFP is not released, indicating that the PV and tubulovesicular network compartments were not disrupted (Figure 3A). The K119-PfEMP1–GFP chimera contains a fragment of the major virulence factor of P. falciparum (PfEMP1) that is directed to the Maurer's clefts and the host cell membrane [21]. These structures are not lost during EqtII lysis (Figure 3B). By contrast, REX3 is exported to the host cell cytoplasm as a soluble protein [20]. We found that the RBC cytoplasm-located REX3–GFP was released during treatment of transfectants with EqtII, leaving only the parasite-associated population (Figure 3C).
Figure 3. Fluorescence microscopy analysis of the selective permeabilization of host membranes by EqtII.
RBCs infected with (A) EXP1–GFP, (B) Lys119-PfEMP1–GFP, or (C) REX3–GFP transfectants were left untreated (left-hand columns) or treated with 2 HUs of EqtII (right-hand columns). The fluorescence and DIC images were collected using a confocal microscope.
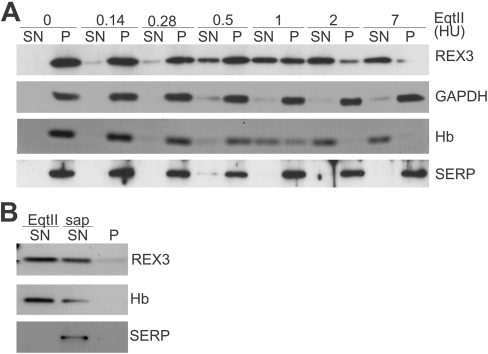
We used Western blot analysis of EqtII-treated mixed cultures to confirm these findings. EqtII (at ≥2 HUs) released haemoglobin and REX3 in a dose-dependent manner from an aliquot of malaria culture (2×108 cells, Figure 4A). In contrast with SLO, both REX3 and haemoglobin were released at similar levels of EqtII. P. falciparum GAPDH was not released from the parasite cytoplasm by this treatment, indicating that EqtII does not lyse the parasite plasma membrane (Figure 4A). SERP is a soluble PV protein [30] that has previously been used to test PV membrane integrity [6]. Importantly, EqtII treatment did not release SERP even with 7 HUs of EqtII (Figure 4A). However, SERP was readily released after lysing the PV membrane with saponin (Figure 4B).
Figure 4. Western blot analysis of the selective permeabilization of host membranes by EqtII.
(A) Aliquots of a malaria culture (2×108 cells; ∼5% parasitaemia) were treated with EqtII (amounts indicated in HUs above each lane) and separated into pellet (P) and supernatant (SN) and subjected to SDS/PAGE. The release of haemoglobin (Hb) was monitored by Coomassie Blue staining (third row). The release of P. falciparum GAPDH (soluble cytoplasmic parasite protein), SERP (PV lumen parasite protein) and REX3 (exported into the infected RBC cytoplasm) was assessed by immunoblot analysis. (B) To confirm that SERP is released if the PV membrane is breached, an aliquot of a malaria culture (2×108 cells; ∼5% parasitaemia) was treated with 1 HU of EqtII and separated into pellet and supernatant 1 (SN EqtII). The pellet was treated with 0.03% saponin in PBS and separated into pellet (P) and supernatant 2 (SN sap). More than 50% of REX3 and haemoglobin are released under these conditions but SERP is only released upon saponin treatment.
EqtII-treated RBCs have variable size pores
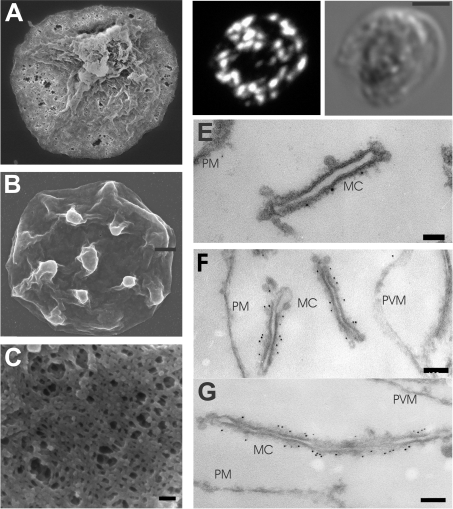
In model membranes, three to four EqtII molecules are thought to oligomerize and to initiate haemolysis by opening cation selective pores with a diameter of 1–2 nm followed by lysis due to colloid-osmotic shock [14]. Under the conditions of our experiments EqtII permeabilization of RBCs allowed efficient release of the intracellular haemoglobin (64 kDa, diameter 5.5 nm; [31]), REX3 (37 kDa) and REX3–GFP (66 kDa) from infected RBCs. This suggests that at least some of the pores are sufficiently large to release proteins. To investigate the nature of the pores formed by EqtII we examined the surface of EqtII-treated cells using scanning EM (Figures 5A and 5C). The surface of EqtII-treated infected RBCs is pitted with pores of sizes ranging up to approx. 100 nm. Mock-treated infected RBCs show some shrinkage during preparation for scanning EM but do not show perforations (Figure 5B).
Figure 5. Immunofluorescence and EM analyses of EqtII-permeabilized RBCs.
(A–C) Scanning EM. Isolated mature stage infected RBCs (3D7 strain, 108 cells) were treated with 2 HUs of EqtII (A, C) or mock-treated (B). The cells were pelleted, fixed in 2% (v/v) glutaraldehyde and prepared for scanning EM. (D) Immunofluorescence labelling. Infected RBCs were fixed with 2% paraformaldehyde, then treated with 2 HUs of EqtII, and labelled with anti-PfEMP1 serum followed by Alexa Fluor® 568 anti-rabbit IgG. (E–G) Immuno-EM. Paraformaldehyde-fixed, EqtII-permeabilized PfEMP1–GFP transfectants were labelled with anti-PfEMP1 (E) serum or anti-GFP serum (F, G), followed by Protein A–gold (6 nm conjugate). Abbreviations: MC, Maurer's clefts; PM, RBC plasma membrane; PVM, PV membrane. Scale bars: (A, B) 1 μm; (C) 100 nm; (D) 2.5 μm; (E) 50 nm; and (F) 100 nm.
Parasite proteins exported into the host cell can be accessed with antibodies in EqtII-permeabilized infected RBCs
To determine whether proteins could be effectively delivered to sites within the host RBC compartment in EqtII-permeabilized infected RBCs, we performed immunofluorescence labelling using an antibody that recognizes the cytoplasmic domain of PfEMP1 [24]. Infected RBCs were lightly fixed with paraformaldehyde, permeabilized with EqtII, then labelled with a rabbit antiPfEMP1 serum followed by a fluorescently labelled secondary antibody. Efficient labelling of infected RBCs was achieved (Figure 5D). A three-dimensional reconstruction of the anti-PfEMP1-labelled cells (Supplementary Figure S1 at http://www.BiochemJ.org/bj/403/bj4030167add.htm) reveals the excellent preservation of the three-dimensional structure of infected RBCs labelled in this manner.
We also performed immunogold labelling of epitopes that are exposed to the cytoplasm in EqtII-permeabilized infected RBCs. Lightly paraformaldehyde-fixed cells were permeabilized with EqtII, then labelled with rabbit anti-PfEMP1 serum followed by Protein A–gold. Excellent preservation of membranous structures such as the Maurer's clefts was achieved (Figures 5E–5G). The RBC plasma membrane and PV membrane were also retained. The anti-PfEMP1 serum decorates the Maurer's clefts (Figure 5E) as previously reported for SLO-permeabilized infected RBCs [9]. We observed even better labelling using anti-GFP to detect a GFP chimaera of PfEMP1 in transfected malaria parasites (Figures 5F and 5G). Lower levels of labelling were achieved when gold-labelled antibodies were used as the secondary agent rather than Protein A–gold (results not shown). In our hands the EqtII-permeabilization protocol was much more reliable for immuno-EM studies than SLO permeabilization due to variable lysis of infected RBCs by SLO even after enrichment (results not shown). We observed very little labelling of parasite structures when the primary antibody was omitted (results not shown).
EqtII-mediated lysis of RBCs is inhibited in the presence of SM-containing vesicles
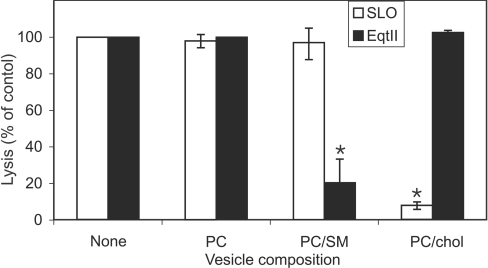
We examined the ability of membrane vesicles of different lipid composition to inhibit lysis of RBC membranes by EqtII and SLO. Insertion of EqtII into membranes has previously been shown to be favoured by the presence of SM in the target bilayer [14,15], while SLO binding is known to be cholesterol-dependent [32,33]. Small phospholipid vesicles comprised of PC or PC/cholesterol added to RBCs (at concentrations equivalent to their levels in the RBCs) had no significant effect on lysis by EqtII. However, PC/SM vesicles markedly reduced the level of lysis (Figure 6; P<0.001). This suggests that PC/SM effectively competes for binding of the toxin. By contrast, PC/cholesterol vesicles markedly reduced the level of lysis of RBCs by SLO (Figure 6; P<0.0001), while PC alone or PC/SM mixtures had no effect.
Figure 6. Effect of phospholipid vesicles of different composition on lysis by SLO and EqtII.
Uninfected RBCs (108 cells) were incubated with 2 HUs of EqtII or 4 HUs of post-activated SLO either alone or in the presence of phospholipid vesicles composed of PC, PC/SM or PC/cholesterol (chol) at concentrations equivalent to those in the RBCs. Cells were pelleted and the supernatants were assayed at 412 nm and compared with a control with no added lipid. The results shown represent the means±S.D. for triplicate determinations. The asterisk (*) indicates a P-value of <0.001.
DISCUSSION
The malaria parasite, P. falciparum, invades human RBCs and induces dramatic changes in the physical properties of the host cell membrane [34]. For example, as the trophozoite stage parasite develops, the host membrane undergoes a permeability change, which is characterized by the appearance of a novel permeation pathway. This permeability change renders mature stage parasitized RBCs susceptible to lysis by hypo-osmotic shock or solute exchange in hypertonic media and forms the basis of commonly employed sorbitol synchronization methods [16,17]. The deposition of knobs at the RBC membrane decreases the tendency of the infected RBCs to form rouleaux, which allows flotation in gelatin-containing media [18]. The RBC membrane also undergoes changes in adhesiveness and deformability and the integral membrane proteins show a marked decrease in diffusional mobility, which may be due to the deposition of oxidized haemichromes at the cytoplasmic surface [35,36]. These changes are initiated in the ring stage of infection and increased during the trophozoite stage of growth [35].
The phospholipid composition of the parasite differs markedly from the host cell membrane. Particularly notable is the apparently reduced level of cholesterol and SM in parasite membranes [37]. The amounts of these lipids are also decreased in the host membrane of infected RBCs compared with uninfected RBCs [38] possibly due to the uptake of ‘raft’-like regions of the RBC membrane [39].
In the present study, we have found that in mixed cultures infected RBCs are much more resistant to lysis by SLO than uninfected RBCs. This may be due to cross-linking of integral membrane proteins, which may inhibit insertion of SLO into the host cell membrane. Alternatively the decrease in the levels of cholesterol and/or polyunsaturated phospholipids in the infected RBC membrane [37] may inhibit insertion of SLO into the infected RBC membrane [32].
It is not possible to achieve parasitaemias of much more than 10% during in vitro culture of P. falciparum. The resulting excess of uninfected RBCs is a drawback for many experiments. Differential sedimentation methods that are currently used to separate infected RBCs from uninfected RBCs rely on changes to the infected RBC that occur approx. 20 h post-invasion. Similarly, magnetic columns used to purify infected RBCs rely on the presence of haemozoin, which accumulates in later stage parasites. There are relatively few changes to the host cell in the first half of the asexual life cycle (comprising ring stages and young trophozoites) and hence it is very difficult to isolate these stages. In the present study, we have shown that SLO can be used to preferentially lyse uninfected RBCs, providing a convenient method of isolating early stage infected RBCs. These isolated ring-infected RBCs could be used to undertake biochemical, genetic or immunochemical analyses of this relatively unstudied stage of the parasite life cycle.
We sought an alternative pore-former that would lyse uninfected and infected RBCs with equal efficiency and found that the eukaryotic toxin, EqtII, performed well for this purpose. EqtII is available as a recombinant protein and is readily purified from E. coli lysates [40]. EqtII does not need to be activated and is highly stable during storage. The toxin induces close to 100% lysis of RBCs at a ratio of approximately one protein molecule per 400 phospholipid molecules. The protein prefers SM-containing target membranes [15] and we found that addition of an equimolar level of SM-containing vesicles inhibited lysis of RBCs. Uninfected RBC membranes have approx. 26% SM [41], while parasite membranes apparently have reduced levels of SM [37]. The host membrane of mature parasite-infected RBCs has been reported to have decreased SM levels [42,43]; however, this decrease does not appear to be sufficient to prevent EqtII-mediated lysis.
We found that EqtII lyses the host cell membrane but not the PV membrane surrounding the intracellular parasite. This was evidenced by the release of soluble protein from the RBC cytoplasm but not from the PV or the parasite cytoplasm. If the pool of bound tetrameric EqtII is in equilibrium with a population of soluble monomers, then some of the toxin molecules would be expected to enter the pores and access the inner membrane. Preservation of the PV membrane may be due to a much lower SM content [37,44]. There is some evidence for incorporation of ceramide into lipids at the PV membrane [45,46]; however, no SM synthase has been identified in the P. falciparum genome.
The ultrastructure of parasitized RBCs was maintained upon EqtII treatment, especially if a light paraformaldehyde fixation was used before or after addition of EqtII. Membranous structures, such as the Maurer's clefts, were well preserved. Scanning EM and transmission EM analyses revealed that the pores formed by EqtII under the conditions employed were up to 100 nm in diameter. This allows efficient release of haemoglobin.
Permeabilized cells were efficiently labelled with fluorescently labelled antibodies recognizing the cytoplasmic domain of the integral membrane protein, PfEMP1. Immunogold labelling of structures in the RBC cytoplasm was also very efficient, especially if Protein A–gold was employed rather than gold-labelled antibodies as the secondary reagent. IgGs are Y-shaped molecules with a longest dimension of approx. 12 nm [47,48]. Thus the pores generated by EqtII are large enough to allow efficient access of unlabelled or fluorescently labelled IgG but addition of 10 nm gold particles decreases the efficiency of entry. The ability of EqtII to efficiently and consistently permeabilize infected RBCs even in the presence of uninfected RBCs renders it a more useful reagent than SLO for use in protocols designed to label the components of the host cell cytoplasm.
Recent studies have indicated that P. falciparum exports over 400 proteins to the infected RBC cytoplasm [49] where they presumably play important roles in promoting the virulence of this major human pathogen. We have provided a number of novel experimental tools that allow better manipulation of infected RBCs and better access to the host cell compartment. These techniques should be useful in determining the organization and function of these exported proteins.
Online data
Acknowledgments
This work was supported by the National Health and Medical Research Council, Australia. T.S. was supported by the Swiss National Science Foundation and the Alexander von Humboldt Stiftung. We thank Sam Deed for technical support and Dr Gregor Anderluh for providing EqtII. We thank Dr Ellen Knuepfer and Professor Alan Cowman (Walter and Eliza Hall Institute, Melbourne, VIC, Australia) for supplying the K119-PfEMP1–GFP transfectants and Professor K. Lingelbach and Dr C. Daubenberger for antibodies.
References
- 1.Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 1985;47:52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckingham L., Duncan J. L. Approximate dimensions of membrane lesions produced by streptolysin S and streptolysin O. Biochim. Biophys. Acta. 1983;729:115–122. doi: 10.1016/0005-2736(83)90462-5. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med. Microbiol. Immunol. (Berlin) 1993;182:167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- 4.Walev I., Bhakdi S. C., Hofmann F., Djonder N., Valeva A., Aktories K., Bhakdi S. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marti M., Rug M., Baum J., Tilley L., Cowman A. F. Signal mediated export of proteins from the malaria parasite to the host erythrocyte. J. Cell Biol. 2005;171:587–592. doi: 10.1083/jcb.200508051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansorge I., Benting J., Bhakdi S., Lingelbach K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem. J. 1996;315:307–314. doi: 10.1042/bj3150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansorge I., Paprotka K., Bhakdi S., Lingelbach K. Permeabilization of the erythrocyte membrane with streptolysin O allows access to the vacuolar membrane of Plasmodium falciparum and a molecular analysis of membrane topology. Mol. Biochem. Parasitol. 1997;84:259–261. doi: 10.1016/s0166-6851(96)02806-x. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister S., Paprotka K., Bhakdi S., Lingelbach K. Selective permeabilization of infected host cells with pore-forming proteins provides a novel tool to study protein synthesis and viability of the intracellular apicomplexan parasites Plasmodium falciparum and Toxoplasma gondii. Mol. Biochem. Parasitol. 2001;112:133–137. doi: 10.1016/s0166-6851(00)00343-1. [DOI] [PubMed] [Google Scholar]
- 9.Kriek N., Tilley L., Horrocks P., Pinches R., Elford B. C., Ferguson D. J., Lingelbach K., Newbold C. I. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol. Microbiol. 2003;50:1215–1227. doi: 10.1046/j.1365-2958.2003.03784.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderluh G., Macek P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40:111–124. doi: 10.1016/s0041-0101(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 11.Athanasiadis A., Anderluh G., Macek P., Turk D. Crystal structure of the soluble form of equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure. 2001;9:341–346. doi: 10.1016/s0969-2126(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 12.Norton R. S., Macek P., Reid G. E., Simpson R. J. Relationship between the cytolysins tenebrosin-C from Actinia tenebrosa and equinatoxin II from Actinia equina. Toxicon. 1992;30:13–23. doi: 10.1016/0041-0101(92)90497-s. [DOI] [PubMed] [Google Scholar]
- 13.Kristan K., Podlesek Z., Hojnik V., Gutierrez-Aguirre I., Guncar G., Turk D., Gonzalez-Manas J. M., Lakey J. H., Macek P., Anderluh G. Pore formation by equinatoxin, a eukaryotic pore-forming toxin, requires a flexible N-terminal region and a stable beta-sandwich. J. Biol. Chem. 2004;279:46509–46517. doi: 10.1074/jbc.M406193200. [DOI] [PubMed] [Google Scholar]
- 14.Belmonte G., Pederzolli C., Macek P., Menestrina G. Pore formation by the sea anemone cytolysin equinatoxin II in red blood cells and model lipid membranes. J. Membr. Biol. 1993;131:11–22. doi: 10.1007/BF02258530. [DOI] [PubMed] [Google Scholar]
- 15.Bonev B. B., Lam Y. H., Anderluh G., Watts A., Norton R. S., Separovic F. Effects of the eukaryotic pore-forming cytolysin Equinatoxin II on lipid membranes and the role of sphingomyelin. Biophys. J. 2003;84:2382–2392. doi: 10.1016/S0006-3495(03)75044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aley S. B., Sherwood J. A., Marsh K., Eidelman O., Howard R. J. Identification of isolate-specific proteins on sorbitol-enriched Plasmodium falciparum infected erythrocytes from Gambian patients. Parasitology. 1986;92:511–525. doi: 10.1017/s0031182000065410. [DOI] [PubMed] [Google Scholar]
- 17.Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 18.Pasvol G., Wilson R. J., Smalley M. E., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann. Trop. Med. Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 19.Adisa A., Rug M., Klonis N., Foley M., Cowman A. F., Tilley L. The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites. J. Biol. Chem. 2003;278:6532–6542. doi: 10.1074/jbc.M207039200. [DOI] [PubMed] [Google Scholar]
- 20.Spielmann T., Hawthorne P. L., Dixon M. W., Hannemann M., Klotz K., Kemp D. J., Klonis N., Tilley L., Trenholme K. R., Gardiner D. L. A cluster of ring stage-specific genes linked to a locus implicated in cytoadherence in Plasmodium falciparum codes for PEXEL-negative and PEXEL-positive proteins exported into the host cell. Mol. Biol. Cell. 2006;17:3613–3624. doi: 10.1091/mbc.E06-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knuepfer E., Rug M., Klonis N., Tilley L., Cowman A. F. Trafficking of the major virulence factor to the surface of transfected P. falciparum-infected erythrocytes. Blood. 2005;105:4078–4087. doi: 10.1182/blood-2004-12-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spielmann T., Gardiner D. L., Beck H. P., Trenholme K. R., Kemp D. J. Organization of ETRAMPs and EXP-1 at the parasite–host cell interface of malaria parasites. Mol. Microbiol. 2006;59:779–794. doi: 10.1111/j.1365-2958.2005.04983.x. [DOI] [PubMed] [Google Scholar]
- 23.Daubenberger C. A., Tisdale E. J., Curcic M., Diaz D., Silvie O., Mazier D., Eling W., Bohrmann B., Matile H., Pluschke G. The N′-terminal domain of glyceraldehyde-3-phosphate dehydrogenase of the apicomplexan Plasmodium falciparum mediates GTPase Rab2-dependent recruitment to membranes. Biol. Chem. 2003;384:1227–1237. doi: 10.1515/BC.2003.135. [DOI] [PubMed] [Google Scholar]
- 24.Frankland S., Adisa A., Horrocks P., Taraschi T. F., Schneider T., Elliott S. R., Rogerson S. J., Knuepfer E., Cowman A. F., Newbold C. I., Tilley L. Delivery of the malaria virulence protein PfEMP1 to the erythrocyte surface requires cholesterolrich domains. Eukaryot. Cell. 2006;5:849–860. doi: 10.1128/EC.5.5.849-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adisa A., Rug M., Foley M., Tilley L. Characterisation of delta-COP homologue in the malaria parasite, Plasmodium falciparum. Mol. Biochem. Parasitol. 2002;123:11–21. doi: 10.1016/s0166-6851(02)00117-2. [DOI] [PubMed] [Google Scholar]
- 26.Goodyer I. D., Johnson J., Eisenthal R., Hayes D. J. Purification of mature-stage Plasmodium falciparum by gelatine flotation. Ann. Trop. Med. Parasitol. 1994;88:209–211. doi: 10.1080/00034983.1994.11812859. [DOI] [PubMed] [Google Scholar]
- 27.Trang D. T., Huy N. T., Kariu T., Tajima K., Kamei K. One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar. J. 2004;3:7. doi: 10.1186/1475-2875-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderluh G., Krizaj I., Strukelj B., Gubensek F., Macek P., Pungercar J. Equinatoxins, pore-forming proteins from the sea anemone, Actinia equina, belong to a multigene family. Toxicon. 1999;37:1391–1401. doi: 10.1016/s0041-0101(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 29.Burghaus P. A., Lingelbach K. Luciferase, when fused to an N-terminal signal peptide, is secreted from transfected Plasmodium falciparum and transported to the cytosol of infected erythrocytes. J. Biol. Chem. 2001;276:26838–26845. doi: 10.1074/jbc.M100111200. [DOI] [PubMed] [Google Scholar]
- 30.Delplace P., Fortier B., Tronchin G., Dubremetz J. F., Vernes A. Localization, biosynthesis, processing and isolation of a major 126 kDa antigen of the parasitophorous vacuole of Plasmodium falciparum. Mol. Biochem. Parasitol. 1987;23:193–201. doi: 10.1016/0166-6851(87)90026-0. [DOI] [PubMed] [Google Scholar]
- 31.Liddington R., Derewenda Z., Dodson E., Hubbard R., Dodson G. High resolution crystal structures and comparisons of T-state deoxyhaemoglobin and two liganded T-state haemoglobins: T(alpha-oxy)haemoglobin and T(met)haemoglobin. J. Mol. Biol. 1992;228:551–579. doi: 10.1016/0022-2836(92)90842-8. [DOI] [PubMed] [Google Scholar]
- 32.Zitzer A., Bittman R., Verbicky C. A., Erukulla R. K., Bhakdi S., Weis S., Valeva A., Palmer M. Coupling of cholesterol and cone-shaped lipids in bilayers augments membrane permeabilization by the cholesterol-specific toxins streptolysin O and Vibrio cholerae cytolysin. J. Biol. Chem. 2001;276:14628–14633. doi: 10.1074/jbc.M100241200. [DOI] [PubMed] [Google Scholar]
- 33.Prigent D., Alouf J. E. Interaction of steptolysin O with sterols. Biochim. Biophys. Acta. 1976;443:288–300. doi: 10.1016/0005-2736(76)90511-3. [DOI] [PubMed] [Google Scholar]
- 34.Cooke B. M., Lingelbach K., Bannister L., Tilley L. Protein trafficking in Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2004;20:581–589. doi: 10.1016/j.pt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Parker P. D., Tilley L., Klonis N. Plasmodium falciparum induces reorganization of host membrane proteins during intraerythrocytic growth. Blood. 2004;103:2404–2406. doi: 10.1182/blood-2003-08-2692. [DOI] [PubMed] [Google Scholar]
- 36.McPherson R. A., Sawyer W. H., Tilley L. Rotational diffusion of the erythrocyte integral membrane protein band 3: effect of hemichrome binding. Biochemistry. 1992;31:512–518. doi: 10.1021/bi00117a030. [DOI] [PubMed] [Google Scholar]
- 37.Vial H. J., Eldin P., Tielens A. G., van Hellemond J. J. Phospholipids in parasitic protozoa. Mol. Biochem. Parasitol. 2003;126:143–154. doi: 10.1016/s0166-6851(02)00281-5. [DOI] [PubMed] [Google Scholar]
- 38.Maguire P. A., Sherman I. W. Phospholipid composition, cholesterol content and cholesterol exchange in Plasmodium falciparum-infected red cells. Mol. Biochem. Parasitol. 1990;38:105–112. doi: 10.1016/0166-6851(90)90210-d. [DOI] [PubMed] [Google Scholar]
- 39.Samuel B. U., Mohandas N., Harrison T., McManus H., Rosse W., Reid M., Haldar K. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J. Biol. Chem. 2001;276:29319–29329. doi: 10.1074/jbc.M101268200. [DOI] [PubMed] [Google Scholar]
- 40.Anderluh G., Pungercar J., Strukelj B., Macek P., Gubensek F. Cloning, sequencing, and expression of equinatoxin II. Biochem. Biophys. Res. Commun. 1996;220:437–442. doi: 10.1006/bbrc.1996.0391. [DOI] [PubMed] [Google Scholar]
- 41.Yawata Y. Weinheim: Wiley-VCH Verlag; 2003. Cell Membrane: The Red Blood Cell as a Model. [Google Scholar]
- 42.Maguire P. A., Prudhomme J., Sherman I. W. Alterations in erythrocyte membrane phospholipid organization due to the intracellular growth of the human malaria parasite, Plasmodium falciparum. Parasitology. 1991;102:179–186. doi: 10.1017/s0031182000062466. [DOI] [PubMed] [Google Scholar]
- 43.Hsiao L. L., Howard R. J., Aikawa M., Taraschi T. F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem. J. 1991;274:121–132. doi: 10.1042/bj2740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vial H. J., Ancelin M. L., Philippot J. R., Thuet M. J. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells. 1990;16:531–555. [PubMed] [Google Scholar]
- 45.Haldar K., Uyetake L., Ghori N., Elmendorf H. G., Li W. L. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol. Biochem. Parasitol. 1991;49:143–156. doi: 10.1016/0166-6851(91)90137-u. [DOI] [PubMed] [Google Scholar]
- 46.Lauer S. A., Chatterjee S., Haldar K. Uptake and hydrolysis of sphingomyelin analogues in Plasmodium falciparum-infected red cells. Mol. Biochem. Parasitol. 2001;115:275–281. doi: 10.1016/s0166-6851(01)00281-x. [DOI] [PubMed] [Google Scholar]
- 47.Leatherbarrow R. J., Stedman M., Wells T. N. Structure of immunoglobulin G by scanning tunnelling microscopy. J. Mol. Biol. 1991;221:361–365. doi: 10.1016/0022-2836(91)80056-z. [DOI] [PubMed] [Google Scholar]
- 48.Silverton E. W., Navia M. A., Davies D. R. Three-dimensional structure of an intact human immunoglobulin. Proc. Natl. Acad. Sci. U.S.A. 1977;74:5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sargeant T. J., Marti M., Caler E., Carlton J. M., Simpson K., Speed T. P., Cowman A. F. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 2006;7:R12. doi: 10.1186/gb-2006-7-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.