Abstract
Eukaryotic cells control the levels of molecular chaperones and folding enzymes in the endoplasmic reticulum (ER) by a transcriptional induction process termed the unfolded protein response (UPR) according to the needs within the ER. In Saccharomyces cerevisiae, expression of the UPR-specific transcription factor Hac1p is tightly regulated at the level of mRNA splicing that depends on an unconventional system. Thus, HAC1 precursor mRNA is constitutively expressed but not translated. A sensor molecule Ire1p/Ern1p-mediated signaling from the ER specifically removes an intron of 252 nucleotides from the precursor mRNA, and the resulting mature mRNA is translated to produce Hac1p. Because the 5′ splice site is located near the C-terminal end of the Hac1p-coding region, this splicing replaces the last 10 codons of the ORF with an exon encoding 18 aa without affecting the N-terminal 220-aa region which contains the DNA-binding domain. Here, we found that this C-terminal 18-aa segment functions as a potent activation domain. Therefore, the splicing event joins the HAC1 DNA-binding domain to its activation domain, allowing rapid posttranscriptional generation of a potent transcriptional activator (238-aa Hac1p) that activates the UPR efficiently. This suggests that the UPR is hardly activated by Hac1p produced without splicing (230-aa Hac1p) which may occur in the absence of Ire1p/Ern1p-mediated signaling from the ER. Based on these and other results, we propose that the control of expression and activity of Hac1p meets the requirements of the ER.
The levels of molecular chaperones and folding enzymes in the endoplasmic reticulum (ER) are adjusted by an intracellular signaling pathway from the ER to the nucleus termed the unfolded protein response (UPR). Thus, when unfolded proteins are accumulated in the ER, the UPR is activated to induce transcription of these luminal protein genes in the nucleus, primarily to increase the folding capacity and thus maintain homeostasis of the ER (1–3). Extensive studies of the molecular mechanism of the UPR in Saccharomyces cerevisiae led to the identification of three genes as essential components of the UPR: the IRE1/ERN1 gene encoding a transmembrane protein kinase Ire1p believed to act as a sensor in the ER (4, 5), the HAC1/ERN4 gene encoding a basic leucine zipper-type transcription factor Hac1p (6–8) which directly recognizes the cis-acting UPR element (UPRE) present in the promoter regions of target genes (9–11), and the RLG1 gene encoding tRNA ligase Rlg1p (12). In addition, the PTC2 gene was identified; Ptc2p is a protein serine/threonine phosphatase that appears to regulate Ire1p negatively (13). In S. cerevisiae, the UPR is coupled to inositol biosynthesis; cells lacking Ire1p or Hac1p are not only defective in the UPR but also require inositol for growth (4–8, 12, 14). It was suggested that production of ER proteins and ER membrane is coordinately regulated by the UPR (15).
The UPR signal transduction system is distinguished by the use of an unconventional mRNA splicing system to generate the UPR-specific transcription factor Hac1p (6, 16, 17). Hac1p is present only in cells in which the UPR is activated because HAC1 mRNA constitutively expressed as a 1.4-kb precursor mRNA (premRNA) is not translated because of the presence of an intron of 252 nucleotides until mRNA splicing is induced in response to accumulation of unfolded proteins in the ER. Ire1p-mediated signaling from the ER specifically removes the intron from HAC1 premRNA, resulting in efficient translation of the 1.2-kb HAC1 mature mRNA thus produced. Synthesized Hac1p activates transcription of UPR target genes in the nucleus through binding to the UPRE. In addition to the observation that the sequences around the splice sites do not match the consensus for conventional mRNA splicing (6, 16), spliceosome-independent, sequence-specific, and nonsequential cleavage of the splice sites as well as direct involvement of tRNA ligase and Ire1p (12, 18–20) indicated that the HAC1 mRNA processing system represents a unique type of mRNA splicing in eukaryotes.
Because the 5′ splice site is located within the Hac1p-coding region, mRNA splicing replaces the C-terminal region of Hac1p without affecting the N-terminal 220 aa; the premRNA and mature mRNA encode proteins of 230 aa (230-aa Hac1p) and 238 aa (238-aa Hac1p), respectively, and therefore the tail of Hac1p switches from 10 aa to the second exon-encoded 18 aa (6, 16) (Fig. 1). Although it was initially proposed that this replacement would markedly affect the stability of the proteins synthesized (6), subsequent analysis has demonstrated that 230-aa Hac1p forced to be expressed in vivo is as unstable as 238-aa Hac1p within cells (half-life ≈2 min) and that the presence of the intron blocks translation of HAC1 pre-mRNA (16, 17). Thus, Hac1p is synthesized as the result of mRNA splicing induced by Ire1p-mediated signaling from the ER, leading to activation of the UPR.
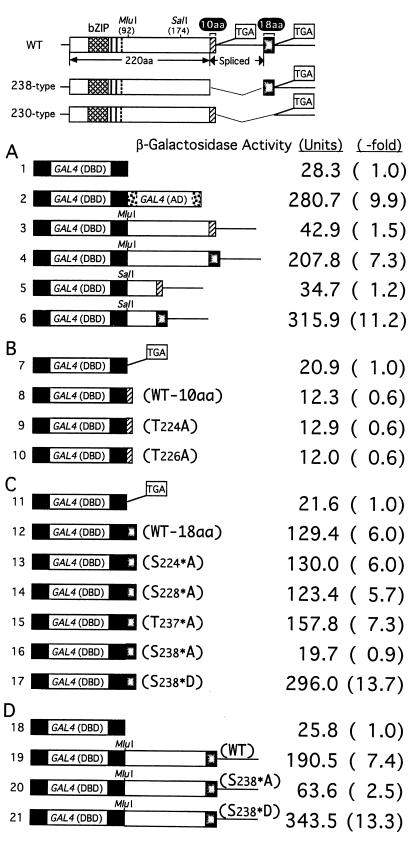
Figure 1.
Transactivation activities of various fragments derived from 230-aa and 238-aa Hac1p. (Upper) Structures of the WT and mutant HAC1 (238 type and 230 type) are shown schematically. Boxed TGAs represent stop codons present in constructs, and thin lines denote nucleotide segments deleted from HAC1. The DBD of HAC1 is marked by basic leucine zipper. The 10-aa segment of 230-aa Hac1p and 18-aa segment of 238-aa Hac1p are indicated by the hatched and stippled boxes, respectively. (A–D) The ire1Δ strain KMY1100 carrying the UASGAL-CYC1-lacZ reporter gene on the chromosome was transformed with various GAL4(DBD) fusion constructs, the structures of which are shown schematically. Transformants were grown at 30°C in SC(−Leu) medium to OD600 of ≈0.5. β-Gal activities determined are presented as means, based on duplicate determinations with three independent transformants. Standard deviation was >10% for all values shown. The ratio of each activity to that of vector control is presented as -fold increase.
In this study, we performed experiments to determine the biological significance of the C-terminal replacement caused by mRNA splicing. From the results obtained, we propose here a model for expression and activity of Hac1p in the yeast UPR.
Materials and Methods
Strains and Microbiological Techniques.
The yeast strains used in this study were KMY1105 (MATα leu2–3, 112 ura3–52 his3-Δ200 trp1-Δ901 lys2–801 ura3–52∷URA3-UPRE-CYC1-lacZ); KMY1115 (KMY1105 ire1Δ∷TRP1), KMY1145 (KMY1105 hac1Δ∷TRP1) (16); and KMY1100 (MATα leu2–3, 112 ura3–52 his3-Δ200 trp1-Δ901 lys2–801 ire1Δ∷TRP1 his3-Δ200∷HIS3-UASGAL-CYC1-lacZ) (this study). The compositions of synthetic complete media used for selection of transformants such as SC(−Leu, Ura) were described (21). Inositol-free media were prepared as described (22). Tunicamycin (TM; Sigma) was used at a concentration of 5 μg/ml. Yeast cells were transformed by the lithium acetate method (23).
Construction of Plasmids.
Recombinant DNA techniques were performed as described (24). YCp-HAC1(WT), YCp-HAC1(230 type), and YCp-HAC1(238 type) were described (16). Site-directed mutagenesis of HAC1 was performed by using a QuikChange site-directed mutagenesis kit (Stratagene). The 0.3-kb fragment of pGADGH (CLONTECH) carrying the activation domain of the yeast transcriptional activator Gal4p [GAL4(AD)] was inserted into the SpeI site of YEp-HAC1 (7) in-frame to generate YEp-GAL4(AD)-HAC1.
The 1.8-kb SnaBI–NaeI fragment of pGBT9 (CLONTECH) containing the ADH1 promoter-DNA-binding domain of Gal4p [GAL4(DBD)]-multicloning site-ADH1 terminator was transferred to YCp-L2 (CEN4-ARS1-based single-copy vector carrying the LEU2 selectable marker) (7). The 0.5-kb HindIII–SalI fragment of the resulting plasmid was replaced with the corresponding fragment of pAS2 (CLONTECH) to introduce a hemagglutinin epitope tag between GAL4(DBD) and the multicloning site. This plasmid is referred to here as YCp-GAL4(DBD) and various fragments of HAC1 or synthetic, double-stranded oligonucleotides encoding the 10-aa or 18-aa segment were inserted into appropriate restriction enzyme sites of YCp-GAL4(DBD).
Assays.
β-Galactosidase (β-gal) assays and Northern blot hybridization analysis were performed as described (5, 7). Labeling of yeast cells at 30°C with [35S]Met and [35S]Cys [100 μCi (3.7 MBq) per cells of 2 OD600 units per sample] with EXPRE35S35S protein-labeling mix (DuPont) and subsequent immunoprecipitation of 35S-labeled Hac1p were performed as described (16).
Results
Molecular Basis for the Increase in Transactivation Activity Caused by C-Terminal Replacement of Hac1p.
It was demonstrated previously that not only the mature mRNA product of the HAC1 gene (238-aa Hac1p) but also the premRNA product (230-aa Hac1p) were synthesized and thus detected when an intronless construct such as YCp-HAC1 (238 type) or YCp-HAC1 (230 type) (Fig. 1 has schematic structures) was introduced into yeast cells (16, 17). Interestingly, the levels of β-gal produced from a reporter gene in cells expressing 238-aa Hac1p were much greater than those in cells expressing 230-aa Hac1p (16, 17), suggesting that 238-aa Hac1p may have higher transactivation activity than 230-aa Hac1p despite the small difference in the C-terminal region.
To identify the region(s) of HAC1 responsible for such a difference in transactivation by 230-aa Hac1p and 238-aa Hac1p, we fused various Hac1p subregions immediately downstream of the DBD of the yeast transcriptional activator Gal4p [referred to as GAL4(DBD)]. Transcriptional activation was determined by measuring the level of β-gal expressed from a UASGAL-CYC1-lacZ reporter gene integrated into the chromosome (Fig. 1). To eliminate endogenous Hac1p-dependent transcription, these experiments were performed in a strain lacking Ire1p required for splicing of HAC1 mRNA (6, 16). When the subregions starting from the MluI site (amino acid 92) and thus lacking the basic leucine zipper domain of Hac1p were fused, the 230-aa type construct increased β-gal activity by only 1.5-fold over the vector control (Fig. 1; compare lines 3 and 1), whereas the 238-type construct did so by 7.3-fold (line 4). Such a difference in transactivation was maintained when the subregions starting from the SalI site (aa 174) were compared (lines 5 and 6). These results suggested that the difference in transactivation could be ascribed to the C-terminal tail regions replaced by splicing.
We therefore joined synthetic oligonucleotides encoding either the last 10 aa of the 230-aa Hac1p or the last 18 aa of the 238-aa Hac1p to the GAL4(DBD) and tested their activation potential. To avoid possible complications arising from aa encoded by the multicloning site sequence, a stop codon was generated at the site of oligonucleotide insertion, which resulted in only a slight decrease in the level of β-gal (lines 7 and 11; compare with line 1). Insertion of the last 10 aa of 230-aa Hac1p decreased β-gal activity (line 8), indicating that these aa do not activate transcription. In marked contrast, insertion of the last 18 aa of 238-aa Hac1p enhanced β-gal activity by 6-fold (line 12). This extent of activation approached 9.9-fold enhancement achieved by the Gal4p activation domain [referred to as GAL4(AD); line 2]. Thus, the second exon-encoded 18-aa segment functions as a potent activation domain.
Examination of aa sequences indicated that the 10-aa segment contained two Thr, whereas the 18-aa segment contained three Ser and one Thr, suggesting that phosphorylation may play a role in transcriptional activity. We therefore mutated each of these aa to Ala, and examined the activation potential of the mutant when fused to the GAL4(DBD). The aa encoded by the second exon are marked by asterisks to discriminate them from those in the 10-aa segment. Changes in neither Thr224 nor Thr226 in the 10-aa segment to Ala affected β-gal expression (compare lines 9 and 10 with line 8). In contrast, the change of Ser238* to Ala completely abolished the increase in β-gal (compare line 16 with line 12), whereas other changes had almost no effect (lines 13–15). Furthermore, the change of Ser238* to Asp more than doubled the increase in β-gal expression (line 17), indicating the importance of a negative charge at aa238*.
To assess the contribution of the 18-aa segment-mediated transactivation to the overall transcriptional activity of Hac1p, we introduced the S238*A or S238*D mutation into the fusion of GAL4(DBD) with the 92–238-aa region of the 238-aa Hac1p. As compared with the wild type (WT; line 19), the change of Ser238* to Ala decreased the β-gal expression to one third (line 20), whereas the change to Asp increased the β-gal expression by 1.8-fold (line 21). These results strongly suggested that the 18-aa segment plays a major role in transactivation activity of Hac1p and that Ser238* in the 18-aa exon is crucial for transactivation, probably because it is phosphorylated.
238-aa Hac1p Has Significantly Higher Transactivation Activity than 230-aa Hac1p.
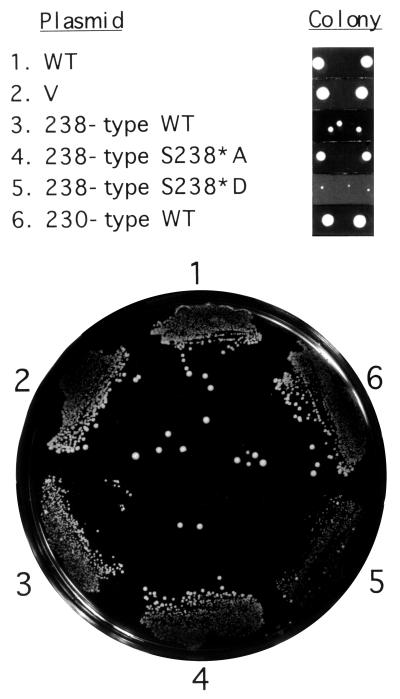
To examine whether transactivation activities exhibited by fusion with GAL4(DBD) actually reflected those of Hac1p, we introduced the S238*A or S238*D mutation into 238-aa Hac1p and examined their effects. Because HAC1 is not essential, hac1Δ cells carrying the vector alone grew as fast as those carrying a single-copy expression plasmid YCp-HAC1(WT) (Fig. 1 Upper for its schematic structure) which produces 238-aa Hac1p in response to accumulation of unfolded proteins in the ER (Fig. 2; compare lines 1 and 2). As reported (6, 16), however, constitutive expression of large amounts of 238-aa Hac1p from the intronless construct YCp-HAC1(238 type) caused slow growth (line 3), presumably because constitutively elevated levels of the UPR target proteins in the absence of unfolded proteins accumulated in the ER are toxic to cells. In contrast, constitutive expression of large amounts of 230-aa Hac1p from the similar intronless construct YCp-HAC1(230 type) did not affect the growth rate (line 6) because of its low transactivation potential (Fig. 3). We found that, as compared with WT (line 3), the change of Ser238* to Asp caused even slower growth (line 5), whereas the change to Ala supported faster growth (line 4). Thus, the relative colony sizes of transformants expressing various mutant forms of Hac1p (Fig. 2) were inversely correlated with their transactivation activities determined by the GAL4(DBD) fusions (Fig. 1).
Figure 2.
Effects of expression of various Hac1p mutants on cell growth. The hac1Δ strain KMY1145 with the UPRE-CYC1-lacZ reporter gene integrated into the chromosome was transformed with vector alone (V, YCp-L2) (7) or a single-copy expression plasmid carrying WT or mutant HAC1, streaked on SC(−Leu, Ura) agar, and grown at 30°C for 3 days. Relative sizes of colonies of typical transformants obtained 4 days after transformation are also shown at the top for comparison.
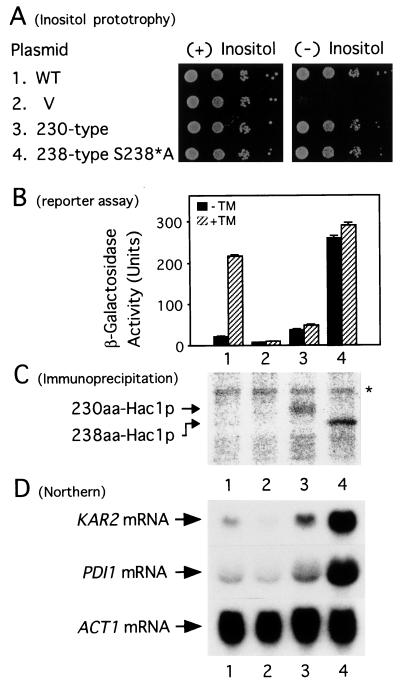
Figure 3.
Comparison of transcriptional activator activity of 230-aa Hac1p and 238-aa Hac1p(S238*A). KMY1145 was transformed with vector alone (V) or a single-copy expression plasmid carrying WT or mutant HAC1. Transformants were grown at 30°C in SC(−Leu, Ura) medium. (A) Overnight cultures were diluted with H2O to OD600 of ≈0.1 and then a series of 10-fold dilutions were prepared. Aliquots (2 μl) each were spotted onto SC(−Leu, Ura) agar containing (+) or lacking (−) inositol and incubated at 30°C for 2 days. (B) Aliquots of cells at midlogarithmic phase were incubated at 30°C for 3 h in the presence (hatched bars) or absence (solid bars) of TM. β-gal activities determined are presented as means ± standard deviation (bars), based on duplicate determinations with three independent transformants. (C) Each transformant was grown in the absence of TM and pulse labeled at 30°C for 6 min with [35S]Met and [35S]Cys. Hac1p was then immunoprecipitated and subjected to SDS/PAGE (12% gel). 35S-labeled bands were visualized and quantified by using a BioImaging Analyzer BAS-2000 (Fuji). The positions of 230-aa Hac1p and 238-aa Hac1p are indicated and the asterisk denotes a nonspecific band. It should be noted that 238-aa Hac1p migrated faster than 230-aa Hac1p because of the difference in number of charged residues in the tail region (16). (D) Total RNA was extracted from each transformant grown at 30°C to midlogarithmic phase in the absence of TM and analyzed by Northern blot hybridization by using a 32P-labeled probe specific to KAR2, PDI1, or ACT1. mRNA bands were visualized by exposing the filter to x-ray film.
Nonetheless, we could not determine the actual difference in transactivation potential between 238-aa Hac1p and 230-aa Hac1p mainly because of the slow growth rate of cells expressing 238-aa Hac1p from the intronless construct YCp-HAC1(238 type). To circumvent this difficulty, we compared transactivation activity of 230-aa Hac1p with that of mutant 238-aa Hac1p. Cells expressing 238-aa Hac1p(S238*A) from YCp-HAC1(238 type-S238*A) showed comparable growth to those expressing 230-aa Hac1p from YCp-HAC1(230 type) (Fig. 2). In addition, both 238-aa Hac1p(S238*A) and 230-aa Hac1p suppressed the inositol requirement of the hac1Δ strain completely (Fig. 3A). In contrast, the level of β-gal constitutively produced from the UPRE-CYC1-lacZ reporter gene in cells expressing 238-aa Hac1p(S238*A) (Fig. 3B; lane 4) was eightfold greater than that in cells expressing 230-aa Hac1p (Fig. 3B; lane 3) when β-gal obtained with the vector control (Fig. 3B; lane 2) was subtracted. Northern blot hybridization analysis showed that the levels of KAR2 mRNA and PDI1 mRNA, targets of the UPR, were correlated well with those of β-gal (Fig. 3D). On the other hand, immunoprecipitation analysis revealed that the amount of 238-aa Hac1p(S238*A) constitutively expressed in the transformant was comparable (only twofold greater) with that of 230-aa Hac1p (Fig. 3C). This quantitative analysis clearly indicated that 238-aa Hac1p(S238*A) possesses 4-fold higher transactivation activity than 230-aa Hac1p. Given the considerable difference in transactivation by 238-aa Hac1p(WT) and 238-aa Hac1p(S238*A) (Figs. 1 and 2), we concluded that mRNA splicing-mediated C-terminal replacement of Hac1p has an impact on the UPR because the transactivation potential of the synthesized protein was markedly enhanced by the replacement.
Hac1p Needs to Acquire an Activation Domain to Activate the UPR Efficiently.
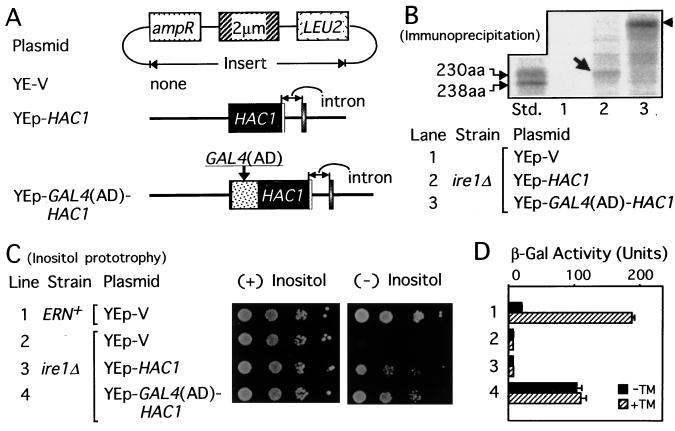
HAC1 was previously isolated as a multicopy suppressor of the ire1Δ strain (6, 8). We also found that when HAC1 was expressed from a multicopy vector designated as YEp-HAC1 (Fig. 4A) in the ire1Δ strain, the inositol requirement was suppressed (7). Because no splicing should take place in the ire1Δ strain (6, 16), these results suggested that multicopy expression could somehow release HAC1 premRNA from the translational block, possibly allowing expression of 230-aa Hac1p. Indeed, small amounts of 230-aa Hac1p were detected by immunoprecipitation from the ire1Δ cells carrying YEp-HAC1 (Fig. 4B, lane 2, arrow), which could grow in the absence of inositol unlike ire1Δ cells carrying a vector alone (Fig. 4C, line 3, compare with line 2). However, this multicopy expression of HAC1 did not result in production of β-gal from the UPRE-CYC1-lacZ reporter gene used to monitor cellular UPR activity (Fig. 4D, line 3) at levels higher than the vector control (line 2). These experiments suggested that the 230-aa Hac1p is competent in inositol biosynthesis but inefficient at activating UPR transcription when expressed at low levels.
Figure 4.
Hac1p of 230-aa efficiently activated the UPR only after fusion with a potent activation domain. (A) Structures of YEp-V (YEp351) (26), YEp-HAC1, and YEp-GAL4(AD)-HAC1 are presented schematically. Strain KMY1105 with normal ER-nuclear signaling activity (ERN+) and ire1Δ strain KMY1115 carrying the UPRE-CYC1-lacZ reporter gene on the respective chromosome were transformed. (B) 35S-labeled Hac1p was immunoprecipitated from KMY1115 transformed with the indicated plasmid and analyzed as described in the legend to Fig. 3C. The arrow and arrowhead indicate 230-aa Hac1p and Gal4p(AD)-Hac1p fusion protein, respectively. The amount of Gal4p(AD)-Hac1p was 3-fold greater than that of 230-aa Hac1p. Aliquots of 35S-labeled 230-aa and 238-aa Hac1p were mixed and run on the same gel for comparison (lane Std.). (C) Inositol prototrophy of each transformant was determined as described in the legend to Fig. 3A. (D) β-Gal assays were performed as described in the legend to Fig. 3B. The line numbers in D correspond to those in C.
Given our finding that the 18-aa exon functions as a potent activation domain, the simplest explanation for inefficient activation of the UPR by 230-aa Hac1p is that this protein lacks an activation domain. To test this hypothesis, we fused the Gal4p activation domain to the N terminus of Hac1p and generated the multicopy vector YEp-GAL4(AD)-HAC1 (Fig. 4A). The presence of Gal4p(AD)-Hac1p fusion protein in the ire1Δ cells (Fig. 4B, lane 3, arrowhead) not only suppressed the inositol requirement (Fig. 4C, line 4) but also caused marked production of β-gal from the UPRE-CYC1-lacZ reporter gene (Fig. 4D, line 4), up to 50% of the level induced by TM in ERN+ cells (Fig. 4D, line 1, hatched bar); TM causes accumulation of unfolded proteins in the ER by inhibiting protein N-glycosylation (1, 2). These results strongly indicated that Hac1p needs to acquire an activation domain to activate the UPR efficiently.
Discussion
Hac1p is the UPR-specific transcription factor controlling the levels of ER chaperones. Hac1p is synthesized as a result of unconventional splicing of HAC1 mRNA, which replaces the C-terminal tail region of Hac1p from 10 to 18 aa. It was previously suggested that the mature mRNA product (238-aa Hac1p) has higher transcriptional activator activity than the premRNA product (230-aa Hac1p) (16, 17). In this study, we analyzed the molecular basis of the difference in their transactivation potential. Whereas the last 10 aa of 230-aa Hac1p did not activate transcription, the last 18 aa of 238-aa Hac1p showed a marked stimulatory effect on transcription. In addition, phosphorylation of a single Ser residue seemed to be crucial for transactivation by the 18-aa segment (Figs. 1 and 2).
Interestingly, the 18-aa exon appeared to resemble the activation domain one of the human heat shock factor one. Acidic and hydrophobic residues, especially Asp-416 and Phe-418, were shown to be important for transactivation by the activation domain one consisting of 20 aa (25). Similarly, the 18-aa segment of 238-aa Hac1p contained three Phe and the change in Ser-238* to Asp more than doubled its activation potential. Phosphorylation of Ser-238* may be important because it would confer acidity on the aa. In marked contrast, the 10-aa segment of 230-aa Hac1p contained neither Phe nor acidic residues, which may explain why the 10-aa segment did not activate transcription.
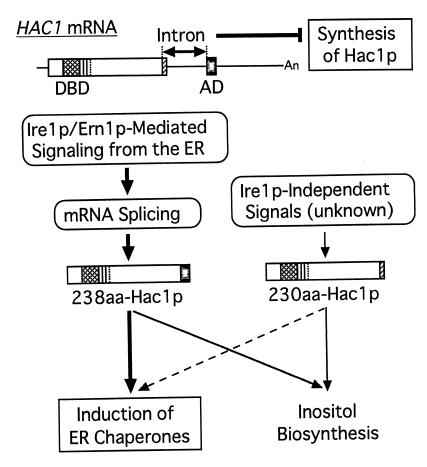
Our current model for expression and activity of Hac1p is summarized in Fig. 5. HAC1 mRNA is constitutively expressed as an intron-containing premRNA, which is blocked for the synthesis of active Hac1p at two levels. The presence of the intron blocks synthesis of Hac1p at the level of translation as has been shown (16, 17). This blockade is relieved by splicing: Ire1p-mediated signaling from the ER specifically removes the intron to produce the mature mRNA (left part of the model). The splicing event also controls Hac1p at a second level as shown here. As a result of mRNA splicing, the DBD encoded by the first exon is detached from the C-terminal 10-aa and joined with the potent activation domain encoded by the second exon. This results in production of a highly active transcription factor 238-aa Hac1p, leading to efficient activation of the UPR (Figs. 2 and 3). We and others also showed that multicopy expression of HAC1 caused a leak from the translational block of HAC1 premRNA without splicing (6–8). However, the UPR was not activated by 230-aa Hac1p thus expressed at low levels mainly because 230-aa Hac1p lacked a potent activation domain (Fig. 4). The separation of the activation domain from the DBD of Hac1p by the intron may therefore provide a “fail-safe” mechanism for controlling Hac1p activity. Even in the event that translation of intron-containing HAC1 premRNA occurs in the absence of Ire1p-mediated signaling from the ER, the resulting protein will activate the UPR only slightly (Fig. 5, right part of the model). Such tight control is necessary because inappropriate induction of the UPR in the absence of unfolded proteins in the ER is toxic to cells (6, 16).
Figure 5.
Model for expression and activity of Hac1p (see text).
Ire1p-mediated expression of Hac1p is required for the synthesis of inositol (15). Thus, when signals come from the ER, not only ER chaperones but also inositol biosynthesis is induced by produced 238-aa Hac1p. Interestingly, the unspliced form of Hac1p (230-aa Hac1p) is also able to suppress the inositol requirement created by loss of Hac1p despite its low transcriptional activator activity (Fig. 3) although its mechanism remains to be clarified. Very importantly, perhaps, these results suggest that yeast cells can stimulate inositol biosynthesis without UPR induction under some conditions not yet defined, if they could relieve the translational block for the intron-containing premRNA without activating splicing.
Recently, we showed that the promoter regions of each of target genes of the UPR contain a single UPRE sequence that is necessary and sufficient for induction and that UPRE contains an E box (CANNTG)-like palindrome separated by a one-nucleotide spacer that is essential for its function as well as specific binding to Hac1p (7, 11). This characteristic feature of UPRE appears to distinguish UPRE from other cis-acting elements recognized by basic region-containing transcription factors and is likely to explain why only ER chaperones are induced in response to accumulation of unfolded proteins in the ER. We propose that strict control of expression, activity, and specificity of Hac1p all meet the requirements of the ER.
Acknowledgments
We thank Masako Nakayama, Rika Takahashi, Seiji Takahara, and Tomoko Yoshifusa for technical assistance.
Abbreviations
- ER
endoplasmic reticulum
- GAL4(AD)
activation domain of Gal4p
- GAL4(DBD)
DNA-binding domain of Gal4p, premRNA, precursor mRNA
- UPR
unfolded protein response
- UPRE
unfolded protein response element
- WT
wild type
- β-gal
β-galactosidase
- TM
tunicamycin
- DBD
DNA-binding domain
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050010197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050010197
References
- 1.McMillan D R, Gething M J, Sambrook J. Curr Opin Biotechnol. 1994;5:540–545. doi: 10.1016/0958-1669(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 2.Shamu C E, Cox J S, Walter P. Trends Cell Biol. 1994;4:56–60. doi: 10.1016/0962-8924(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman R J. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 4.Cox J S, Shamu C E, Walter P. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 5.Mori K, Ma W, Gething M J, Sambrook J. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 6.Cox J S, Walter P. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T. Genes Cells. 1996;1:803–817. doi: 10.1046/j.1365-2443.1996.d01-274.x. [DOI] [PubMed] [Google Scholar]
- 8.Nikawa J, Akiyoshi M, Hirata S, Fukuda T. Nucleic Acids Res. 1996;24:4222–4226. doi: 10.1093/nar/24.21.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori K, Sant A, Kohno K, Normington K, Gething M J, Sambrook J F. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohno K, Normington K, Sambrook J, Gething M J, Mori K. Mol Cell Biol. 1993;13:877–890. doi: 10.1128/mcb.13.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. J Biol Chem. 1998;273:9912–9920. doi: 10.1074/jbc.273.16.9912. [DOI] [PubMed] [Google Scholar]
- 12.Sidrauski C, Cox J S, Walter P. Cell. 1996;87:405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 13.Welihinda A A, Tirasophon W, Green S R, Kaufman R J. Mol Cell Biol. 1998;18:1967–1977. doi: 10.1128/mcb.18.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikawa J, Yamashita S. Mol Microbiol. 1992;6:1441–1446. doi: 10.1111/j.1365-2958.1992.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 15.Cox J S, Chapman R E, Walter P. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara T, Yanagi H, Yura T, Mori K. Mol Biol Cell. 1997;8:1845–1862. doi: 10.1091/mbc.8.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman R E, Walter P. Curr Biol. 1997;7:850–859. doi: 10.1016/s0960-9822(06)00373-3. [DOI] [PubMed] [Google Scholar]
- 18.Sidrauski C, Walter P. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 19.Kawahara T, Yanagi H, Yura T, Mori K. J Biol Chem. 1998;273:1802–1807. doi: 10.1074/jbc.273.3.1802. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez T N, Sidrauski C, Dorfler S, Walter P. EMBO J. 1999;18:3119–3132. doi: 10.1093/emboj/18.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman F, Fink G R, Hicks J B. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 22.Culbertson M R, Henry S A. Genetics. 1975;80:23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito H, Fukuda Y, Murata K, Kimura A. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 25.Newton E M, Knauf U, Green M, Kingston R E. Mol Cell Biol. 1996;16:839–846. doi: 10.1128/mcb.16.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill J E, Myers A M, Koerner T J, Tzagoloff A. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]