Abstract
Nicotine acting centrally increases bronchomotor tone and airway secretion, suggesting that airway-related vagal preganglionic neurons (AVPNs) within the rostral nucleus ambiguus (rNA) express nicotinic acetylcholine receptors (nAChRs). In the present study, we examined the three main functionally characterized subtypes of nAChRs in the CNS, the α7 homomeric and α4β2 heteromeric receptors. First, we characterized the expression of these subunits at the message (mRNA) and protein levels in brain tissues taken from the rNA region, the site where AVPNs are located. In addition, double labeling fluorescent immunohistochemistry and confocal laser microscopy were used to define the presence of α7, α4, and β2 nAChRs on AVPNs that were retrogradely labeled with cholera toxin h subunit (CTb), injected into the upper lung lobe (n = 4) or extrathoracic trachea (n = 4). Our results revealed expression of all three studied subunits at mRNA and protein levels within the rNA region. Furthermore, virtually all identified AVPNs innervating intrapulmonary airways express α7 and α4 nAChR subunits. Similarly, a majority of labeled AVPNs projecting to extrathoracic trachea contain α7 and β2 subunits, but less than half of them show detectable α4 nAChR traits. These results suggest that AVPNs express three major nAChR subunits (α7, α4, and β2) that could assemble into functional homologous or heterologous pentameric receptors, mediating fast and sustained nicotinic effects on cholinergic outflow to the airways.
Keywords: Rat; Airway control; α7, α4, and β2 nAChR subunits; Vagal preganglionic neurons; Rostral nucleus ambiguus; Retrograde tracing; Cholera toxin β subunit (CTb)
1. Introduction
In humans, as in other mammals, nicotine resorbed from inhaled tobacco smoke may act centrally, to induce a vagally mediated increase in airway smooth muscle tone (Hartiala et al., 1984). Nicotine administered topically to the ventrolateral region of medulla oblongata where the airway-related vagal preganglionic neurons (AVPNs) are located, elevates cholinergic outflow to the airways, inducing bronchoconstriction and airway submucosal gland secretion. These effects could be blocked by centrally administered hexamethonium (Haxhiu et al., 1986; Haxhiu et al., 1991), a general blocker of nicotinic acetylcholine receptors (nAChRs), suggesting the presence of nAChRs on AVPNs.
In rats (Haxhiu et al., 1993), as in other species (reviewed in Kalia, 1987; Jordan, 2001; Haxhiu et al., 2005), the motor preganglionic component of the network innervating the airways arises mainly from the nucleus ambiguus and to a lesser extent from the dorsal motor nucleus of the vagus (DMV). Of these two groups of neurons, AVPNs within the rostral nucleus ambiguus (rNA) play a greater role in generating cholinergic outflow to airway smooth muscle (Haselton et al., 1992), secretory glands, and blood vessels (Haxhiu et al., 2000). Hence, resorbed nicotine and nicotine-like products may act directly on AVPNs, via nAChRs.
nAChRs are pentameric ligand-gated cation channels assembled from a combination of α and β subunits (Cooper et al., 1991; Zoli et al., 1998; Karlin, 2002). In the central nervous system (CNS), there are at least 9α (α2–α10) and three β(β2–β4) subunits. The homomeric assembly of subunits such α7, α8, and α9 has been shown to be functional in mammalian cells, whereas α2–α6 and α10 become operational only when coexpressed with β-subunits, as detailed in recent reviews (Berg and Conroy, 2002; Hogg et al., 2003; Dajas-Bailador and Wonnacott, 2004; Gotti and Clementi, 2004).
It is agreed that the two most abundant functionally characterized subtypes of nAChRs within the CNS are the α7 homomeric receptors that bind a-Bungarotoxin with high affinity, called αBgtx-nAChRs, and α4β2 heteromeric nAChRs that express high binding affinity for nicotine, named nAChRs (Lukas and Bencherif, 1992; Gotti and Clementi, 2004). To date, however, subunit composition of nicotinic ligand-gated ion channels expressed by AVPNs has not been investigated. Therefore, in the present studies, we tested the hypothesis that AVPNs innervating extrathoracic trachea and/or intrapulmonary airways express the major nAChR subunits. Our results showed that, in brain tissues of the rNA region, traits of nAChR are present at message and protein levels, and identified AVPNs express α7, α4, and β2 nAChR subunits, which could assemble into functional receptors with homologous or heterologous pentameric channels.
2. Materials and methods
2.1. Animals
In these studies, we used male Sprague–Dawley rats (250–350g; Harlan, Indianapolis, IN). Male animals were utilized in order to minimize potential physiological changes due to hormonal variations associated with the reproductive cycle. All experimental procedures and protocols were approved by the Howard University Institutional Animal Care and Use Committee.
2.2. RNA extraction and RT-PCR
In the first series of experiments, we employed the reverse transcription-polymerase chain reaction (RT-PCR) to determine whether nAChR mRNAs of α7, α4, and β2 subunits are measurable in the tissues of the rostral ventrolateral medulla oblongata, where AVPNs are located. For these experiments, four adult rats were deeply anesthetized with 100mg/kg pentobarbital, brains were quickly removed, placed in sterile 0.9% saline solution on ice and then tissue was taken from the rNA region and stored at 80°C, until used for RNA extraction, as previously described (Zaidi et al., 2005).
Dissected tissues were pooled, homogenized in TRIZOL reagent for RNA extraction (Invitrogen Corporation, CA). The contaminating genomic DNA in the RNA samples was digested using RQ1 RNase free DNase (Promega Corporation, WI). Total cellular RNA was reverse transcribed and amplified with the one-tube and two-enzyme Access RT-PCR System (Promega Corporation, WI).
Briefly, 250ng of total RNA was added to a mixture of reverse transcription/amplification buffer, dNTP mixture, gene specific primer pair, AMV reverse transcriptase and Tfl DNA polymerase. Oligonucleotide primers for rat α7, α4, and β2 nAChR subunits were synthesized, corresponding to amino acids sequences selected for each nAChR subunit [a7 (Forward): GTGGAACATGTCTGAGTACCCCGGAGTGAA, α7(Reverse): GAGTCTGCAGGCAGCAAGAATACCAGCA; α4 (Forward): GTTCTATGACGGAAGGGTGCAGTGGACA, α4 (Reverse): GGGATGACCAGCGAGGTGGACGGGATGAT; β2 (Forward): ACGGTGTTCCTGCTGCTCATC, β2 (Reverse): CACACTCTGGTCATCATCCTC] as previously published (Lena et al., 1999). β-actin mRNA was used as an internal control to verify the quality of the RNA sample and its subsequent RT-PCR analysis. Primer sequences used were: β-actin (Forward): AACCCTAAGGCCAACCGTGAAAAG, β-actin (Reverse): CTAGGAGCCAGGGCAGTAATCT. The RT-PCR cycling profiles using a Thermal Cycler (GeneAmp PCR System 9700; Applied Biosystems, CA) were as follows: 1 cycle of reverse transcription at 48°C for 45min, 1 cycle at 94°C for 5 min, 35 cycles at 94°C for 1 min, 60°C for 1 min, 72°C for 1min, and a final cycle at 72°C for 7 min. A 10-μl aliquot of each sample was electrophoresed on a 2.0% agarose gel containing 0.5μg/ml ethidium bromide. The bands of α7, α4, and β2 nAChR subunit mRNAs were presented in parallel with β-actin mRNA levels that were determined from separate RT-PCR reactions.
2.3. Western blotting
The pooled rostral nucleus ambiguus tissue samples of four rats were homogenized in a buffer containing 50mM Tris pH 7.4, 1% NP40, 0.25% Nadeoxycholate, 150mM NaCl and 1mM EDTA using a glass-Teflon homogenizer. The buffer was supplemented with Complete protease inhibitor cocktail (Roche Molecular Biochemicals, IN) and 1 mM Phenylmethylsulfonyl fluoride. The homogenate was rocked on an orbital shaker in the cold room for 15 min. The tissue debris was removed from the homogenate by centrifugation at 14,000×g for 15 min. The supernatant, representing the tissue lysate was immediately transferred to a fresh centrifuge tube. All steps involved in the tissue lysis were carried out at 4°C. An aliquot of the lysate was mixed with an equal volume of 2×Laemmli Sample Buffer (Bio-Rad Laboratories, CA) and the mixture was boiled for 5 min. The protein concentration in the sample was estimated by the Bio-Rad protein assay reagent.
The proteins (50μg) were separated by size on a 9% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane in Tris-glycine–methanol buffer containing 25-mM Tris, 192-mM glycine and 20% methanol. For immunoblot detection of nAChRα4, nAChRα7, nAChRαh2 and β-Actin, membranes were blocked in Tris-buffered saline (TBS) containing 5% nonfat dried milk for 1h at room temperature with agitation. The membrane was incubated with primary antibodies diluted in TBS (nAChRα4,1μg/ml; nAChRα7,1μg/ml; nAChRh2,1μg/ml and β-Actin, 0.5μg/ml) containing 5% nonfat dried milk overnight at 4°C. All antibodies were purchased from Santa Cruz Biotechnology, Inc., CA. The membrane was washed in Tween 20-TBS (TTBS) containing TBS and 0.075% Tween 20 and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (Bio-Rad Laboratories, CA) diluted 1:15,000 in TBS containing 5% nonfat dried milk. After this incubation, the membranes were washed again in TTBS. The antigen–antibody peroxidase complex was then finally detected by ImmunStar chemiluminescence kit (Bio-Rad Laboratories, CA) according to the manufacturer’s instructions and visualized by exposure to Hyperfilm (Amersham Biosciences, NJ).
2.4. Expression of nAChRs by vagal preganglionic neurons innervating intrapulmonary airways or extrathoracic trachea
For these studies, under pentobarbital anesthesia (50mg/kg, ip), the trachea was injected with cholera toxin β-subunit (CTb), along the tracheal wall (n = 4) beginning with the third intercartilaginous space (Haxhiu et al., 1993). To label AVPNs innervating intrapulmonary airways, CTb was injected into the right upper lobe (n = 4), as previously described (Hadziefendic and Haxhiu, 1999). After four to five survival days, the animals were anesthetized with pentobarbital (50mg/kg, ip) and perfused through the left ventricle with 300ml of saline, containing 10,000U of heparin. This was subsequently followed by 300ml of 1% paraformaldehyde in 0.1M phosphate buffer (PB, pH 7.4). The brains were removed and stored in the same fixative for 1h, then transferred to 30% sucrose in 0.1M PB overnight. Transverse sections of the medulla oblongata from pontomedullary border to the decussation of pyramids were cut at 40μm using a Bright OTF Cryostat (Hacker Instruments Inc., NJ) and stored in 0.5% sodium azide in 0.1M PB (pH 7.4).
2.4.1. Immunohistochemistry
The expression of specific subunits of nAChRs by identified AVPN innervating the trachea, or the intra-pulmonary airways, was evaluated using sequential double-labeling immunocytochemistry as previously described (Dehkordi et al., 2004; Dehkordi et al., 2005). Briefly, 1-in-5 series of tissue sections of the medulla oblongata were rinsed three times in 0.1M phosphate buffer saline (PBS) at pH 7.4. Tissues were then incubated for 5min in 0.3% Triton-X in PBS and underwent sequential treatment with a series of ethanol and acetone to further increase the penetration of the primary antibody. Endogenous peroxidase activity was quenched before the immunostaining by exposing the sections to 0.1 M PBS containing 0.3% hydrogen peroxide for 30min. Tissues were then placed into a blocking medium containing TNB blocking buffer and 0.1% normal donkey serum (NDS, Santa Cruz Biotechnology, Inc., CA) for 30min. Following these steps, the sections were processed for detection of the α7, α4 and/or β2 subunits of nAChRs using the Tyramide Signal Amplification (TSA) Fluorescence System (PerkinElmer Life Sciences, MA), and then for visualizing CTb labeled AVPNs.
In the first sequence, sections were incubated for 24h at 4°C with the α7 (rabbit polycolonal, Santa Cruz Biotechnology, 1:10), α4 (rabbit polycolonal, Santa Cruz Biotechnology, CA, 1:50) or β2 primary antisera (rabbit polyclonal, Santa Cruz Biotechnology, CA, 1:50). Sections were then washed 3 times, 5min each in 0.1M PBS and incubated with a biotinylated donkey anti-rabbit secondary antibody (Santa Cruz Biotechnology, CA, 1:50) in 0.1M PBS for 1–2h. After washing in PBS (3×5min each), streptavidin conjugated horseradish peroxidase (SA-HRP) was applied to the tissue sections for 30min. The sections were then washed in PBS (3×5min each) and incubated in Flurophore Tyramide (amplification reagent) for 10min. After the detection of nAChR protein, the tissues were then washed in PBS (3×5min each) and incubated in a 1:10,000 dilution of goat anti-CTB (List Biological Laboratories, Inc., CA) for 48h. Subsequently, the sections were washed in PBS (3×5min ea.) and incubated for 2h in a 1:200 solution of donkey anti-goat IgG conjugated with Alexa Fluro 594 (Texas red, TR; Molecular Probes, Eugene, OR). Finally, the sections were rinsed in PBS (3×5min each), mounted and cover-slipped using Vecta Shield (Vector Laboratories Inc., CA) antifade mounting media.
The control experiments for each of the labeling were done by omission of the primary antibodies, determining whether the secondary antibodies produced falsepositive results. Omission of primary antibodies resulted in the absence of nAChR protein and CTb labeling; thereby demonstrating that no false-positive results were obtained with these reagents.
2.4.2. Fluorescent and laser scanning confocal microscopy
First, slides were viewed with a fluorescent microscope (Olympus AX70, Olympus America, NY) equipped with appropriate filter systems to observe the green (receptor) and red (CTb) fluorescence. Colocalization of nAChR protein with the CTb was identified by viewing the sections and alternating between fluorescence filters. The contrasting immunoprecipitates were readily distinguishable. Sections were also examined, digitized, and indirect immunofluorescence images were collected by use of an Olympus laser scanning confocal microscope. In these experiments, receptor was detected by fluorophore-labeled tyramides (Green) in the presence of biotinylated secondary antibody and SA-HRP as previously described (Dehkordi et al., 2004, 2005). The CTb was detected employing a donkey anti-goat IgG conjugated with Alexa Fluro 594 (Texas red; Molecular Probes, OR). Signals were acquired from the same area of the section, digitized, and stored as tiff files. The Olympus software produced overlay (superimposed) images in which the overlap of the red and green signals generated yellowish color, thereby indicating the degree to which the staining patterns arising from the different antibodies were codistributed.
2.4.3. Cell counting
A minimum of 8 to 10 rostrocaudal sections of the medulla oblongata (Bregma: -11.60mm to -14.08mm; Paxinos and Watson, 1986) were used for counting of retrogradely labeled vagal preganglionic neurons within the ventrolateral medulla, following CTb injection into the extrathoracic trachea or the upper right lung lobe. We counted the total number of cells containing CTb and the number of neurons that coexpressed CTb and α7, CTb and α4, or CTb and β2 subunits, and calculated the percentage of CTb-containing neurons expressing α7, α4 or β2 nAChRs.
3. Results
3.1. Expression of α7, α4, and b2 subunits at mRNA and protein levels
RT-PCR experiments showed the presence of α7, α4, and β2 subunit mRNAs and immunoblotting demonstrated specific receptor proteins in the tissues of the ventrolateral medulla oblongata where AVPNs are located (Fig. 1, left and right panels). These findings formed the foundation for studies on expression of nAChR subunits by identified AVPNs.
Fig. 1.
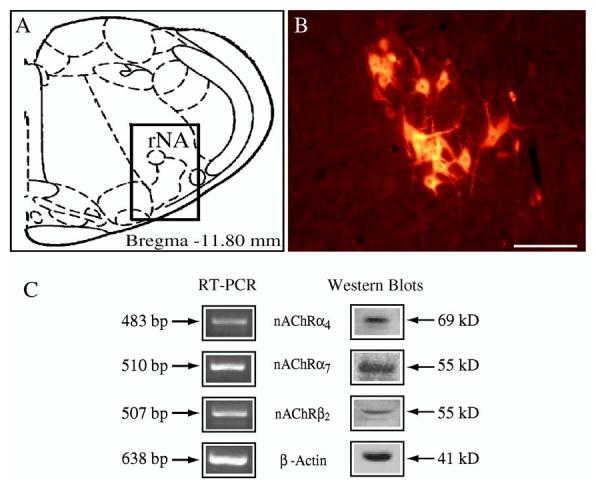
RT-PCR and Western blot analysis of nicotinic acetylcholine receptor mRNA and protein expression in the rostral nucleus ambiguus (rNA) where airway-related vagal preganglionic neurons are located. (A) Schematic representation of a coronal section (Paxinos and Watson, 1986) and (B) the distribution of CTb-labeled AVPNs visualized with rhodamine indicating the location of AVPN within the rNA regions (C), RT-PCR analysis of nicotinic acetylcholine receptor (nAChR) subunits: α4, α7, β2, and β-actin seen at 483, 510, 507, and 638bp, respectively and Western blot analysis of α4, α7, β2, and β-actin, seen at 69, 55, 55, and 41kDa, respectively (Scale bar: 100μm).
3.2. Expression of α7, α4, or b2 subunits at single cell level
The expression of α7, α4, or β2 was analyzed at the protein level, using specific antibodies that recognize each of these subunits. Adult rat brain sections were incubated with the primary antibody and exposed to the secondary antibody, or were incubated with the secondary antibody alone. Sections treated with a single employed primary antibody and the corresponding secondary antibody gave a consistent pattern of labeling in the brain stem, as previously described (Dehkordi et al., 2004). No cell labeling was found in the absence of the primary antibody of α7, α4or β2.
Using confocal laser microscopy, within the rostral nucleus ambiguus region, the labeling intensity of the analyzed neurons was found to be clearly higher (more than two times) than the background noise. This was observed throughout the entire analyzed region where AVPNs are located. Labeling pattern of α7, α4 and β2 receptor subunits was comparable. Immunolabeling of cells within the rNA region resulted in the staining of neurons within the compact and loose portion of the nucleus ambiguus. Labeled cells varied in their morphologies. Extensive α7 labeling was visible throughout cell perikaryon. Labeling of soma surface and specific cellular processes were also observed (Fig. 2). A cellular staining for α4 and β2 subunits was not distinguishable from distribution of signals identifying the presence of the α7 subunit.
Fig. 2.
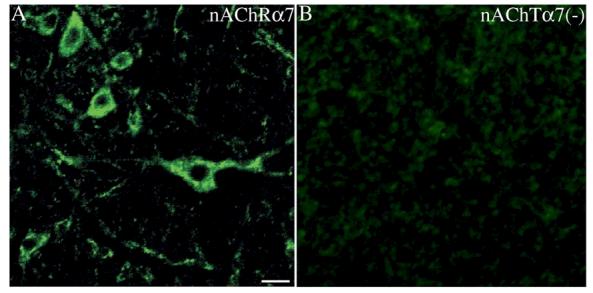
(A) An example of a confocal image of the α7-nAChR subunit expressed by neurons within the rostral nucleus ambiguus region (rNA). The α7-nAChR like-immunoreactive product is localized on the cell membrane and dendrites. However, it was also observed in the cytoplasm, suggesting internalization of receptor protein. (B) After omission of primary antibody no labeling of neurons or the fibers was observed (Scale bar: 20μm).
3.3. Expression of α7, α4, or b2 subunits by identified AVPNs
Following injection of CTb into the extrathoracic tracheal wall or intrapulmonary airways and lung parenchyma, majority of retrogradely labeled parasympathetic preganglionic neurons were seen within the rNA: namely the compact portion of the nucleus ambiguus and the area ventral to it. A few neurons were observed in the most rostral part of the dorsal motor nucleus. This labeling pattern correlated well with previous studies in rats, describing vagal preganglionic innervation of the extrathoracic trachea and most distal intrapulmonary airways (Haxhiu et al., 1993; Hadziefendic and Haxhiu, 1999; Perez Fontan and Velloff, 2001).
Double labeling studies showed that virtually every identified vagal preganglionic neuron innervating intrapulmonary or extrathoracic airways expressed α7 like immunoreactivity. Specifically, 151 out of 157 CTb positive neurons, following injections of retrograde tracer into the upper right lung lobe, expressed the α7 nAChR subunit (96%). Similarly, almost all retrogradely labeled neurons innervating extrathoracic trachea demonstrated strong immunoreactivity for the alpha 7 subunit of nAChRs; 151 out of 161 CTb positive cells (94%) showed strong α7 immunoreactive signal (Fig. 3); differences in expression of α7 subunit by AVPNs providing cholinergic outflow to the intrapulmonary or extrathoracic airways were negligible.
Fig. 3.
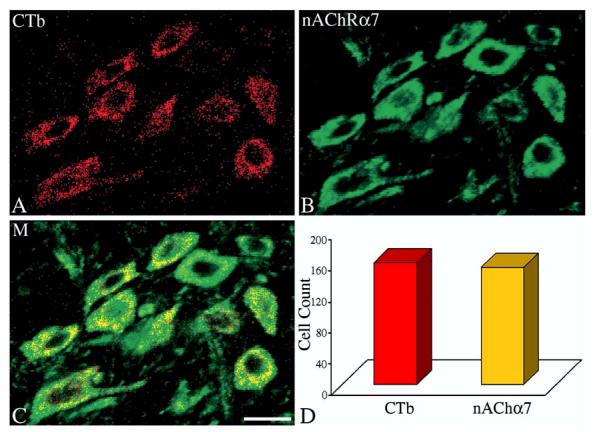
Confocal images of (A), retrogradely labeled airway-related vagal preganglionic neurons (AVPNs) within the rNA, following microinjection of cholera toxin β subunit (CTb) tracer into the upper right lung lobe; (B) α7-nAChR subunit immunoreactive neurons in the same field. (C) The merged (M) image showing the CTb colocalized with an α7-nAChR traits. (D) Total number of counted CTb labeled neurons and the number of the double labeled cells (CTb and α7-nAChR). Scale bar: 20μm.
In the same group of rats, studies revealed that 91% (113 out of 123) of vagal preganglionic neurons projecting to the intrapulmonary airways expressed the α4 nicotinic receptor subunit (Fig. 4). However, the number of identified preganglionic neurons innervating extrathoracic trachea that expressed the α4 subunit was somehow lower. Counting of retrogradely labeled cells and double labeled neurons showed that only 38% (67 out of 176) of neurons innervating the extrathoracic trachea express α4 nAChR subunit.
Fig. 4.
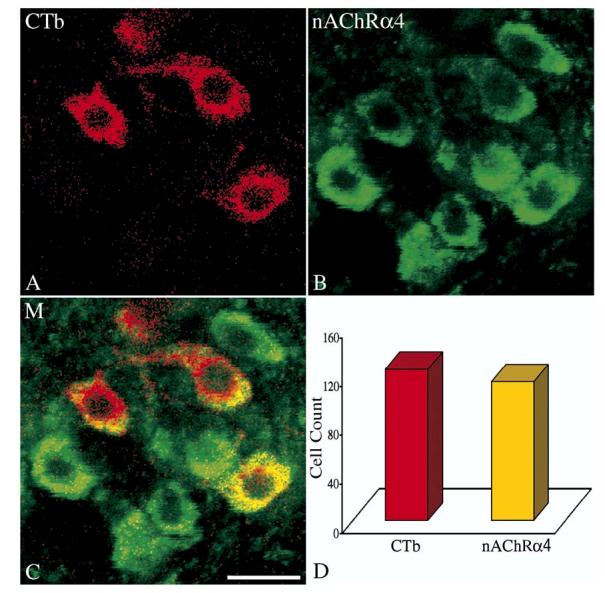
Confocal images of (A), retrogradely labeled airway-related vagal preganglionic neurons (AVPNs) within the rNA, following microinjection of cholera toxin β subunit (CTb) tracer into the upper right lung lobe; (B) α4-nAChR subunit immunoreactive neurons in the same field. (C) The merged (M) image showing the CTb colocalized with an α4-nAChR traits. (D) Total number of counted CTb labeled neurons and the number of the double labeled cells (CTb and α4-nAChR). Scale bar: 20μm.
Vagal preganglionic neurons projecting to the extrathoracic trachea were also evaluated for expression of the β2 subunit which is known to assemble with α4 to form heteromeric α4β2 nAChRs. Of the 217 CTb positive neurons in the rNA, 74% were immunoreactive for the β2 subunit of nAChRs (Fig. 5).
Fig. 5.
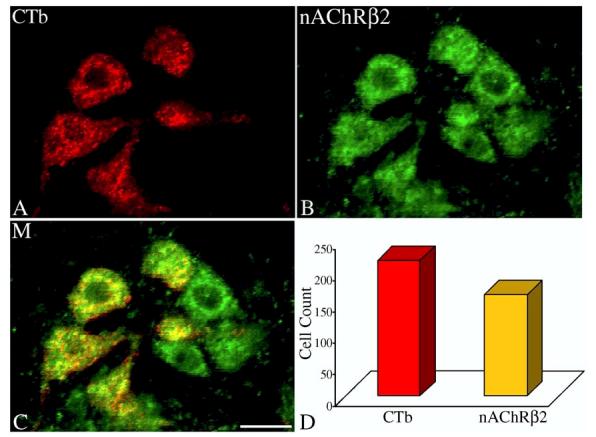
Confocal images of (A), retrogradely labeled airway-related vagal preganglionic neurons (AVPNs) within the rNA, following microinjection of cholera toxin β subunit (CTb) tracer into the extrathoracic trachea; (B) β2-nAChR subunit immunoreactive neurons in the same field. (C) The merged (M) image showing the CTb colocalized with a β2-nAChR traits. (D) Total number of counted CTb labeled neurons and the number of the double labeled cells (CTb and β2-nAChR). Scale bar: 20μm.
4. Discussion
4.1. Limitations of the techniques
Prior to discussing the findings of the present studies, we will mention the limitations of the techniques used. The results obtained by RT-PCR and Western blotting showed that the all three nAChR subunits are present in the region of the rNA, at mRNA and protein levels. Although RT-PCR and Western blot techniques have high specificity, these findings require careful interpretation. Excised rNA region contains not only vagal preganglionic neurons but also interneurons that lie side by side with those cells that innervate extrathoracic trachea and intrapulmonary airways (Haxhiu et al., 1993). Therefore, observed signals underling specific message and protein subunits may originate from cells other than AVPNs. Hence, these findings were complemented by immunolabeling data.
In addition, the results of immunohistochemistry studies employing subunit specific primary antibodies should be interpreted with caution. A number of control experiments are required to avoid possible nonspecific labeling (Saper and Sawchenko, 2003). In our experiments specificity of the used antibodies was tested using Western blotting, and these antibodies have been previously characterized and successfully used in immnunohistohemistry (Schroder et al., 1989; Dominguez del Toro et al., 1994; Ferreira et al., 2001; Dehkordi et al., 2004). In addition in our control experiments, after omitting the primary antisera, no neuronal labeling above the background was observed in the region of rostral nucleus ambiguus or the brain stem sites known to express nAChRs traits at message (Wada et al., 1989) and protein forms (Ferreira et al., 2001; Dehkordi et al., 2004). Hence, we consider that the employed primary antibodies specifically labeled targeted α7, α4, or β2 antigen-like molecules.
4.2. Expression of nAChRs by identified AVPNs
The findings of the present study show for the first time that AVPNs express α7, α4 and β2 nAChR subunits that could assemble into functional receptors with homologous or heterologous pentameric channels. This could explain nicotine-induced activation of AVPNs, associated with a concomitant increase in airway smooth muscle tone and submucosal gland secretion (Haxhiu et al., 1986; Haxhiu et al., 1991).
4.2.1. The α7 subunit of nAChRs
Fluorescent and laser confocal microscopic analysis of immunolabeling within the rostral nucleus ambiguus region, revealed for the first time that the α7 subunit of nAChRs is postsynaptically expressed by vagal preganglionic neurons innervating intrapulmonary and extrathoracic airways. Furthermore, our findings indicate that in rats almost every AVPN expresses α7 receptor protein. Clearly, without electron microscopic immunolabeling studies, the precise location of nicotinic receptors cannot be ascertained (Fabian-Fine et al., 2001). However, using confocal laser microscopy, heavy labeling was observed within the membranous elements, including dendritic and postsynaptic cytoplasmic components. Labeling was also observed within perikaryon that could be due to internalized receptor protein. The abundant expression of α7 subunit of nAChRs suggests that nicotinic homomeric receptor channels composed of this subunit could play an important role in regulation of cholinergic outflow to the airways. This assumption is supported by studies using whole-cell recordings. By contrast to the motoneurons of the VII and XII cranial nerves, it was found that the ACβ-induced current in vagal preganglionic neurons was mediated by α7-containing nAChRs. Furthermore, autoradiography revealed dense binding sites for [125I] a-bungarotoxin in vagal preganglionic neurons, a selective ligand of homomeric α7-containing nAChRs (Zaninetti et al., 1999). Taken together, these findings indicate the importance of α7-nAChRs, but do not exclude the possibility of existence of the α7 as a component of a pentameric nAChR consisting of the α7 and β2(Khiroug et al., 2002), or α7 and β3 subunits (Palma et al., 1999). This assumption is supported by recent findings showing that the α7-nAChR subtype that is expressed by the neurons of the rat DMV, is functionally and pharmacologically distinct from homomeric α7-nAChRs (Ferreira et al., 2001).
As on autonomic ganglionic neurons (Dajas-Bailador and Wonnacott, 2004), the α7 receptor subunit could be present on perisynaptic sites of the AVPNs, and on presynaptic axon terminals. In both human and rats these sites could be involved in transducing effects of ACh and/or choline upon neurotransmitter release and synaptic function (Albuquerque et al., 2000; De Filippi et al., 2005). Activation of perisynaptic nAChRs could cause increase in excitability or depolarization of AVPNs, whereas stimulation of presynaptic receptors localized on axonal terminals innervating vagal preganglionic neurons could modulate the release of several substances, including release of glutamate and facilitation of glutamatergic neurotransmission, as shown in other CNS sites (Aramakis and Metherate, 1998; Alkondon et al., 2003; Jones and Wonnacott, 2004).
4.2.2. Expression of the α4 and the β2 subunits of the nAChRs by AVPNs
The results of the present study support previous findings, showing that individual neurons may express multiple classes of nAChRs (as reviewed by Le Novere et al., 2002; Alkondon and Albuquerque, 2004). We found that a majority of identified AVPNs innervating intrapulmonary airways also express the α4 subunit. However, it should be mentioned that only a third of CTb labeled cells, following injection of the retrograde tracer into extrathoracic tracheal wall, showed a clearly detectable α4 immunoreactive signal. The observed difference cannot be fully explained by recent tracer studies, demonstrating at the single cell level, that neurons innervating extrathoracic trachea also provide cholinergic input to intrapulmonary airways (Perez Fontan and Velloff, 2001). Therefore, further investigation, using in situ hybridization technique and physiological approaches, is needed to characterize this phenomenon.
The α4 subunit of nAChRs most often makes a heteromeric assembly with a β2 subunit (Flores et al., 1992). Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nAChRs (Luetje and Patrick, 1991), which could be influenced by the ratio of α4/β2(Zwart and Vijverberg, 1998). Expression of α4β2 nAChRs can be affected by multiple factors. For example, chronic exposure of animals to nicotine, or humans to cigarette smoke (Breese et al., 1997; Benwell et al., 1988; Buisson and Bertrand, 2001), leads to upregulation of surface α4β2 nicotinic receptors in a dose-and time-dependent fashion (Ke et al., 1998), which partly results from a decrease in the rate of receptor turnover (Peng et al., 1994). This will enhance the response of α4β2 nAChRs to agonists, leading to depolarization of neurons, via an increase in the permeability of nicotinic channels to both Na+ and Ca2+, that in turn could affect synaptic transmission (Matsubayashi et al., 2004). In asthmatic patients this could increase airway responsiveness and cause worsening of pulmonary function even by passive smoking (Dahms et al., 1981; Leuenberger et al., 1994).
4.3. Physiological relevance of nAChR expression by AVPNs
In the CNS, the nAChR system participates in multiple regulatory brain functions (Clementi et al., 2000), including neuronal signaling (reviewed in Tribollet et al., 2001; Dajas-Bailador and Wonnacott, 2004), synaptic transmission and plasticity (reviewed in Albuquerque et al., 2000; Mansvelder and McGehee, 2000), transmitter release (Rowell, 1995; MacDermott et al., 1999; Radcliffe et al., 1999), development (Rossi et al., 2001) and survival of neurons, including spinal cord motor cells (Messi et al., 1997). Furthermore, deficiency of nAChRs is associated with changes in autonomic control. For example, absence of the α7 subunit leads to altered baroreflex responses (Franceschini et al., 2000), whereas a lack of β4(Wang et al., 2003), or β2 and β4 subunits (Xu et al., 1999) causes multiorgan autonomic dysfunction.
The role of nAChRs in regulating cholinergic outflow to the airways is not fully elucidated. Furthermore, the origin of ACh or choline, as endogenous and selective agonists for nAChRs (Alkondon et al., 1997; Mike et al., 2000) is not clear. CNS cells regulating parasympathetic outflow to the airways are located in the brain stem and in several higher brain regions, containing excitatory and inhibitory neurotransmitters (reviewed in Haxhiu et al., 2005), but no distinct cholinergic cell group that provide monosynaptic inputs to AVPNs was found. It could be postulated that ACh is released by varicosities of passing fibers from pontine structures (Jones, 1990) that are not engaged in synaptic contacts with AVPNs (Haxhiu et al., 1993). ACh released into the extracellular fluid by diffusion will reach nAChRs on AVPNs. This type of neurotransmitter release (Castel et al., 1996) and nonsynaptic transmission (Agnati and Fuxe, 2000) has already been established.
Recently, the role of nAChRs in the transmission of afferent constricting inputs from bronchopulmonary receptors to the nucleus tractus solitarius (nTS) and in the mediation of reflex airway constriction was assessed. In ferrets, it was shown that nTS neurons receiving inputs from the airway sensory system express the nAChR α3 subtype. Whereas activation of these receptors by nicotine increases cholinergic outflow to the airways, their blockade has no significant effect on reflex bronchoconstriction (Ferguson et al., 2000), suggesting that the ACβ-nAChR signaling pathway is not required for transmission of information from the airways to the nTS and from the nTS to AVPNs.
Vagal preganglionic neurons innervating the intrapulmo-nary and extrathoracic airways synthesize ACh (Kc et al., 2004), involved in communication of AVPNs with airway intramural ganglionic cells. ACh can be secreted by soma and dendrites of AVPNs. It was shown that ACh is synthesized and acts as an autocrine growth factor for small cell carcinoma (Song et al., 2003). In analogy, ACh nAChR-induced modulation of vagal preganglionic neuronal discharge could occur in an autocrine manner, through somatodendritic release of ACh, activating perisynaptic receptors, and probably nAChRs localized at glutamatergic nerve terminals innervating AVPNs.
The nAChRs could modulate activity of the AVPNs by mediating effects of endogenous substances such as choline, the tryptophan metabolite kynurenic acid, neurosteroids, and of exogenous compounds, including nicotine and the so-called nicotinic allosteric potentiating ligands and psychotomimetic drugs such as phencyclidine, ketamine (reviewed in Pereira et al., 2002), and antidepressants (Fryer and Lukas, 1999).
In summary, endogenous and exogenous substances may activate α7, α7β2, and/or α4β2 nAChRs expressed by AVPNs and/or those located on the glutamatergic axon terminals innervating AVPNs. While activation of nicotinic receptors expressed by AVPNs will result in depolarization of and in an increase in their firing rate, ACβ- or nicotine-induced activation of presynaptic receptors could facilitate excitatory synaptic neurotransmission (Fig. 6). Hence, blood borne nicotine may trigger bronchoconstriction through activation of presynaptic and/or postsynaptic mechanisms. Further investigation of the functional role of different nAChRs could provide better understanding of the importance of nAChR diversity and the central mechanisms involved in airway hyperresponsiveness induced by repeated exposures to tobacco smoke.
Fig. 6.
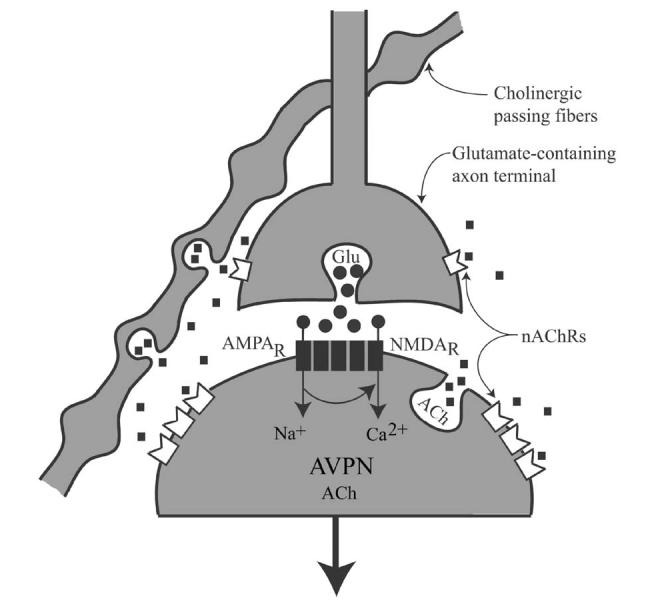
Summary diagram. Possible involvement of acetylcholine (ACh)-nicotinic acetylcholine receptors (nAChRs) signaling pathway in regulating the excitability of AVPNs. ACh released from passing fibers or from AVPNs could reach perisynaptic nAChRs (a7 homologous, α7β2, and/or α4β2 heterologous pentameric channels) causing activation of the cells, or it could activate α7 homomeric receptor expressed by axon terminals innervating AVPNs and facilitate glutamate release and further augment cholinergic outflow to the airways. Nicotine and nicotine-like substances use the same receptors to influence activity of AVPNs.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute Grant HL-50527 (to M. A. Haxhiu), National Institute of Neurological Disorders and Stroke and National Center for Research and Resources Grant 1U54 NS-39407 (to M. A. Haxhiu). We thank Dr. Richard J. Martin for reading the manuscript.
References
- Agnati LF, Fuxe K. Volume transmission as a key feature of information handling in the central nervous system possible new interpretative value of the Turing’s B-type machine. Prog. Brain Res. 2000;125:3–19. doi: 10.1016/S0079-6123(00)25003-6. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Mike A, Eisenberg HM, Maelicke A, Alkondon M. Neuronal nicotinic receptors in synaptic functions in humans and rats: physiological and clinical relevance. Behav. Brain Res. 2000;113:131–141. doi: 10.1016/s0166-4328(00)00208-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog. Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. (Review). [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur. J. Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. NMDA and AMPA receptors contribute to the nicotinic cholinergic excitation of CA1 interneurons in the rat hippocampus. J. Neurophysiol. 2003;90:3–1613. doi: 10.1152/jn.00214.2003. [DOI] [PubMed] [Google Scholar]
- Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J. Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J. Neurobiol. 2002;53:512–523. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J. Pharmacol. Exp. Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J. Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. NeuroReport. 1996;7:543–547. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- Clementi F, Fornasari D, Gotti C. Neuronal nicotinic receptors, important new players in brain function. Eur. J. Pharmacol. 2000;393:3–10. doi: 10.1016/s0014-2999(00)00066-2. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Dahms TE, Bolin JF, Slavin RG. Passive smoking. Effects on bronchial asthma. Chest. 1981;80:530–534. doi: 10.1378/chest.80.5.530. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- De Filippi G, Baldwinson T, Sher E. Nicotinic receptor modulation of neurotransmitter release in the cerebellum. Prog. Brain Res. 2005;148:307–320. doi: 10.1016/S0079-6123(04)48024-8. (Review). [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Haxhiu MA, Millis RM, Dennis GC, Kc P, Jafri A, Khajavi M, Trouth CO, Zaidi SI. Expression of alpha-7 nAChRs on spinal cord-brainstem neurons controlling inspiratory drive to the diaphragm. Respir. Physiol. Neurobiol. 2004;141:21–34. doi: 10.1016/j.resp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Millis RM, Dennis GC, Coleman BR, Johnson SM, Changizi L, Trouth CO. Alpha-7 and alpha-4 nicotinic receptor subunit immunoreactivity in genioglossus muscle motoneurons. Respir. Physiol. Neurobiol. 2005;145:153–161. doi: 10.1016/j.resp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Dominguez del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J. Comp. Neurol. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DG, Haxhiu MA, To AJ, Erokwu B, Dreshaj IA. The alpha3 subtype of the nicotinic acetylcholine receptor is expressed in airway related neurons of the nucleus tractus solitarius, but is not essential for reflex bronchoconstriction in ferrets. Neurosci. Lett. 2000;287(2):141–145. doi: 10.1016/s0304-3940(00)01166-6. [DOI] [PubMed] [Google Scholar]
- Ferreira M, Ebert SN, Perry DC, Yasuda RP, Baker CM, Davila-Garcia MI, Kellar KJ, Gillis RA. Evidence of a functional alpha7-neuronal nicotinic receptor subtype located on motoneurons of the dorsal motor nucleus of the vagus. J. Pharmacol. Exp. Ther. 2001;296:260–269. [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol. Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Franceschini D, Orr-Urtreger A, Yu W, Mackey LY, Bond RA, Armstrong D, Patrick JW, Beaudet AL, De Biasi M. Altered baroreflex responses in alpha7 deficient mice. Behav. Brain Res. 2000;113:3–10. doi: 10.1016/s0166-4328(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J. Neurochem. 1999;72:1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol., Dec. 2004;74(6):363–396. doi: 10.1016/j.pneurobio.2004.09.006. (Review). [DOI] [PubMed] [Google Scholar]
- Hadziefendic S, Haxhiu MA. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J. Auton. Nerv. Syst. 1999;76:135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Hartiala J, Mapp C, Mitchell RA, Shields RL, Gold WM. Cigarette smoke-induced bronchoconstriction in dogs: vagal and extravagal mechanisms. J. Appl. Physiol. 1984;57(4):1261–1270. doi: 10.1152/jappl.1984.57.4.1261. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Solomon IC, Motekaitis AM, Kaufman MP. Bronchomotor vagal preganglionic cell bodies in the dog: an anatomic and functional study. J. Appl. Physiol. 1992;73:1122–1129. doi: 10.1152/jappl.1992.73.3.1122. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Deal EC, Jr., Norcia MP, van Lunteren E, Mitra J, Cherniack NS. Medullary effects of nicotine and GABA on tracheal smooth muscle tone. Respir. Physiol. 1986;64:351–363. doi: 10.1016/0034-5687(86)90128-3. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Van Lunteren E, Cherniack NS. Influence of ventrolateral surface of medulla on tracheal gland secretion. J. Appl. Physiol. 1991;71:1663–1668. doi: 10.1152/jappl.1991.71.5.1663. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Jansen AS, Cherniack NS, Loewy AD. CNS innervation of airway-related parasympathetic preganglionic neurons: a transneuronal labeling study using pseudorabies virus. Brain Res. 1993;618:115–134. doi: 10.1016/0006-8993(93)90435-p. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Chavez JC, Pichiule P, Erokwu B, Dreshaj IA. The excitatory amino acid glutamate mediates reflexly increased tracheal blood flow and airway submucosal gland secretion. Brain Res. 2000;883:77–86. doi: 10.1016/s0006-8993(00)02890-0. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Kc P, Moore CT, Acquah SS, Wilson CG, Zaidi SI, Massari VJ, Ferguson DJ. Brain stem excitatory and inhibitory signaling pathways regulating bronchoconstrictive responses. J. Appl. Physiol. 2005;98:1961–1982. doi: 10.1152/japplphysiol.01340.2004. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev. Physiol., Biochem. Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J. Comp. Neurol. 1990;295:485–514. doi: 10.1002/cne.902950311. [DOI] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J. Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Central nervous pathways and control of the airways. Respir. Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Kalia MP. Organization of central control of airways. Annu. Rev. Physiol. 1987;49:595–609. doi: 10.1146/annurev.ph.49.030187.003115. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev., Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Kc P, Mayer CA, Haxhiu MA. Chemical profile of vagal preganglionic motor cells innervating the airways in ferrets: the absence of noncholinergic neurons. J. Appl. Physiol. 2004;97:1508–1517. doi: 10.1152/japplphysiol.00282.2004. [DOI] [PubMed] [Google Scholar]
- Ke L, Eisenhour CM, Bencherif M, Lukas RJ. Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes: I. Dose- and time-dependent effects of nicotine treatment. J. Pharmacol. Exp. Ther. 1998;286:825–840. [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J. Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Lena C, de Kerchove D’Exaerde A, Cordero-Erausquin M, Le-Novere N, del Mar Arroyo-Jimenez M, Changeux JP. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger P, Schwartz J, Ackermann-Liebrich U, Blaser K, Bolognini G, Bongard JP, Brandli O, Braun P, Bron C, Brutsche M. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study). Swiss Study on Air Pollution and Lung Diseases in Adults, SAPALDIA Team. Am. J. Respir. Crit. Care Med. 1994;150:1222–1228. doi: 10.1164/ajrccm.150.5.7952544. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Bencherif M. Heterogeneity and regulation of nicotinic acetylcholine receptors. Int. Rev. Neurobiol. 1992;34:25–131. doi: 10.1016/s0074-7742(08)60097-5. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu. Rev. Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Matsubayashi H, Amano T, Seki T, Sasa M, Sakai N. Postsynaptic alpha 4 beta 2 and alpha 7 type nicotinic acetylcholine receptors contribute to the local and endogenous acetylcholine-mediated synaptic transmissions in nigral dopaminergic neurons. Brain Res. 2004;1005:1–8. doi: 10.1016/j.brainres.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Messi ML, Renganathan M, Grigorenko E, Delbono O. Activation of alpha7 nicotinic acetylcholine receptor promotes survival of spinal cord motoneurons. FEBS Lett. 1997;411:32–38. doi: 10.1016/s0014-5793(97)00600-5. [DOI] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX. Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain Res. 2000;882:155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the alpha7 and beta3 subunits. J. Biol. Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rate Brain in Stereotaxic Coordinates. Academic Press; 1986. [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol. Pharmacol. 1994;46:523–530. [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002;53(4):479–500. doi: 10.1002/neu.10146. (Review). [DOI] [PubMed] [Google Scholar]
- Perez Fontan JJ, Velloff CR. Labeling of vagal motoneurons and central afferents after injection of cholera toxin B into the airway lumen. Am. J. Physiol., Lung Cell. Mol. Physiol. 2001;280:L152–L164. doi: 10.1152/ajplung.2001.280.1.L152. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann. N. Y. Acad. Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell PP. Nanomolar concentrations of nicotine increase the release of [3H]dopamine from rat striatal synaptosomes. Neurosci. Lett. 1995;189:171–175. doi: 10.1016/0304-3940(95)11471-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J. Comp. Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Schroder H, Zilles K, Maelicke A, Hajos F. Immunohisto- and cytochemical localization of cortical nicotinic cholinoceptors in rat and man. Brain Res. 1989;502:287–295. doi: 10.1016/0006-8993(89)90624-0. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Raggenbass M. Role of neuronal nicotinic receptors in the transmission and processing of information in neurons of the central nervous system. Pharmacol. Biochem. Behav. 2001;70:457–466. doi: 10.1016/s0091-3057(01)00700-6. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang N, Orr-Urtreger A, Chapman J, Rabinowitz R, Korczyn AD. Deficiency of nicotinic acetylcholine receptor beta 4 subunit causes autonomic cardiac and intestinal dysfunction. Mol. Pharmacol. 2003;63:574–580. doi: 10.1124/mol.63.3.574. [DOI] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SI, Jafri A, Doggett T, Haxhiu MA. Airway-related vagal preganglionic neurons express brain-derived neurotrophic factor and TrkB receptors: implications for neuronal plasticity. Brain Res. 2005;1044:133–143. doi: 10.1016/j.brainres.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Presence of functional neuronal nicotinic acetylcholine receptors in brainstem motoneurons of the rat. Eur. J. Neurosci. 1999;11:2737–2748. doi: 10.1046/j.1460-9568.1999.00689.x. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J. Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]


