Abstract
A number of studies, mostly performed ex vivo, suggest that lysosomes are involved in apoptosis as a result of a release of their cathepsins into the cytosol. These enzymes could then contribute to the permeabilization of the outer mitochondrial membrane; they could also activate effector caspases. The present study aims at testing whether the membrane of liver lysosomes is disrupted during Fas-mediated cell death of hepatocytes in vivo, a process implicated in several liver pathologies. Apoptosis was induced by injecting mice with aFas (anti-Fas antibody). The state of lysosomes was assessed by determining the proportion of lysosomal enzymes (β-galactosidase, β-glucuronidase, cathepsin C and cathepsin B) present in homogenate supernatants, devoid of intact lysosomes, and by analysing the behaviour in differential and isopycnic centrifugation of β-galactosidase. Apoptosis was monitored by measuring caspase 3 activity (DEVDase) and the release of sulfite cytochrome c reductase, an enzyme located in the mitochondrial intermembrane space. Results show that an injection of 10 μg of aFas causes a rapid and large increase in DEVDase activity and in unsedimentable sulfite cytochrome c reductase. This modifies neither the proportion of unsedimentable lysosomal enzyme in the homogenates nor the behaviour of lysosomes in centrifugation. Experiments performed with a lower dose of aFas (5 μg) indicate that unsedimentable lysosomal hydrolase activity increases in the homogenate after injection but with a marked delay with respect to the increase in DEVDase activity and in unsedimentable sulfite cytochrome c reductase. Comparative experiments ex vivo performed with Jurkat cells show an increase in unsedimentable lysosomal hydrolases, but much later than caspase 3 activation, and a release of dipeptidyl peptidase III and DEVDase into culture medium. It is proposed that the weakening of lysosomes observed after aFas treatment in vivo and ex vivo results from a necrotic process that takes place late after initiation of apoptosis.
Keywords: apoptosis, caspase 3, Fas, Jurkat cell, liver, lysosome
Abbreviations: Ac-DEVD-AMC, N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin; aFas, anti-Fas antibody; DPPIII, dipeptidyl peptidase III
INTRODUCTION
The involvement of lysosomes in apoptosis as suggested by several studies is still highly debatable (for a review, see [1]). Lysosome-mediated apoptosis would result from a destabilization of the lysosomal membrane causing a release of cathepsins into the cytosol. These proteases would stimulate the mitochondrial pathway of apoptosis by contributing to the permeabilization of the outer mitochondrial membrane, perhaps as a result of Bid cleavage by these enzymes [2]. They could also trigger the cytosolic pathway, by activating effector caspases. However, several caspases, caspase 3 for example, seem to be poor substrates for lysosomal cathepsins in vitro [3]. The aim of the present study was to investigate whether lysosomes are involved in apoptosis of hepatocytes in vivo due to Fas ligation. The Fas apoptotic pathway was selected because it has been extensively investigated as a model system for mammalian apoptosis. Moreover, Fas-mediated cell death of hepatocytes is observed in several severe liver pathologies. Knowing whether lysosomal enzymes are involved in vivo in the Fas apoptotic pathway may be of some help to explore specific therapeutic targets for cell death inhibition in these pathologies [4–6]. Until now, results published on the possible involvement of lysosomes in Fas-induced apoptosis have been mostly obtained ex vivo and are controversial. While some authors suggest that lysosomes are involved [7–10], others suggest that they are not involved [11,12] in apoptotic death caused by agonistic aFas (anti-Fas antibody). The study presented here aims at testing whether after an injection of aFas the lysosomal membrane is disrupted in mouse liver. Our method is simple and it involves determining the proportion of lysosomal enzymes found in high-speed supernatants of liver homogenates originating from controls and from animals injected with aFas. If lysosomes are involved in apoptosis after injection of aFas, one would expect that lysosomal enzymes would be released into the cytosol as a result of the injection and that an increase in the proportion of unsedimentable lysosomal hydrolases in homogenates should definitely be observed, as has been seen, for example, in ischaemic liver necrosis [13,14]. Hepatocyte apoptosis was assessed by measuring the activity of the major executioner caspase, caspase 3, with a site-specific tetrapeptide substrate (DEVDase activity) and by determining the percentage of sulfite oxidase released in the high-speed supernatant of the homogenate. This enzyme is located in the intermembrane space of mitochondria [15] and is co-released with cytochrome c into the cytosol during apoptosis [16]. The measurement of this enzymatic activity with cytochrome c as electron acceptor (sulfite cytochrome c reductase) allows quantification of the proportion of mitochondria whose outer membrane is permeabilized. This is easier than the determination of immunoprecipitated cytochrome c. In a limited number of experiments, the state of lysosomes was assessed by analysing their behaviour in differential and isopycnic centrifugation. Experiments were first performed by injecting 10 μg of aFas into animals. Under these conditions, a rapid and massive apoptosis of the liver cells takes place as assessed by morphological and biochemical observations [17–19] and causes the death of most animals after 1–2 h. Our results show that, in that case, aFas injection causes a large and rapid increase in DEVDase activity and in unsedimentable sulfite cytochrome c reductase without modifying the proportion of unsedimentable activity of lysosomal enzymes. If the amount of aFas injected is lower (5 μg), the development of apoptosis is slower, the peak of DEVDase activity and of unsedimentable sulfite cytochrome c reductase is observed later, and the animal can be kept alive for several hours. Under these conditions, the percentage of unsedimentable lysosomal enzymes begins to increase, 2–3 h after injection, indicating a progressive damage of lysosomes with time. For the sake of comparison, similar experiments were performed ex vivo with Jurkat cells. In these cells, involvement of lysosomes in Fas-mediated apoptosis has been described [9]. Our results show that aFas treatment of these cells causes an increase in the unsedimentable activity of lysosomal enzymes but that takes place with a marked delay with respect to caspase 3 activation (DEVDase). Such an increase almost coincides with a release into the culture medium of DPPIII (dipeptidyl peptidase III) and DEVDase, two cytosolic enzymes.
EXPERIMENTAL
In vivo experiments
In vivo experiments were performed with female NMRI mice weighing 20–25 g. Animals were cared for using protocols approved by the FUNDP Commission d'éthique en expérimentation animale. Mice were injected intravenously via tail vein with aFas (BD Biosciences PharMingen) in a volume of 200 μl of saline or with 200 μl of saline for control experiments. Mice were killed after selected times, and the liver was removed and homogenized in ice-cold 0.25 M sucrose with a Potter–Elvehjem-type homogenizer consisting of a smooth-walled glass tube fitted with a Teflon pestle (Arthur Thomas, Philadelphia, NJ, U.S.A.), rotating at 3000 rev./min. Part of the homogenate was centrifuged at 35000 rev./min for 40 min in a Beckman rotor type 40 to obtain a high-speed supernatant for determination of unsedimentable enzyme activity. Fractionation of the homogenate by differential centrifugation was achieved using the method of de Duve et al. [20]. A nuclear fraction N, a heavy mitochondrial fraction M, a light mitochondrial fraction L, a microsomal fraction P and a soluble fraction S were successively isolated by integrated forces respectively of 10000, 33000, 250000 and 3000000 g·min using the fractionation scheme described by these authors [16]. For isopycnic centrifugation, preformed linear density gradients of Percoll (27–90%) with 0.25 M sucrose as a solvent were used and centrifugation was performed at 25000 rev./min in a Beckman SW 65 rotor for 60 min.
Ex vivo experiments
Ex vivo experiments were carried out with Jurkat cells (clone E 6-1) cultured in RPMI 1640 medium supplemented with 10% (v/v) foetal calf serum, 10 mM Hepes, penicillin (100 units/ml), streptomycin (0.1 mg/ml) and L-glutamine (0.03%). For the experiments, incubation was performed at a density of 2×106cells/ml, in the absence or presence of human aFas (clone CH-11; Bio Connect, Huissen, The Netherlands). For the preparation of homogenates, cells were collected by centrifugation, suspended in 0.25 M sucrose and sonicated for 5 s (SONIC Vibra cells, amplitude probe S&M 0702, 40% of maximum). The efficiency of homogenization was checked by phase-contrast microscopy. In addition, the percentage of DPPIII, a cytosolic enzyme, recovered in high-speed supernatants of homogenates was regularly measured; it was always greater than 90% of the homogenate total activity, indicating that most of the cells are disrupted by the homogenization procedure. On the other hand, as shown in the results, only 10–15% of lysosomal enzyme activities were found in high-speed supernatant of homogenates originating from control cells, illustrating that homogenization did not disrupt most of the normal lysosomes. High-speed supernatants were prepared as described for the liver.
Enzyme measurements
To measure caspase-3-like activity (DEVDase activity), aliquots of homogenates were incubated at 37 °C with 50 μM Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin), 50 mM Tris/HCl buffer (pH 7.5), 0.1 M NaCl, 0.1% Triton X-100 and 10 mM dithiothreitol in a volume of 0.2 ml. The reaction was stopped by adding 1.3 ml of 50 mM glycine buffer (pH 10.5) containing 5 mM EDTA and 0.5% Triton X-100. To assay β-glucuronidase activity, the medium contained 1 mM 4-methylumbelliferyl-β-D-glucuronide, 20 mM sodium acetate buffer (pH 5) and 0.05% Triton X-100 in a volume of 0.2 ml. Incubation was performed at 37 °C and the reaction was stopped by adding 1.3 ml of 50 mM glycine buffer (pH 10.5) containing 5 mM EDTA and 0.5% Triton X-100. The medium for the determination of cathepsin B contained 250 μM benzyloxycarbonyl-Arg-Arg-7-amido-4-methylcoumarin, 0.1 M sodium phosphate buffer (pH 6), 5 mM aminoethanethiol, 1 mM EDTA and 0.05% Triton X-100 in a volume of 0.5 ml. The reaction was stopped by adding 1.3 ml of 50 mM glycine buffer (pH 10.5) containing 5 mM EDTA and 0.5% Triton X-100. For DPPIII, incubation was carried out in a medium containing 0.25 mM Arg-Arg-naphthylamide and 25 mM Tris buffer (pH 8) in a volume of 0.2 ml. The reaction was stopped by adding 1.3 ml of 50 mM glycine buffer (pH 10.5) containing 5 mM EDTA and 0.5% Triton X-100. In each determination, the released fluorogenic group (aminomethyl-coumarin, 4-methyl umbelliferone or naphthylamine) was determined with a Versa Fluor fluorimeter (Bio-Rad, Nazareth-Eke, Belgium), excitation filter 170-2420 and emission filter 170-2421. β-Galactosidase and cathepsin C were measured as described by Lecocq et al. [21], sulfite cytochrome c reductase by the method of Wattiaux-De Coninck and Wattiaux [15] and cytochrome oxidase as described by de Duve et al. [20].
RESULTS
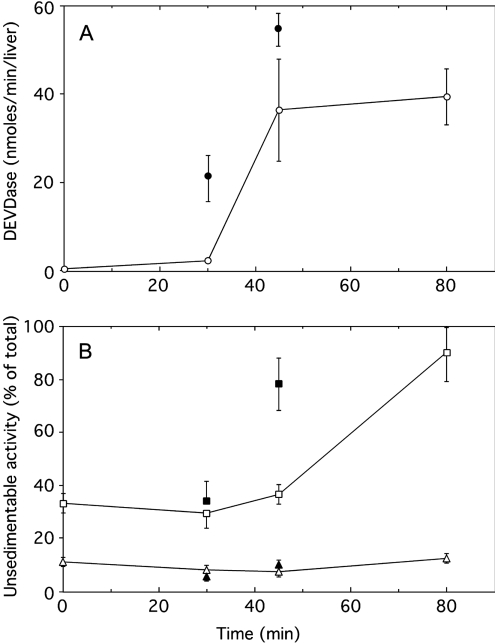
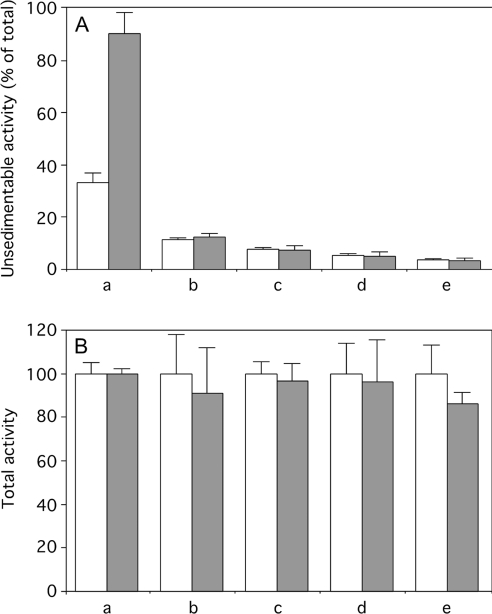
Initially, experiments were performed by injecting 10 μg of aFas. Such an injection causes a massive apoptosis of hepatocytes as assessed by biochemical and morphological criteria [17–19] and the death of the animals 90–120 min after injection. To monitor liver apoptosis, we examined the chronological changes of DEVDase activity and of unsedimentable sulfite cytochrome c reductase taking place after aFas injection. The unsedimentable activity of β-galactosidase, a reference hydrolase of lysosomes, was estimated to see whether there is a possible rupture of the membranes of these organelles. As shown in Figure 1, injection of aFas initially causes a rapid increase in homogenate DEVDase activity up to a maximum 60–80 min after injection. With time, the percentage of unsedimentable sulfite cytochrome c reductase increases, and by approx. 80 min, it is up to 95% of homogenate activity, indicating that at that time most of the mitochondria have a permeabilized outer membrane. The percentage of unsedimentable β-galactosidase is low before aFas injection (≤10%) and is not modified by such an injection. Even when apoptosis caused by aFas injection is stimulated by a fructose injection [22], one does not observe an increase in unsedimentable β-galactosidase activity. The total and unsedimentable activities of three other lysosomal hydrolases (β-glucuronidase, cathepsin C and cathepsin B) were measured in the control homogenates and in the homogenates obtained 80 min after aFas injection. As shown in Figure 2(A), the proportion of these enzyme activities recovered in the homogenate supernatants is not increased by aFas injection, as observed for β-galactosidase. Moreover, the total liver activities of the four lysosomal hydrolases are not significantly decreased after the injection (Figure 2B). This indicates that the low proportion of their unsedimentable activity does not result from a loss or an inhibition of the hydrolases after they would have been released into the cytosol of apoptotic cells.
Figure 1. Caspase-3-like protease (DEVDase) activation (A) and unsedimentable activities (B) of sulfite cytochrome c reductase (□) and β-galactosidase (△) at increasing time after injection of aFas (10 μg/animal).
Determinations were performed on total homogenate for DEVDase, on total homogenate and on high-speed supernatant (35000 rev./min for 40 min; Beckman rotor type 40) for sulfite cytochrome c reductase and β-galactosidase. DEVDase activity was given in nmol of methylumbelliferone released/min per liver. The unsedimentable activity was expressed as percentage of the total activity present in the homogenate. Error bars represent the S.E.M. for at least three animals. Closed symbols give the values obtained after stimulation of apoptosis by an intraperitoneal injection of fructose (250 mg) 15 min before aFas injection.
Figure 2. Unsedimentable (A) and total (B) activities of sulfite cytochrome c reductase (a), β-galactosidase (b), β-glucuronidase (c), cathepsin C (d) and cathepsin B (e) present in liver homogenates originating from mice injected with saline (open bars) or with aFas (10 μg/animal) (closed bars) and killed 80 min after injection.
Unsedimentable activity was expressed as a percentage of the activity present in the homogenate. For sulfite cytochrome c reductase and β-galactosidase, values indicated in Figure 1 have been used. Values for unsedimentable activity of sulfite cytochrome c reductase of treated versus control mice are significantly different (P<0.01) but not for unsedimentable lysosomal hydrolases (P>0.05). To make the presentation uniform, total activity measured in the aFas-injected mice liver homogenates is given as a percentage of the activity found in control mice liver homogenates supposed to be equal to 100. Error bars represent the S.E.M. for at least three animals. Values found in homogenates of injected and control mice do not significantly differ (P>0.05).
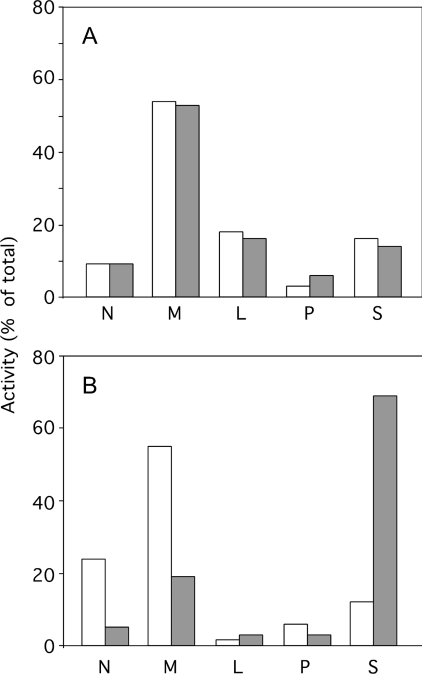
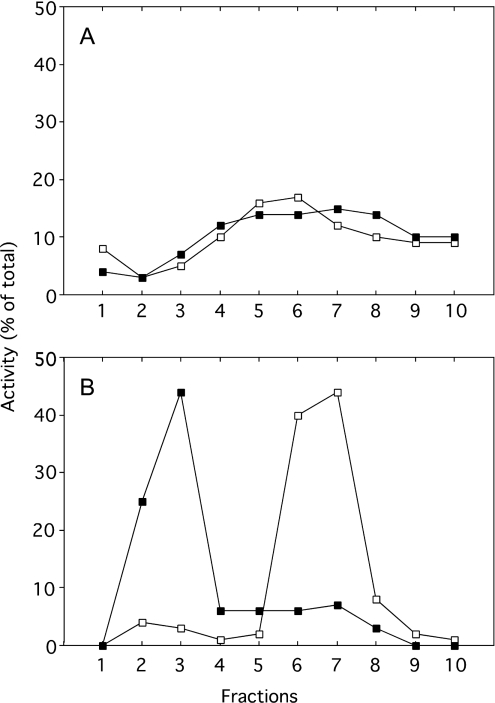
The aforementioned results strongly suggest that liver lysosomes remain intact until the animal's death, during apoptosis resulting from an injection of 10 μg of aFas. To obtain additional information on the state of lysosomes under these conditions, we have determined the behaviour of these organelles when they are subjected to differential and isopycnic centrifugation in iso-osmotic sucrose. Such an experimental approach allows us to determine whether the size and the density of the particles are modified after aFas injection. Figure 3 illustrates the distribution of β-galactosidase taken as a reference enzyme for lysosomes, after differential centrifugation in 0.25 M sucrose performed as described by de Duve et al. [20]. This was done on a liver homogenate originating from a control mouse and from a mouse injected with aFas that was killed 60 min after injection. Obviously, both distribution patterns are similar; β-galactosidase is mainly recovered in M and L fractions and is present in low amount in the S fraction. This is in contrast with the distribution of sulfite cytochrome c reductase, as aFas injection strongly increases the percentage of that enzyme associated with the unsedimentable S fraction to the detriment of the N and M sedimentable fractions. These results indicate that aFas injection does not modify the average sedimentation coefficient of lysosomes in iso-osmotic sucrose and therefore probably does not cause a swelling of these organelles as is seen in the change from apoptosis to necrosis during cell death [23]. That the liver lysosomes are not swollen after aFas injection is supported by the results of isopycnic centrifugation reported in Figure 4. M+L fractions isolated from the liver of a control and from an aFas-injected mouse killed 60 min after injection, were subjected to an isopycnic centrifugation in a Percoll gradient with 0.25 M sucrose as a solvent. This procedure allows one to determine the density of a particle under iso-osmotic conditions. The distribution curve of β-galactosidase is the same whether the M+L fraction originates from the control or the aFas-injected mouse, indicating that the lysosome mean density under iso-osmotic conditions is not affected by the injection. This is contrary to the behaviour of mitochondria as ascertained by the distribution curve of reference enzyme cytochrome oxidase. Such a curve is markedly shifted towards the low-density zones of the gradient after aFas injection, probably as a result of the swelling of the mitochondria that takes place during apoptosis [16]. It is also worthwhile to mention that these results show that sedimentable lysosomal enzymes, present in the liver homogenates after aFas injection, are truly associated with lysosomes. They could not be an artefact arising due to readsorption after their release on sedimentable structures such as mitochondria, fragments of endoplasmic reticulum, Golgi or plasma membrane. In summary, our results show that an injection of 10 μg of aFas to mice causes a massive apoptosis of the liver leading to a quick death of the animal which does not cause a leakage of lysosomes.
Figure 3. Distribution of β-galactosidase (A) and sulfite cytochrome c reductase (B) after differential centrifugation in 0.25 M sucrose.
N, nuclear fraction; M, heavy mitochondrial fraction; L, light mitochondrial fraction; P, microsomal fraction; S, soluble fraction. Liver originated from a saline-injected mouse (open bars) or from an aFas-injected mouse killed 60 min after injection (closed bars). DEVDase activities of homogenates were respectively 0.1 nmol/min per liver for the control and 90.1 nmol/min per liver for the aFas-injected mouse.
Figure 4. Distribution of β-galactosidase (A) and cytochrome oxidase (B) after isopycnic centrifugation in a Percoll gradient, with 0.25 M sucrose as a solvent, of a total mitochondrial fraction (M+L) isolated from the liver of a saline-injected mouse (□) or of an aFas-injected mouse killed 60 min after injection (■).
The M+L fraction was layered at the top of the gradient. Centrifugation was performed at 25000 rev./min for 60 min in the Beckman rotor SW 65. Fractions are numbered from the top to the bottom of the gradient. DEVDase activities of homogenates were respectively 0 nmol/min per liver for the control and 0 and 66.1 nmol/min per liver for the aFas-injected mouse.
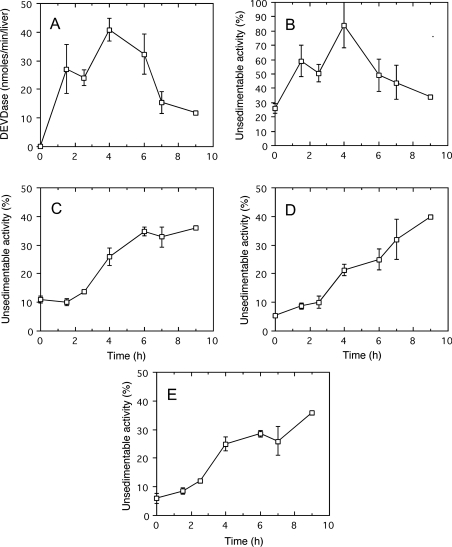
In a second set of experiments, we investigated the possible influence of the agonist dose on liver apoptosis caused by aFas injection. We found that apoptosis was delayed when the amount of injected aFas was decreased along with an increase in the duration of life of the treated animals. As illustrated in Figure 5, after an injection of 5 μg of aFas, the increase in DEVDase and in unsedimentable sulfite cytochrome c reductase with time is slower than after a 10 μg injection; a maximum value is obtained only after 4 h and is followed by a marked decrease in activity for both enzymes. Unsedimentable activity of the lysosomal hydrolases β-galactosidase, cathepsin C and β-glucuronidase does not change until approx. 2 h after injection. Later, a steady increase occurs indicating a progressive leakage of lysosomes.
Figure 5. Caspase-3-like protease (DEVDase) activation (A) and unsedimentable activities of sulfite cytochrome c reductase (B), β-galactosidase (C), cathepsin C (D) and β-glucuronidase (E) at increasing time after injection of aFas (5 μg/animal).
Determinations were performed on total homogenate for DEVDase, on total homogenate and on high-speed supernatant (35000 rev./min for 40 min; Beckman rotor type 40) for sulfite cytochrome c reductase and acid hydrolases. DEVDase activity was given in nmol of methylumbelliferone released/min per liver. The unsedimentable activity was expressed as a percentage of the total activity present in the homogenate. Error bars represent the S.E.M. for at least three animals. The last values (9 h) were obtained in a single experiment.
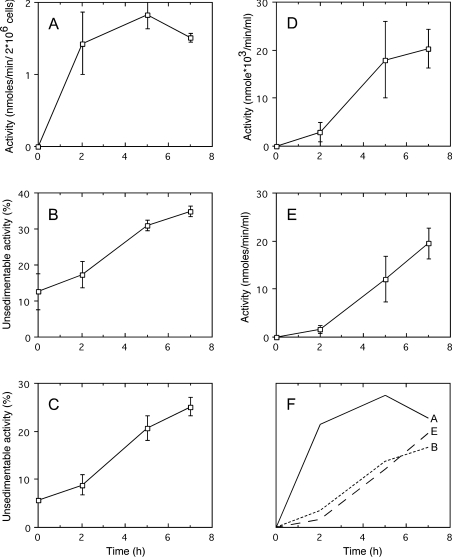
The cell death pathway triggered by Fas ligation in Jurkat cells is a prototypic model of apoptosis ex vivo. An involvement of lysosomes has been described in that process [9]. This is why we were interested in seeing if a leakage of lysosomes can be observed with our methodology in Jurkat cells treated with aFas. We used homogenates originating from Jurkat cells that had been cultured in the presence of aFas (50 ng/ml) for increasing times. Apoptosis was monitored by measuring DEVDase; leakage of lysosomes was assessed by determining the percentage of unsedimentable β-galactosidase and cathepsin C. In addition, permeabilization of plasma membrane was appraised by measuring, in the culture medium, two cytosolic enzymes, DPPIII [24] and DEVDase [25]. Results are presented in Figure 6. An increase in DEVDase activity is observed after aFas addition to the medium and approx. 2 h are required to obtain a maximum value. On the other hand, an increase in unsedimentable lysosomal hydrolases occurs in the homogenate but with a delay with respect to DEVDase increase. Interestingly, in parallel, an increase in DPPIII activity is seen in the culture medium. The same is true for DEVDase, an enzymatic activity that can only originate from cells in which caspase 3 activation, and therefore apoptosis, occurred. These results strongly suggest that the leakage of lysosomes we observed after aFas treatment is associated with a permeabilization of the plasma membrane. Similar results (results not shown) were obtained in a more limited number of experiments by treating Jurkat cells with aFas at a concentration of 20 ng and 200 ng/ml.
Figure 6. Caspase-3-like protease (DEVDase) activation in cells (A), unsedimentable activities of β-galactosidase (B) and of cathepsin C (C) in cell homogenates, activities of DPPIII (D) and of DEVDase (E) in culture medium, at increasing times after addition of aFas (50 ng/ml) to Jurkat cell culture medium.
Determinations were performed on total homogenate of Jurkat cells and on the culture medium for DEVDase, on total homogenate and on high-speed supernatant for β-galactosidase and cathepsin C. DEVDase activity was given in nmol of methylumbelliferone released·min−1·(2×106 cells)−1 or ml of culture medium. DPPIII activity is given in nmol of naphthylamine released·min−1·(ml of culture medium)−1. The unsedimentable activity was expressed as a percentage of the total activity present in the homogenate. Error bars represent the S.E.M. for five cell preparations. (F) Outline of (A, B, E) to compare the time courses of apoptosis (A), lysosome leakage (B) and cell lysis (E).
DISCUSSION
Our results show that if the injection into mice of aFas (10 μg/animal) induces a fast and massive apoptosis of the liver leading to a rapid death of the animals, liver lysosomes remain intact and their enzymes are not required to cause death of hepatocytes. If the dose of aFas is lower (5 μg/animal) and leads to a slower development of apoptosis as well as a prolonged survival of animals, an increase in unsedimentable activity of lysosomal enzymes in the homogenates can be observed 2–3 h after the injection, indicating a disruption of lysosomes inside the cells or that the organelles become more fragile making them more susceptible to homogenization procedures. On the other hand, after 4 h, DEVDase activity decreases. That could result from an inactivation of the enzyme in dead cells [26] or from a release from liver cells owing to a plasma membrane leakage. A similar explanation could be used to explain the decrease in unsedimentable sulfite cytochrome c reductase activity. In Jurkat cells, aFas causes an increase in the unsedimentable activity of lysosomal hydrolases but that begins later than the caspase-3 activation (DEVDase).
Our results, in vivo and ex vivo, could be explained by supposing that aFas can induce cell damage by two processes: one that does not involve lysosomes and begins relatively early and a second one that takes place later and implies lysosomes. According to previous biochemical and morphological observations [17–19] and our observations, it is probable that the first process corresponds to apoptosis. In Jurkat cells, the second process goes with a rupture of plasma membrane as illustrated by the release of cytosolic enzymes into the medium. That strongly suggests that it is a necrotic one and could explain the leakage of lysosomes, a phenomenon frequently observed in cell necrosis [1,13,14,23,27]. The striking similarity that we observe between the time courses of cytosolic enzyme release and the lysosome leakage is in agreement with the recent observations that plasma membrane disruption and necrosis are linked to the susceptibility of lysosomes to rupture [23]. The same reasoning can probably be applied to liver. Leist et al. [28] have shown that after aFas injection into mice, apoptosis of hepatocytes, characterized by DNA fragmentation, largely precedes lysis of these cells, monitored by serum alanine aminotransferase levels. Our interpretation is strongly supported by several observations, suggesting the presence of a necrotic death pathway in Fas receptor signalling. Matsumura et al. [29] have reported that, in Jurkat cells, both apoptotic and necrotic death pathways are activated through the Fas receptor. As shown by Vercammen et al. [26], Fas triggering in fibrosarcoma cells leads first to a rapid and strong apoptotic response followed, after 3 h, by a necrotic process, these two death pathways co-existing in the same cell. According to Kawahara et al. [30], FADD (Fas-associated death domain) can cause a necrotic death of Jurkat cells. Leist et al. [28] have shown that activation of Fas in vivo and ex vivo causes first apoptosis of hepatocytes followed by their necrosis. That we do not observe a leakage of lysosomes in liver after an injection of 10 μg of aFas is probably explained by the fact that, under these conditions, the massive apoptosis of hepatocytes induces a rapid death of the animal due to a fulminant liver failure, before the development of the slower necrotic process could occur.
It is to be noted that the increase in unsedimentable lysosomal enzyme after aFas treatment is relatively modest. The value found in the control liver and Jurkat cell homogenates is more or less 10–12% of the total activity. After aFas treatment, it reaches 30–35%. If we refer to the postulate of biochemical homogeneity of subcellular structures [20], that would mean that 20–25% of the lysosomes (on a mass basis) become fragile with the treatment. This could originate from the fact that, on average, 20–25% of lysosomes are leaky in each cell or that all the lysosomes in 20–25% of the cells have become fragile.
What causes the leakage of lysosomes induced by Fas triggering? There are probably several factors that are involved. First, activated caspases could destabilize lysosomes by proteolysis of their membrane. Many years ago, it was shown that proteolytic enzymes could disrupt lysosomal membranes in vitro [31]. More recently, it has been reported that incubation of lysosomes with caspase 8 makes the lysosomal membrane more fragile [11]. A second possibility is that reactive oxygen radicals that accumulate in cells after stimulation of Fas receptor [26] disrupt the lysosomal membrane as observed in vitro [32]. A third factor that could play a role is the strong decrease in cellular ATP concentration. aFas treatment brings about a progressive depletion of cytochrome c in mitochondria with, consequently, a mitochondrial respiratory dysfunction [18] and a decrease in ATP production. As shown by Leist et al. [33], ATP depletion induces a change from apoptosis to necrosis. Such a depletion is associated with an increase in lysosome size, making these organelles fragile and favouring their rupture [23].
Most of the studies aimed at investigating whether lysosomes are involved in Fas-mediated apoptosis have been done on isolated cells. Our observations allow us to compare results obtained in vivo and ex vivo. They show, in both cases, the importance of taking into account the time between the stimulation of Fas and the determination of the possible leakage of lysosomes. Such a leakage is a late phenomenon that we suggest is associated not with apoptosis but with the slow necrotic process induced by Fas.
Acknowledgments
This work was supported by the FRFC (Fonds de la Recherche Fondamentale Collective). M.C.G. holds a fellowship from CUD (Coopération Universitaire au Développement). We are indebted to Sandra Misquith for helping in the preparation of the manuscript and to Michel Savels for the artwork.
References
- 1.Guicciardi M. E., Leist M., Gores G. J. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 2.Cirma T., Oresi K., Mazovec G. D., Turk V., Reed J. C., Myers R. M., Salvesen G. S., Turk B. Selective disruption of lysosomes in Hela cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 2003;279:3578–3587. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- 3.Ferri K. F., Kroemer G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 4.Green D. R., Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J. Clin. Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G., Martin S. J. Caspase-independent cell-death. Nat. Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 6.Czaja M. J. TNF toxicity – death from caspase or cathepsin, that is the question. Hepatology. 2001;34:20–22. doi: 10.1053/jhep.2001.0340844. [DOI] [PubMed] [Google Scholar]
- 7.Brunk U. T., Svensson I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999;4:1–11. doi: 10.1179/135100099101534675. [DOI] [PubMed] [Google Scholar]
- 8.Brunk U. T., Neuzil J., Eaton J. W. Lysosomal involvement in apoptosis. Redox Rep. 2001;6:91–97. doi: 10.1179/135100001101536094. [DOI] [PubMed] [Google Scholar]
- 9.Neuzil J., Svensson I., Weber T., Weber C., Brunk U. T. α-Tocopheryl succinate-induced apoptosis in Jurkat T cells involves caspase-3 activation and both lysosomal and mitochondrial destabilisation. FEBS Lett. 1999;445:295–300. doi: 10.1016/s0014-5793(99)00141-6. [DOI] [PubMed] [Google Scholar]
- 10.Tardy C., Autefage H., Garcia V., Levade Th., Andrieu-Labadie N. Mannose 6-phosphorylated proteins are required for Tumor Necrosis Factor-α-induced apoptosis. J. Biol. Chem. 2004;279:52914–52923. doi: 10.1074/jbc.M408261200. [DOI] [PubMed] [Google Scholar]
- 11.Guicciardi M. E., Deussing J., Miyoshi H., Bronk S. F., Svingen P. A., Peters C., Kaufmann S. H., Gores G. J. Cathepsin B contributes to TNFα-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werneburg N. W., Guicciardi M. E., Bronk S. F., Gores G. J. Tumor necrosis factor-α-associated lysosomal permeabilization is cathepsin B dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G947–G956. doi: 10.1152/ajpgi.00151.2002. [DOI] [PubMed] [Google Scholar]
- 13.Wattiaux R., Wattiaux-De Coninck S. Effect of a transitory ischemia on the structure-linked latency of rat-liver acid phosphatase and β-galactosidase. Biochem. J. 1981;196:861–866. doi: 10.1042/bj1960861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wattiaux R., Wattiaux-De Coninck S. Effects of ischaemia on lysosomes. Int. Rev. Exp. Pathol. 1984;26:85–106. [PubMed] [Google Scholar]
- 15.Wattiaux-De Coninck S., Wattiaux R. Subcellular distribution of sulfite cytochrome c reductase in rat-liver tissue. Eur. J. Biochem. 1971;19:552–556. doi: 10.1111/j.1432-1033.1971.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 16.Kluck R. M., Degli Esposti M., Perkins G., Renken C., Kuwana T., Bossy-Wetzel E., Goldberg M., Allen T., Barber M. J., Green D. R., Newmeyer D. D. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo M., Hakem A., Elia A. J., Hakem R., Duncan G. S., Patterson B. J., Mak T. W. In vivo evidence that caspase-3 is required for Fas-mediated apoptosis of hepatocytes. J. Immunol. 1999;163:4909–4916. [PubMed] [Google Scholar]
- 18.Mootha V. K., Wei M. C., Buttle K. F., Scorrano L., Panoutsakopoulou V., Mannella C. A., Korsmeyer S. J. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J. 2001;20:661–671. doi: 10.1093/emboj/20.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez I., Matsuura K., Ody C., Shigekazu N., Vassalli P. Systemic injection of tripeptide inhibits the intracellular activation of CPP32-like proteases in vivo and fully protects mice against Fas-mediated fulminant liver destruction and death. J. Exp. Med. 1996;184:2067–2072. doi: 10.1084/jem.184.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Duve C., Pressman B. C., Gianetto R., Wattiaux R., de Duve C. Tissue fractionation studies. VI. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecocq M., Andrianaivo F., Warnier M. Th., Wattiaux-De Coninck S., Wattiaux R., Jadot M. Uptake by mouse liver and intracellular fate of plasmid DNA after a rapid tail vein injection of a small or a large volume. J. Gene Med. 2002;5:142–156. doi: 10.1002/jgm.328. [DOI] [PubMed] [Google Scholar]
- 22.Latta M., Künstle G., Leist M., Wendel A. Metabolic depletion of ATP by fructose inversely controls CD95 and tumor necrosis factor receptor 1-mediated hepatic apoptosis. J. Exp. Med. 2000;191:1975–1985. doi: 10.1084/jem.191.11.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono K., Kim S. O., Han J. Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor α induced cell death. Mol. Cell. Biol. 2003;23:665–676. doi: 10.1128/MCB.23.2.665-676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons M. E., Pennington R. J. T. Separation of rat muscle aminopeptidases. Biochem. J. 1976;195:375–383. doi: 10.1042/bj1550375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler J. M., Cohen G. M., MacFarlane M. Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J. Biol. Chem. 1998;273:10815–10818. doi: 10.1074/jbc.273.18.10815. [DOI] [PubMed] [Google Scholar]
- 26.Vercammen D., Brouckaert G., Denecker G., van de Craen M., Declercq W., Fiers W. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. I. Exp. Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Duve C., Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 28.Leist M., Gantner F., Künstle G., Bohlinger I., Tiegs G., Bluethmann H., Wendel A. The 55-kD tumor necrosis factor receptor and CD95 independently signal murine hepatocyte apoptosis and subsequent liver failure. Mol. Med. 1996;1:109–124. [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumura H., Shimizu Y., Oshawa Y., Kawahara A., Uchiyama Y., Nagata S. Necrotic death pathway in Fas receptor signaling. J. Cell Biol. 2000;151:1247–1255. doi: 10.1083/jcb.151.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawahara A., Ohsawa Y., Matsumura H., Uchiyama Y., Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beaufay H., de Duve C. Tissue fractionation studies. 9. Enzymic release of bound hydrolases. Biochem. J. 1959;73:604–609. doi: 10.1042/bj0730604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decharneux T. H., Dubois F., Beauloye C., Wattiaux-De Coninck S., Wattiaux R. Effect of various flavonoids on lysosomes subjected to oxidative and osmotic stress. Biochem. Pharmacol. 1992;44:1243–1248. doi: 10.1016/0006-2952(92)90521-j. [DOI] [PubMed] [Google Scholar]
- 33.Leist M., Single B., Castoldi A. F., Kûhnle S., Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]