Abstract
Since its’ discovery over 20 years ago, BDNF has been shown to play a key role in neuronal survival, in promoting neuronal regeneration following injury, regulating transmitter systems and attenuating neural-immune responses. Estrogen’s actions in the young and mature brain, and its role in neurodegenerative diseases in many cases overlaps with those observed for BDNF. Reduced estrogen and BDNF are observed in patients with Parkinson’s disease and Alzheimer’s disease, while high estrogen levels are a risk factor for development of multiple sclerosis. Estrogen receptors, which transduce the actions of estrogen, colocalize to cells that express BDNF and its receptor trkB, and estrogen further regulates the expression of this neurotrophin system. This review describes the distribution of BDNF and trkB expressing cells in the forebrain, and the roles of estrogen and the BDNF/trkB neurotrophin system in Parkinson’s disease, Alzheimer’s disease and multiple sclerosis.
1. Introduction
The neurotrophin family is a group of small, basic, secreted proteins that aid in survival and maintenance of specific neuronal populations. This family includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5). BDNF was first isolated from the pig brain and found to support neuronal survival and outgrowth of cultured embryonic chick sensory neurons [5] as well as embryonic dorsal root ganglion and nodose ganglion neurons [47]. The cloning of the cDNA for BDNF [68] opened the door for determining its expression in the central nervous system.
One of the most prominent functions of BDNF defined in earlier studies was that of promoting survival of embryonic primary sensory neurons [5,26,71], cholinergic neurons of the basal forebrain [64], dopaminergic neurons of the substantia nigra [51] and retinal ganglion cells [61]. In the adult brain, BDNF promotes regeneration of adult sensory neurons [72], retinal ganglion cells [126] and basal forebrain cholinergic neurons [65,85] following injury. Interestingly, the actions of the three other neurotrophins are both overlapping and distinct, depending on the neuronal population. For instance, both BDNF [1] and NGF [1,44] support neuronal survival of basal forebrain cholinergic neurons, but only BDNF increases survival of cultured retinal ganglion cells [61] and both BDNF [64] and NT-3 [52] activate dopamine uptake in ventral mesencephalic dopamine cells. Additionally, in developing motoneurons BDNF, NT-3 and NT-4/5, but not NGF, stimulate choline acetyltransferase [137].
2. Location of BDNF/TrkB cells
BDNF is widely distributed throughout the brain in diverse cell types (see Table 1 for a partial list) in both animals and humans. BDNF mRNA is localized to the cortex, hippocampus [48,134], midbrain, hindbrain, cerebellum, olfactory bulb, spinal cord [48] and the hypothalamus [130]. In particular, the hippocampal formation, the cerebral and cerebellar cortex, the thalamic and hypothalamic nuclei and the pontine nuclei show prominent labeling [48]. In the rat basal forebrain, BDNF mRNA is expressed in major targets of the septum/diagonal band cholinergic system to include the olfactory bulb, hippocampus, amygdala and neocortex [97]. In these initial reports, BDNF labeling was associated most closely with neurons [48,97,134], however as we will discuss later, BDNF is also present in support cells such as glia and peripheral immune cells.
Table 1.
Cell types expressing BDNF
| Cell Type | Protein/mRNA | Ref(s) |
|---|---|---|
| Neurons* | mRNA | [48,97,134] |
| Astrocytes | mRNA/protein | [31,32,105] |
| Microglia | mRNA | [31,83,124] |
| Oligodendrocytes | mRNA | [105] |
| protein | [31] | |
| Endothelial cells | mRNA | [7,88] |
| Macrophages | protein | [31,63] |
| T cells, B cells and monocytes | protein | [63] |
| Blood mononuclear cells | mRNA | [39,119] |
| Platelets | protein | [140] |
| Vascular Smooth Muscle Cells | mRNA | [30] |
Not an exhaustive list
The action of BDNF is transduced through the high affinity tyrosine kinase receptor, trkB. In the forebrain, trkB mRNA expression overlaps with many of the same targets that express BDNF mRNA to include the olfactory bulb, the cerebral cortex, hippocampal formation, amygdala and cerebellar cortex [77]. TrkB expression is also present in the thalamus, hypothalamus, brainstem and spinal cord motorneurons [79].
3. Regulation of BDNF
The BDNF gene consists of 5 exons, although the mature protein is entirely coded by one exon (exon 5). All untranslated exons possess individual promoters, resulting in a complex pattern of tissue- and cell-specific expression of this protein. The number of alternate transcripts is further increased by the presence of two polyadenylation sites and the recent discovery of a new untranslated exon [76]. Several factors regulate the expression of this gene. Significantly, compounds that influence or alter neural development exert a profound effect on the expression of this gene and protein. These include teratogens such as cocaine [142], alcohol [75] and nicotine [62], steroid hormones such as glucocorticoids [144] and estrogen [115,117], as well as calcium [133,138] and other signaling molecules such as cAMP [131].
4. Estrogen and BDNF
Hormonal status can greatly influence the expression of BDNF and/or trkB expression (Table 2). We were the first to show that BDNF-synthesizing neurons co-localized estrogen receptors in the forebrain [82], suggesting a biological substrate for the regulation of this gene by a gonadal hormone. Estrogen replacement in young adult, ovariectomized, female rats increases BDNF expression in the olfactory bulb [57,58,117], hippocampus [2,37,38,74,115,147], cortex [2,117], amygdala [74,147], septum [38,74] dorsolateral area of the bed nucleus terminalis and the lateral habenular nucleus [74]. In ovariectomized, aged (23-24 mo.) female rats, estrogen treatment increases BDNF, NGF and NT3 in the entorhinal cortex [11]. Estrogen also increases BDNF expression in primary midbrain cultures of mixed gender E15 mice [55] and in the hippocampus of gonadectomized male mice [118]. However, in some reports, estrogen has no effect on several cortical brain regions [16,38,115], the hippocampus [16] or the nucleus/ventral pallidum [38]. Interestingly, while acute [117] and long term [115] estrogen replacement increase BDNF mRNA in select cortical regions, Gibbs [37] and Cavus and Duman [16] reported that high endogenous estrogen levels during the estrus cycle are associated with decreased BDNF mRNA in the hippocampus [16,37] and prefrontal cortex [16]. Moreover, BDNF protein expression in the forebrain region is also down-regulated by exogenous estrogen replacement [57]. The mechanisms underlying the discrepancies related to estrogen/BDNF interactions are poorly understood and will require more efforts to fully resolve. Not enough effort is focused on other ovarian or other steroid hormones, which may significantly influence estrogen/BDNF interactions. For example, Gibbs [37] noted that the timing of high progesterone levels during the estrous cycle may be a critical switch for increased or decreased BDNF expression and in the study by Bimonte-Nelson [11], when estrogen treatment was combined with progesterone, neurotrophin protein expression in the entorhinal cortex decreased. Further, there are some differences in methodology in each of these studies that may influence the outcome of estrogen’s actions on BDNF, such as age, the brain region, or the treatment paradigm. For example, Cavus and Duman [16] used a chronic ovariectomy treatment regimen in which females were ovariectomized for 3 months prior to estrogen treatment whereas females in studies by Singh et al. [115] and Zhou et al. [147] were ovariectomized for less than a month. The females in the study by Cavus and Duman [16] physiologically might have more closely resembled ovarian-aged females, where we have reported that estrogen replacement also fails to increase BDNF in both the olfactory bulb and the horizontal limb [58].
Table 2.
Estrogen Modulation of BDNF
| Animal Model | Effect on BDNF Expression | Brain Region | Refs |
|---|---|---|---|
| Estrogen-replaced, OVX young adult female rat | ↑ BDNF mRNA No effect on BDNF | CA3, CA4, dentate gyrus frontal, temporal and parietal cortex | [115] |
| Estrogen-replaced, OVX young adult female rat | ↑BDNF mRNA | olfactory bulb, cerebral cortex | [117] |
| Estrogen-replaced, OVX young adult female rat | ↑BDNF mRNA | dentate gyrus, CA1,CA3, CA4 | [37] |
| ↑BDNF mRNA | pyriform cortex, hippocampus | [38] | |
| ↑BDNF protein | septum | ||
| No effect on BDNF | olfactory bulb | ||
| No effect on BDNF | frontal cortex, nucleus/ventral pallidum | ||
| Estrogen-replaced, OVX young adult female rat | ↑BDNF protein | olfactory bulb, hlDBB | [57,58] |
| Estrogen-replaced, OVX young adult female rat | ↓BDNF protein | cingulate cortex | [57] |
| Estrogen-treated, E14 chick embryo slice cultures | No effect on BDNF | hypothalamic slice cultures | [130] |
| Estrogen-treated, male/female E15 mouse fetus cultures | ↑BDNF mRNA & protein | midbrain primary cultures | [55] |
| Estrogen-replaced, OVX ovarian-aged female rat | ↓BDNF protein | olfactory bulb, hlDBB | [58] |
| Estrogen-replaced, OVX female prairie vole | ↑BDNF mRNA | dentate gyrus, CA3 | [74] |
| ↑BDNF mRNA | basolateral nucleus of the amygdala | ||
| ↑BDNF mRNA | lateral septum, lateral habenular nucleus | ||
| ↑BDNF mRNA | dorsolateral area of the bed nucleus terminalis | ||
| Estrogen-replaced, gonadectomized male mice | ↑BDNF mRNA & protein | hippocampus | [118] |
| Estrogen-replaced, OVX young adult female rat | No effect on BDNF | hippocampus, medial prefrontal & parietal cortex | [16] |
| Estrogen-replaced, OVX young adult female rat | ↑BDNF mRNA | hippocampus, cortex, spinal cord | [2] |
| OVX young adult female rat | ↑BDNF mRNA & protein | CA1, CA3, medial and basomedial amygdala | [147] |
| No effect on BDNF | CA2, dentate gyrus, hilus | ||
| No effect on BDNF | central or basolateral amygdala |
Abbreviations: hlDBB = horizontal limb of the diagonal band of Broca; OVX: ovariectomized
The BDNF gene contains a sequence with close homology to the estrogen response element and estrogen-ligand complexes are capable of binding this sequence and protecting it from DNase degradation [117]. This sequence consists of a palindromic pentamer and differs from the canonical ERE only in one base pair and in the length of the spacer arm separating the two pentamers. Interestingly the ERE-like motif is also located on the 5′ end of exon 5, which does not have its own promoter. A large separation of the response element from the promoter site is thought to enable intervention of other regulatory factors on gene expression [35]. Another possibility is that an ERE at this site is not a conventional transcriptional element but instead is a site for estrogen receptor complexes to stabilize DNA during transcription, especially in genes with long intronic sequences, such as the one that codes for BDNF.
Both estrogen and the neurotrophins have overlapping actions in the forebrain especially in the regulation of transmitter systems such as the forebrain cholinergic system. One possible explanation for this overlap may be that estrogen and the neurotrophins stimulate common second messengers. For example, in cortical explant cultures, estrogen phosphorylates the MAP kinases ERK1 and ERK2 in a time frame similar to that of the neurotrophins [116]. An alternative possibility is that estrogen may employ this growth factor as a mechanism to regulate neural cell function [82], in much the same way as estrogen interacts with epidermal growth factor (EGF) to regulate uterine growth and function [53] and enhances DNA synthesis in mammary epithelial cells [129]. Hormone-neurotrophin interactions have been demonstrated in the central nervous system as well. In the canary forebrain, where new neurons are added each season in males but not females, testosterone (a related steroid) increases BDNF levels in females and also promotes the addition of new neurons. Furthermore, injections of neutralizing antibodies to BDNF prevent the testosterone-induced addition of new neurons [102]. In the case of the forebrain cholinergic system, both estrogen and the neurotrophins are known to affect cholinergic function via regulation of choline acetyltransferase and high affinity choline uptake. Injections of a neutralizing antibody to the BDNF receptor trkB reduces CREB-phosphorylation in a forebrain circuit and combined injections of anti-trkB and the receptor for nerve growth factor, anti-trkA reduces estrogen-mediated increases in ChAT expression [59], indicating that estrogen may exert its actions via a neurotrophin receptor complex. A related example of a potential hormone-neurotrophin interaction has recently been reported in an animal model for depression, where female Wistar rats showed an increased vulnerability to depression [122]. In this model, peak estrogen levels are associated with the lowest expression of BDNF mRNA levels in the hippocampus and frontal cortex [122]. Interestingly, in the uterus where sympathetic neurite innervation is suppressed by high estrogen levels, BDNF appears to mediate this neurite suppression. Estrogen induces BDNF expression in the uterus, which can be abolished by blocking antibodies to BDNF thus suppressing sympathetic neurite outgrowth [66].
Recent studies also show that estrogen may regulate BDNF expression via non-receptor dependent mechanisms, involving disinhibition of GABA-ergic neurons [13]. This is more fully discussed in the accompanying review by Scharfman and Maclusky.
5. Transport of Neurotrophins
Anterograde and retrograde transport of neurotrophins is a powerful mechanism exploited by the central nervous system that allows these trophic factors to act on distant brain regions. Retrograde transport of BDNF was suggested for basal forebrain cholinergic projections by Wetmore et al. [135] and DiStefano et al. [27] who determined that hippocampal BDNF and NT-3 could be retrogradely transported from the hippocampus, an area rich in these neurotrophins, to the basal forebrain regions of the medial septum and diagonal band nuclei. BDNF retrograde transport also occurs following intrastriatal infusions in a distinct subset of neurons within the substantia nigra [25]. Interestingly, distinct populations of neurons selectively transport NGF, BDNF and NT-3. For example, NGF is not selectively transported by spinal cord motor neurons [27,120] or neurons in the entorhinal cortex, thalamus or neurons within the hippocampus [27]. However, these same neurons do selectively transport BDNF and NT-3 [27]. Similarly, anterograde transport of BDNF has also been documented, as in the case of striatal BDNF that is derived from its cortical afferents [3]. Anterograde transport may have therapeutic value as was shown in a recent study where injection of BDNF into the eye following a visual cortex lesion reduced cell loss and preserved function in the lateral geniculate nucleus, presumably via anterograde transport of exogenously applied BDNF [14].
Neurotrophin transport is mediated by its receptors, which include the tyrosine kinase family of receptors (trks) that bind one or more neurotrophins and p75, the pan-neurotrophin receptor. Both p75 and trkB, the trk receptor that binds BDNF, mediate BDNF transport [25,132]. Estrogen up-regulates trkB in the forebrain [58], and a recent study by this laboratory showed that estrogen enhances transport of exogenous BDNF from the olfactory bulb to the diagonal band region of the basal forebrain [59]. Antibodies to trkB attenuated this transport, suggesting that the mechanism underlying estrogen-mediated neurotrophin transport is hormonal regulation of the receptor. Thus neurotrophin/estrogen interactions may allow hormone action to be transduced in distal regions lacking hormone receptors.
6. Changes in BDNF Regulation with Age
In several brain regions, BDNF and/or trkB expression changes with age and the impact of these changes have functional consequences for neuronal pathways. In the inferior colliculus, a major auditory pathway, aged animals show reductions in inhibitory and excitatory synaptic terminals [45] and this loss is coincident with reduced trkB protein expression [109]. BDNF mRNA is decreased in the pons and BDNF protein decreases in the midbrain of aged rats [23]. Additionally, reductions in trkB mRNA are even more widespread in that the retrosplenial cortex, thalamus, hypothalamus and hippocampus are affected [23]. The consequence of these reductions in trkB and BDNF expression are decreased memory ability and impaired learning as measured with the Morris water maze [23]. Age also affects the efficacy of estrogen treatment in ovarian-aged female rats. In the forebrain, estrogen replacement increases BDNF and trkB protein expression in young adult females but not in ovarian-aged (11-13 mos.) reproductive senescent females [58].
Aging also influences the effectiveness of BDNF to protect motoneurons following injury. In neonatal rats, BDNF is retrogradely transported by motor neurons [141] and local application of BDNF prevents motoneuron cell death following transection of the sciatic nerve [141] or the facial nerve [43,111]. Similarly, in young adult female rats, infusion of BDNF and NT-3, following spinal cord transection [139] or application of BDNF following cervical spinal cord hemisection [143] results in axonal regeneration. However, in aged female rats (30 mos.), mRNA and protein expression of trkA, trkB and trkC are reduced in the cervical and lumbar dorsal root ganglia [10]. Further, following axotomy of the sciatic nerve, aged rats (30 mos.) respond differently than young rats (2-3 mos.). In young rats but not aged rats, trkB increases in lumbar motoneurons following axotomy [60]. Thus, the benefits afforded by BDNF may be attenuated with increasing age.
Aging also affects BDNF’s ability to protect neuronal activity. BDNF infusion in young rats triggers LTP which increases activation of trkB and extracellular signal-regulated kinase (ERK) and enhances evoked release of glutamate in synaptosomes [41]. However, in aged rats both BDNF induced LTP and the associated signaling is reduced [41]. Interestingly, in male rats, Lapchak et al. [67] did not observe changes in BDNF mRNA or trkB mRNA with age, suggesting that gender or loss of ovarian hormones may influence the levels of BDNF/trkB in the brain.
This loss of BDNF regulation may be particularly significant for the etiology of neurodegenerative diseases that have a significant impact on the aging brain such as Alzheimer’s disease, Parkinson’s disease, and autoimmune diseases like multiple sclerosis. These diseases are good examples of how gender and ovarian steroids influence the risk of developing the disease. A summary of how BDNF levels change with these disease states and the effects of hormone replacement therapy on the symptoms associated with these diseases are summarized in Table 3 and discussed in the following sections.
Table 3.
Summary of Clinical Data on BDNF Expression and Effects of Hormone Replacement Therapy in Specific Neurodegenerative Diseases
| Disease | BDNF Regulation | Ref(s) | Hormone Replacement Therapy | Ref(s) |
|---|---|---|---|---|
| Alzheimer’s | ↓protein expression | [22,34,46,80,89] | ↓risk for AD | [92,125,146] |
| Disease | ↓mRNA expression | [50,87,98] | ↑risk for dementia | [33,114] |
| BDNF present in cells surrounding plaques | [34] | no benefit | [86] | |
| Parkinson’s | ↓protein expression | [54,84,94] | ↓risk for PD | [24] |
| Disease | ↓in PD symptoms | [9,108] | ||
| ↓in PD symptoms | [90] | |||
| no benefit | [121] | |||
| Multiple Sclerosis | ↑protein expression w/increased demyelination | [63,119] | ↑MS symptoms | [4,99] |
| ↑mRNA expression | [39] | ↑risk for MS | [128] |
7. Estrogen and Parkinson’s Disease
Estrogen may alleviate some of the symptoms associated with Parkinson’s disease, but clinical studies have shown mixed benefits to estrogen replacement therapy. In two small clinical studies, circulating estrogen correlated with reduced dyskinesias [9] and reduction of conjugated estrogen therapy led to exacerbation of symptoms in patients with Parkinson’s disease [108]. In a retrospective study, post-menopausal estrogen replacement therapy was associated with a decreased risk for developing Parkinson’s disease [24] and in some Parkinson’s female patients, the menstrual cycle influences the severity of motor fluctuations [40,101]. However, oral contraceptives, especially in patients with pre-existing striatal abnormalities was implicated as a risk factor for increased dyskinesia [90] and, in a placebo-controlled, double-blind trial, estrogen treatment had no effect on Parkinsonian symptomology [121].
Animal models suggest that estrogen may be important for regulation of the dopaminergic system which is affected in Parkinson’s disease. In adult female rats, ovariectomy decreases striatal dopamine release, while estrogen replacement restores these levels in an in vitro superfusion model [8]. Similarly, an acute physiological dose of estrogen increases dopamine synthesis and tyrosine hydroxylase, the rate limiting enzyme in the dopamine biosynthetic pathway [95]. Estrogen replacement following ovariectomy also increases rat striatal D-2 dopamine receptors [70,106] and dopamine cells in young adult African green monkeys [69].
Gender differences have also been observed in injury models that mimic Parkinson’s disease, such as striatal application of the neurotoxins, metamphetamine and 1-methyl-4-phenyl-1,2,3,5-tetrahydropyridine (MPTP). Higher levels of dopamine depletion are observed in male mice following treatment with methamphetamine [29,145] or MPTP [81] when compared to age-matched females. Following MPTP treatment, estrogen treatment also reduces the concentration of glial fibrillary acidic protein (GFAP) [81], an indicator of astrogliosis. Pretreatment with estrogen, prior to MPTP treatment, prevented the reduction in striatal dopamine in both female and male mice [28,15] and pretreatment with the ovarian steroids, estrogen, progesterone or a selective estrogen receptor modulator, raloxifene limited MPTP-induced dopamine depletion [42]. Interestingly, although estrogen treatment protected striatal dopamine cells against dopamine depletion, in the study by Callier et al. [15] estrogen pretreatment did not attenuate the loss of dopamine terminals or cell body loss following MPTP treatment.
8. BDNF and Parkinson’s Disease
One of the mechanisms by which estrogen might affect striatal neurons is through the production of neurotrophins such as BDNF. Post mortem analysis of brain tissue from patients with Parkinson’s disease suggests that striatal neurodegeneration correlates with reductions in BDNF expression. Moreover, in brain tissue from patients with Parkinson’s [54,84,94] or with Lewy body dementia [54] striatal BDNF immunoreactivity is reduced as compared to age- and sex-matched controls.
In animal models, a striatal stab wound in 6-8 week old mice increases BDNF and GDNF expression around the wound site and appear to be localized to activated microglia and macrophages [6]. Further, production of BDNF and GDNF occurs in concert with sprouting dopaminergic fibers and dopamine-transporter positive neurites [6]. In rhesus monkeys, elevated BDNF levels can be observed in young adults (8-9.5 yrs) but not middle aged (15-17 yrs) or aged (21-31 yrs) animals following MPTP treatment [20]. Thus, these studies suggest that increased BDNF expression following striatal damage is beneficial to dopaminergic neurons, but that these compensatory changes in growth factor expression may be lost with age.
9. Estrogen and Alzheimer’s Disease
Estrogen may play a role in protecting women from dementia, like Alzheimer’s disease. In several prospective, studies [92,125,146] prior hormone replacement therapy (HRT) was associated with reduced risk for AD. Further, in a placebo-controlled, double-blind study, women who had undergone an oophorectomy, a procedure equivalent to a surgical menopause, and who had taken estrogen following the procedure, displayed no loss on tests for short-term memory, long-term memory and logical reasoning as compared to women who had undergone the surgery but did not receive estrogen [112]. However, Mulnard et al., [86] reported no benefit to estrogen replacement in women with mild to moderate dementia and in a recent study, estrogen/progesterone replacement therapy [114] and estrogen replacement therapy alone [33] increased the adverse effects on global cognitive function in women over 65 years old. Some of this dichotomy could be related to the timing of estrogen replacement. In the study conducted by Zandi et al. [146], the reduced risk for dementia included women who were current HRT users and women who had used HRT for > 10 years. In the most recent study [33,114], in which there was an increased risk for dementia, HRT was initiated in post-menopausal women.
10. BDNF and Alzheimer’s Disease
Age-related neurodegeneration associated with Alzheimer’s disease also correlates with changes in BDNF expression. BDNF protein expression is reduced in the hippocampus [46], the temporal cortex [22], the parietal cortex [80], the frontal cortex [34] and the entorhinal cortex [89] of Alzheimer’s disease patients as compared to age-matched control patients. BDNF mRNA is also reduced in the hippocampus [87,98] and parietal cortex [50] and, reductions in trkA, trkB, and trkC have also been reported in the human nucleus basalis of Meynert [107]. Decreased levels of hippocampal BDNF have potentially damaging effects on neurons in the basal forebrain, as BDNF is retrogradely transported from the hippocampus to the basal forebrain [27] a region that shows significant cell loss in Alzheimer’s disease patients. BDNF production also correlates with plaque formation. BDNF immunostaining is present in dystrophic neurites surrounding senile plaques while trkB immunoreactivity is localized strongly to reactive glial cells including those surrounding senile plaques in Alzheimer’s disease as compared to age-matched controls [34].
The connection between estrogen, BDNF and Alzheimer’s disease is mainly correlative, namely that estrogen depletion is a risk factor for Alzheimer’s disease and that it is a regulatory factor for BDNF, which is decreased in Alzheimer’s patients. However, studies that directly tie estrogen use to BDNF action in Alzheimer’s disease are lacking.
11. Estrogen, BDNF and AutoImmune Diseases
Gender is believed to be a risk factor for development of autoimmune diseases such as multiple sclerosis (MS) and rheumatoid arthritis and many reports suggest that estrogen is a key player in disease progression of multiple sclerosis, a chronic demyelinating disease. The clinical data suggests that estrogen and/or progesterone may actually exacerbate MS symptoms. When estradiol serum concentrations are high and progesterone is low, there is an increase in the number of gadolinium enhancing lesions detected by MRI [4,99] and, contraceptives containing high estrogen concentrations are a risk factor for MS [128].
In animal models, however, estrogen replacement can attenuate the development of experimental allergic encephalomyelitis (EAE), a commonly used model for multiple sclerosis. Estrogen suppresses EAE disease severity and proinflammatory cytokine production in female rats [49] and in female [56] and male mice [93], although, the effects of estrogen are attenuated with age [78]. One mechanism by which estrogen could lessen disease severity is through protection of an important myelin-forming cell, the oligodendrocyte. Estrogen application prevents the cytotoxic effects of the peroxynitrite generator 3-(4-morpholinyl)-sydnonimine on oligodendrocytes and these neuroprotective effects are likely mediated through activation of the estrogen receptors [123]. BDNF mRNA is expressed in oligodendrocytes, astrocytes [105] and microglia [83,124] and, activation of these estrogen receptors could potentially lead to production of BDNF. Additionally, trkB mRNA is present in microglia [21] and represents another checkpoint for estrogenic actions on BDNF.
Clinical studies suggest that BDNF is up-regulated in patients with leukoencephalitis and multiple sclerosis. In some patients, perivascular infiltrates are immunoreactive for BDNF and, in patients with multiple sclerosis BDNF immunoreactive macrophages and lymphocytes are distributed throughout the lesion with enhanced expression in areas of extensive demyelination [63]. Brain tissue taken from patients with multiple sclerosis also express BDNF in immune cells (T cells, macrophages/microglia) as well as reactive astrocytes and, BDNF immunoreactivity positively correlates with demyelinating lesions [119]. Interestingly, neuronal trkB immunoreactivity is present in neurons in the immediate vicinity of the multiple sclerosis plaques but not in immune cells [119]. In unstimulated, peripheral blood mononuclear cells from multiple sclerosis patients in remission, Gielen et al. [39] found that BDNF mRNA is significantly elevated by approximately 60% in MS patients as compared to patients with other neurological diseases or healthy controls. BDNF expression in context of multiple sclerosis is likely a compensatory mechanism to promote recovery/repair of damaged neurons.
Several reports suggest that another means by which estrogen regulates disease progression is through modulation of the dendritic cells. Estrogen interrupts dendritic cell activation of T cell proliferation [96] and prevents dendritic cells from presenting antigen to myelin basic protein-specific T cells [73,96]. In microglia, BDNF inhibits interactions with major histocompatibility (MHC) II molecules [91], thus estrogen’s actions on dendritic cells could potentially be mediated through BDNF.
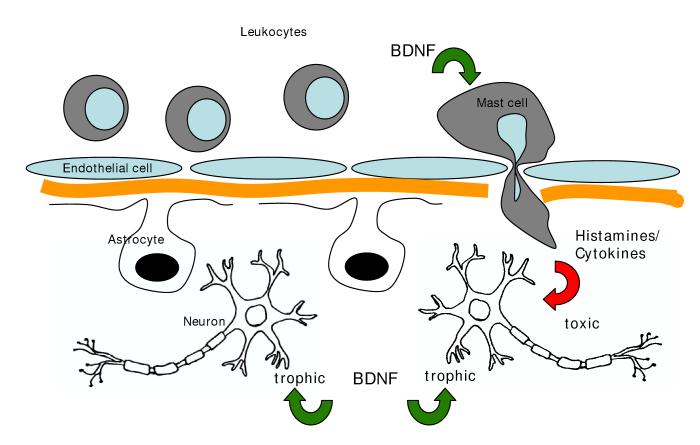
Alternatively, estrogen may act by regulating the blood brain barrier. Endothelial cells and their tight junctions form the functional blood-brain barrier. Additionally, astrocytic endfeet interact with the microvasculature and are believed to maintain the integrity of the blood-brain barrier [103,136]. The literature regarding estrogen’s effects on the blood-brain barrier is mixed. Ethinyl estradiol, a synthetic estrogen commonly used in birth control pills, increases permeability of the brain to albumin [36], water [104], inulin and sucrose [148]. The natural estrogen, 17β-estradiol, on the other hand, increases glucose uptake and transport [12,113], reduces ischemia [19] and VEGF-induced [18] leakiness of the blood-brain barrier in the cortex [17]. However, estrogen acts synergistically with myelin basic protein to cause mast cell infiltration into the brain parenchyma [127]. Mast cell activation is a critical determinant of the severity of the response to EAE [110] and mast cells respond to neurotrophins with an increased release of inflammatory mediators [100]. Hence in this instance, estrogens permissive actions on both BDNF expression and mast cell invasion could result in an adverse affect, and may provide an explanation for why MS symptoms are exacerbated in women with high serum estradiol levels (see Figure 1).
Figure 1.
Schematic representation of trophic and toxic actions resulting from hormone-BDNF interactions: Estrogen stimulates BDNF synthesis in several neural cell types (neurons, astrocytes, endothelial cells). Direct action of mature BDNF on neurons is usually trophic, however, BDNF action on other cells, such as mast cells, which stimulates the production of inflammatory mediators, may indirectly result in toxicity to neurons.
12. Summary and conclusions
BDNF is widely distributed in the brain and significantly impacts neuronal survival and function in the adult brain through a variety of cell types to include neurons, astrocytes, oligodendrocytes, microglia and endothelial cells. Studies have shown that estrogen regulates BDNF expression, potentially through transcription. We hypothesize that this regulation has the potential to be trophic or toxic to neurons (Figure 1) and is cell type-, region- and age-dependent. Neurodegenerative diseases are good examples of how estrogen regulation of BDNF is less effective in sustaining the neuronal health in the aging brain. Thus, therapeutic use of estrogen should be carefully considered in context of the age of the patient, prior ERT history, dose, and timing of estrogen administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alderson R, Alterman A, Barde Y-A, Lindsay R. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- [2].Allen A, McCarson K. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinol. 2005;81:193–199. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- [3].Altar C, Cai N, Bliven T, Juhasz M, Conner J, Acheson A, Lindsay R, Wiegand S. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- [4].Bansil S, Lee H, Jindal S, Holtz C, Cook S. Correlation between sex hormones and magnetic resonance imaging lesions in multiple sclerosis. Acta Neurol. Scand. 1999;99:91–94. doi: 10.1111/j.1600-0404.1999.tb00663.x. [DOI] [PubMed] [Google Scholar]
- [5].Barde Y-A, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Batchelor P, Liberatore G, Wong J, Porritt M, Frerichs F, Donnan G, Howells D. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bayas A, Hummel V, Kallmann B, Karch C, Toyka K, Rieckmann P. Human cerebral endothelial cells are a potential source for bioactive BDNF. Cytokine. 2002;19:55–58. doi: 10.1006/cyto.2002.0892. [DOI] [PubMed] [Google Scholar]
- [8].Becker J. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- [9].Bedard P, Langelier P, Villeneuve A. Oestrogens and extrapyramidal system. Lancet. 1977;2:1367–1368. doi: 10.1016/s0140-6736(77)90429-9. [DOI] [PubMed] [Google Scholar]
- [10].Bergman E, Fundin B, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J. Comp. Neurol. 1999;410:368–386. doi: 10.1002/(sici)1096-9861(19990802)410:3<368::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- [11].Bimonte-Nelson H, Nelson M, Granholm A-C. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- [12].Bishop J, Simpkins J. Estradiol enhances brain glucose uptake in ovariectomized rats. Brain Res. Bull. 1995;36:315–320. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]
- [13].Blurton-Jones M, Kuan P, Tuszynski M. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J. Comp. Neurol. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- [14].Caleo M, Medini P, von Bartheld C, Maffei L. Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J. Neurosci. 2003;23:287–296. doi: 10.1523/JNEUROSCI.23-01-00287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Callier S, Morissette M, Grandbois M, Di Paolo T. Stereospecific prevention by 17β-estradiol of MPTP-induced dopamine depletion in mice. Synapse. 2000;37:245–251. doi: 10.1002/1098-2396(20000915)37:4<245::AID-SYN1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [16].Cavus I, Duman R. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol. Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- [17].Chi O, Liu X, Weiss H. Horm. Metab. Res. 2002;34:530–534. doi: 10.1055/s-2002-34794. [DOI] [PubMed] [Google Scholar]
- [18].Chi O, Barsoum S, Wen Y, Liu X, Weiss H. 17beta-estradiol prevents blood-brain barrier disruption induced by VEGF. Horm. Metab. Res. 2004;36:272–276. doi: 10.1055/s-2004-814478. [DOI] [PubMed] [Google Scholar]
- [19].Chi O, Hunter C, Liu X, Weiss H. Effects of VEGF and nitric oxide synthase inhibition on blood-brain barrier disruption in the ischemic and non-ischemic cerebral cortex. Neurol. Res. 2005;27:864–868. doi: 10.1179/016164105X49418. [DOI] [PubMed] [Google Scholar]
- [20].Collier T, Ling Z, Carvey P, Fletcher-Turner A, Yurek D, Sladek J, Jr, Kordower J. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exp. Neurol. 2005;191:S60–S67. doi: 10.1016/j.expneurol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [21].Condorelli D, Salin T, Dell’ A, Mudo G, Corsaro M, Timmusk T, Metsis M, Belluardo N. Neurotrophins and their trk receptors in cultured cells of the glial lineage and in white matter of the central nervous system. J. Mol. Neurosci. 1995;6:237–248. doi: 10.1007/BF02736783. [DOI] [PubMed] [Google Scholar]
- [22].Connor B, Young D, Yan Q, Faull R, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Mol. Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- [23].Croll S, Ip N, Lindsay R, Wiegand S. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- [24].Currie L, Harrison M, Trugman J, Bennett J, Wooten F. Postmenopausal estrogen use affects risk for Parkinson disease. Arch. Neurol. 2004;61:886–888. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- [25].Curtis R, Adryan K, Stark J, Park J, Compton D, Weskamp G, Huber L, Chao M, Jaenisch R, Lee K. Differential role of the low affinity neurotrophin receptor (p75) in retrograde axonal transport of the neurotrophins. Neuron. 1995;14:1201–1211. doi: 10.1016/0896-6273(95)90267-8. [DOI] [PubMed] [Google Scholar]
- [26].Davies A, Thoenen H, Barde Y-A. The response of chick sensory neurons to brain-derived neurotrophic factor. J. Neurosci. 1986;6:1897–1904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].DiStefano P, Friedman B, Radziejewski C, Alexander C, Boland P, Schick C, Lindsay R, Wiegand S. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- [28].Dluzen D, McDermott J, Liu B. Estrogen as a neuroprotectant against MPTP-Induced neurotoxicity in C57/B1 mice. Neurotoxicol. Teratol. 1996;18:603–606. doi: 10.1016/0892-0362(96)00086-4. [DOI] [PubMed] [Google Scholar]
- [29].Dluzen D, McDermott J. Developmental and genetic influences upon gender differences in methamphetamine-induced nigrostriatal dopaminergic neurotoxicity. Ann. NY Acad. Sci. 2004;1025:205–220. doi: 10.1196/annals.1316.026. [DOI] [PubMed] [Google Scholar]
- [30].Donovan M, Miranda R, Kraemer R, McCaffrey T, Tessarollo L, Mahadeo D, Sharif S, Kaplan D, Tsoulfas P, Parada L. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am. J. Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- [31].Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol. Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- [32].Dreyfus C, Dai X, Lercher L, Racey B, Friedman W, Black I. Expression of neurotrophins in the adult spinal cord in vivo. J. Neurosci. Res. 1999;56:1–7. doi: 10.1002/(SICI)1097-4547(19990401)56:1<1::AID-JNR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [33].Espeland M, Rapp S, Shumaker S, Brunner R, Manson J, Sherwin B, Hsia J, Margolis K, Hogan P, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- [34].Ferrer I, Marin X, Rey M, Ribalta T, Goutan E, Blanco R, Tolosa E, Marti E. BDNF and full-length truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J. Neuropath. Exp. Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- [35].Fuller P. The steroid receptor superfamily: mechanisms of diversity. FASEB J. 1991;5:3092–3099. doi: 10.1096/fasebj.5.15.1743440. [DOI] [PubMed] [Google Scholar]
- [36].Gammal E, Zuk A. Effect of ethinyl estradiol on endothelial permeability to 125I-labeled albumin in female rats. Exp. Mol. Pathol. 1980;32:91–101. doi: 10.1016/0014-4800(80)90046-5. [DOI] [PubMed] [Google Scholar]
- [37].Gibbs R. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- [38].Gibbs R. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- [39].Gielen A, Khademi M, Muhallab S, Olsson T, Piehl F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand. J. Immunol. 2003;57:493–497. doi: 10.1046/j.1365-3083.2003.01260.x. [DOI] [PubMed] [Google Scholar]
- [40].Giladi N, Honigman S, Mizuta E, Kuno S. Hormones and Parkinson’s disease. Neurol. 1995;45:1028–1029. doi: 10.1212/wnl.45.5.1028-a. [DOI] [PubMed] [Google Scholar]
- [41].Gooney M, Messaoudi E, Maher F, Bramham C, Lynch M. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiol. Aging. 2004;25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [42].Grandbois M, Morissette M, Callier S, Di Paolo T. Ovarian steroids and raloxifene prevent MPTP-induced dopamine depletion in mice. Neuroreport. 2000;11:343–346. doi: 10.1097/00001756-200002070-00024. [DOI] [PubMed] [Google Scholar]
- [43].Gravel C, Gotz R, Lorrain A, Sendtner M. Adenoviral gene transfer of ciliary neurotrophic factor and brain-derived neurotrophic factor leads to long-term survival of axotomized motor neurons. Nature Med. 1997;3:765–770. doi: 10.1038/nm0797-765. [DOI] [PubMed] [Google Scholar]
- [44].Hartikka J, Hefti F. Development of septal cholinergic neurons in culture: plating density and glial cells modulate effects of NGF on survival, fiber growth, and expression of transmitter-specific enzymes. J. Neurosci. 1988;8:2967–2985. doi: 10.1523/JNEUROSCI.08-08-02967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Helfert R, Sommer T, Meeks J, Hofstetter P, Hughes L. Age-related synaptic changes in the central nucleus of the inferior colliculus of Fischer-344 rats. J. Comp. Neurol. 1999;406:285–298. [PubMed] [Google Scholar]
- [46].Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease. Arch. Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- [47].Hofer M, Barde Y-A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988;331:261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- [48].Hofer M, Pagliusi S, Hohn A, Leibrock J, Barde Y-A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hoffman G, Le W, Murphy A, Koski C. Divergent effects of ovarian steroids on neuronal survival during experimental allergic encephalitis in Lewis rats. Exp. Neurol. 2001;171:272–282. doi: 10.1006/exnr.2001.7783. [DOI] [PubMed] [Google Scholar]
- [50].Holsinger R, Schnarr J, Henry P, Castelo V, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Mol. Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- [51].Hyman C, Hofer M, Barde Y-A, Juhasz M, Yancopoulos G, Squinto S, Lindsay R. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- [52].Hyman C, Juhasz M, Jackson C, Wright P, Ip N, Lindsay R. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J. Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ignar-Trowbridge D, Nelson K, Bidwell M, Curtis S, Washburn T, McLachlan J, Korach K. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc. Natl. Acad. Sci. USA. 1992;89:4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Imamura K, Hishikawa N, Ono K, Suzuki H, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Cytokine production of activated microglia and decrease in neurotrophic factors of neurons in the hippocampus of Lewy body disease brains. Acta Neuropathol. 2005;109:141–150. doi: 10.1007/s00401-004-0919-y. [DOI] [PubMed] [Google Scholar]
- [55].Ivanova T, Kuppers E, Engele J, Cordian B. Estrogen stimulates brain-derived neurotrophic factor expression in embryonic mouse midbrain neruons through a membrane-mediated and calcium-dependent mechanism. J. Neurosci. Res. 2001;66:221–230. doi: 10.1002/jnr.1214. [DOI] [PubMed] [Google Scholar]
- [56].Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J. Neuroimmunol. 1994;53:203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- [57].Jezierski M, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Mol. Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- [58].Jezierski M, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol. Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- [59].Jezierski M, Sohrabji F. Estrogen enhances retrograde transport of brain-derived neurotrophic factor in the rodent forebrain. Endocrinol. 2003;144:5022–5029. doi: 10.1210/en.2003-0724. [DOI] [PubMed] [Google Scholar]
- [60].Johnson H, Hokfelt T, Ulfhake B. Expression of p75, trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Mol. Brain Res. 1999;69:21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- [61].Johnson J, Barde Y-A, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J. Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kenny P, File S, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res. Mol. Brain Res. 2000;85 doi: 10.1016/s0169-328x(00)00246-1. [DOI] [PubMed] [Google Scholar]
- [63].Kerschensteiner M, Gallmeier E, Behrens L, Leal V, Misgeld T, Klinkert W, Kolbeck R, Hoppe E, Oropeza-Wekerle R-L, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J. Exp. Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Knusel B, Winslow J, Rosenthal A, Burton L, Seid D, Nikolics K, Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc. Natl. Acad. Sci. USA. 1991;88:961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Knusel B, Beck K, Winslow J, Rosenthal A, Burton L, Widmer H, Nikolics K, Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J. Neurosci. 1992;12:4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Krizsan-Agbas D, Pedchenko T, Hasan W, Smith P. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur. J. Neurosci. 2003;18:2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- [67].Lapchak P, Araujo D, Beck K, Finch C, Johnson S, Hefti F. BDNF and trkB mRNA expression in the hippocampal formation of aging rats. Neurobiol. Aging. 1993;14:121–126. doi: 10.1016/0197-4580(93)90087-r. [DOI] [PubMed] [Google Scholar]
- [68].Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y-A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- [69].Leranth C, Roth R, Elsworth J, Naftolin F, Horvath T, Redmond D., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J. Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Levesque D, Di Paolo T. Modulation by estradiol and progesterone of the GTP effect on striatal D-2 dopamine receptors. Biochem. Pharmacol. 1993;45:723–733. doi: 10.1016/0006-2952(93)90148-p. [DOI] [PubMed] [Google Scholar]
- [71].Lindsay R, Thoenen H, Barde Y-A. Placode and neural crest-derived sensory neurons are responsive at early developmental stages to brain-derived neurotrophic factor. Dev. Biol. 1985;112:319–328. doi: 10.1016/0012-1606(85)90402-6. [DOI] [PubMed] [Google Scholar]
- [72].Lindsay R. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu H, Buenafe A, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark A, Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- [74].Liu Y, Fowler C, Young L, Yan Q, Insel T, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J. Comp. Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- [75].Maier S, West J. Drinking patterns and alcohol-related birth defects. Alcohol Res. Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- [76].Malkovska I, Kernie S, Parada L. Differential expression of the four untranslated BDNF exons in the adult mouse brain. J. Neurosci. Res. 2006;83:211–221. doi: 10.1002/jnr.20728. [DOI] [PubMed] [Google Scholar]
- [77].Masana Y, Wanaka A, Kato H, Asai T, Tohyama M. Localization of trkB mRNA in postnatal brain development. J. Neurosci. Res. 1993;35:468–479. doi: 10.1002/jnr.490350503. [DOI] [PubMed] [Google Scholar]
- [78].Matejuk A, Hopke C, Vandenbark A, Hurn P, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J. Immunol. 2005;174:2387–2395. doi: 10.4049/jimmunol.174.4.2387. [DOI] [PubMed] [Google Scholar]
- [79].Merlio J-P, Ernfors P, Jaber M, Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neurosci. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- [80].Michalski B, Fahnestock M. Pro-brain derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Mol. Brain Res. 2003;111:148–154. doi: 10.1016/s0169-328x(03)00003-2. [DOI] [PubMed] [Google Scholar]
- [81].Miller D, Ali S, O’Callaghan J, Laws S. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann. NY Acad. Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- [82].Miranda R, Sohrabji F, Toran-Allerand C. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc. Natl. Acad. Sci. USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Miwa T, Furukawa S, Nakajima K, Furukawa Y, Kohsaka S. Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. J. Neurosci. Res. 1997;50:1023–1029. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1023::AID-JNR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [84].Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived neurotrophic factor and nerve-growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- [85].Morse J, Wiegand S, Anderson K, You Y, Cai N, Carnahan J, Miller J, DiStefano P, Altar C, Lindsay R. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J. Neurosci. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mulnard R, Cotman C, Kawas C, van Dyck C, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal L. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- [87].Murray K, Gall C, Jones E, Isackson P. Differential regulation of brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase messenger RNA expression in Alzheimer’s disease. Neurosci. 1994;60:37–48. doi: 10.1016/0306-4522(94)90202-x. [DOI] [PubMed] [Google Scholar]
- [88].Nakahashi T, Fujimura H, Altar C, Li J, Kambayashi J.-i., Tandon N, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- [89].Narisawa-Saito M, Wakabayashi K, Takahashi H, Nawa H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport. 1996;7:2925–2928. doi: 10.1097/00001756-199611250-00024. [DOI] [PubMed] [Google Scholar]
- [90].Nausieda P, Koller W, Weiner W, Klawans H. Chorea induced by oral contraceptives. Neurol. 1979;29:1605–1609. doi: 10.1212/wnl.29.12.1605. [DOI] [PubMed] [Google Scholar]
- [91].Neumann H, Misgeld T, Matsumuro K, Wekerle H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc. Natl. Acad. Sci. USA. 1998;95:5779–5784. doi: 10.1073/pnas.95.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Paganini-Hill A, Henderson V. Estrogen replacement therapy and risk of Alzheimer disease. Arch. Intern. Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- [93].Palaszynski K, Liu H, Loo K, Voskuhl R. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 2004;149:84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- [94].Parain K, Murer M, Yan Q, Faucheux B, Aid Y, Hirsch E, Raisman-Vozari R. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport. 1999;10:557–561. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- [95].Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J. Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- [96].Pettersson A, Ciumas C, Chirsky V, Link H, Huang Y-M, Xiao B-G. Dendritic cells exposed to estrogen in vitro exhibit therapeutic effects in ongoing experimental allergic encephalomyelitis. J. Neuroimmunol. 2004;156:58–65. doi: 10.1016/j.jneuroim.2004.07.004. [DOI] [PubMed] [Google Scholar]
- [97].Phillips H, Hains J, Laramee G, Rosenthal A, Winslow J. Widespread expression of BDNF but not NT-3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- [98].Phillips H, Hains J, Armanini M, Laramee G, Johnson S, Winslow J. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- [99].Pozzilli C, Falaschi P, Mainero C, Martocchia A, D’Urso R, Proietta A, Frontoni M, Bastianello S, Filippi M. MRI in multiple sclerosis during the menstrual cycles; relationship with sex hormone patterns. Neurol. 1999;53:622–624. doi: 10.1212/wnl.53.3.622. [DOI] [PubMed] [Google Scholar]
- [100].Purcell W, Westgate C, Atterwill C. Rat brain mast cells: an in vitro paradigm for assessing the toxic effects of neurotropic therapeutics. Neurotoxicol. 1996;17:845–850. [PubMed] [Google Scholar]
- [101].Quinn N, Marsden C. Menstrual-related fluctuations in Parkinson’s disease. Mov. Disord. 1986;1:85–87. doi: 10.1002/mds.870010112. [DOI] [PubMed] [Google Scholar]
- [102].Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- [103].Raub T, Kuentzel S, Sawada G. Permeability of bovine brain microvessel endothelial cells in vitro: barrier tightening by a factor released from astroglioma cells. Exp. Cell. Res. 1992;199:330–340. doi: 10.1016/0014-4827(92)90442-b. [DOI] [PubMed] [Google Scholar]
- [104].Reid A, Teasdale G, McCulloch J. Hormonal influence on water permeability across the blood-brain barrier. Clin. Exp. Neurol. 1983;19:50–53. [PubMed] [Google Scholar]
- [105].Riley C, Cope T, Buck C. CNS neurotrophins are biologically active and expressed by multiple cell types. J. Mol. Histol. 2004;35:771–783. doi: 10.1007/s10735-004-0778-9. [DOI] [PubMed] [Google Scholar]
- [106].Roy E, Buyer D, Licari V. Estradiol in the striatum: effects on behavior and dopamine receptors but no evidence for membrane steroid receptors. Brain Res. Bull. 1990;25:221–227. doi: 10.1016/0361-9230(90)90064-7. [DOI] [PubMed] [Google Scholar]
- [107].Salehi A, Verhaagen J, Dijkhuizen P, Swaab D. Co-localization of high-affinity neurotrophin receptors in nucleus basalis of Meynert neurons and their differential reduction in Alzheimer’s disease. Neurosci. 1996;75:373–387. doi: 10.1016/0306-4522(96)00273-4. [DOI] [PubMed] [Google Scholar]
- [108].Sandyk R. Estrogens and the pathophysiology of Parkinson’s disease. Intern. J. Neurosci. 1989;45:119–122. doi: 10.3109/00207458908986223. [DOI] [PubMed] [Google Scholar]
- [109].Sato T, Wilson T, Hughes L, Konrad H, Nakayama M, Helfert R. Age-related changes in levels of tyrosine kinase B receptor and fibroblast growth factor receptor 2 in the rat inferior colliculus: implications for neural senescence. Neurosci. 2001;103:695–702. doi: 10.1016/s0306-4522(01)00022-7. [DOI] [PubMed] [Google Scholar]
- [110].Secor V, Secor W, Gutekunst C, Brown M. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde Y. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- [112].Sherwin B. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendo. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- [113].Shi J, Zhang Y, Simpkins J. Effects of 17beta-estradiol on glucose transporter 1 expression and endothelial cell survival following focal ischemia in the rats. Exp. Brain Res. 1997;117:200–206. doi: 10.1007/s002210050216. [DOI] [PubMed] [Google Scholar]
- [114].Shumaker S, Legault C, Rapp S, Thal L, Wallace R, Ockene J, Hendrix S, Jones B, III, Assaf A, Jackson R, Kotchen J, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The women’s health initiatitive memory study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- [115].Singh M, Meyer E, Simpkins J. The effect of ovariectomy and estradiol replacement on brain derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinol. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- [116].Singh M, Setalo G, Guan X, Warren M, Toran-Allerand C. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sohrabji F, Miranda R, Toran-Allerand C. Identification of a putative estrogen response element in the gene coding for BDNF. Proc. Natl. Acad. Sci. USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Solum D, Handa R. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stadelmann C, Kerschensteiner M, Misgeld T, Bruck W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. 2002;125:75–85. doi: 10.1093/brain/awf015. [DOI] [PubMed] [Google Scholar]
- [120].Stoeckel K, Thoenen H. Retrograde axonal transport of nerve growth factor: specificity and biological importance. Brain Res. 1975;85:337–341. doi: 10.1016/0006-8993(75)90092-x. [DOI] [PubMed] [Google Scholar]
- [121].Strijks E, Kremer J, Horstink M. Effects of female sex steroids on Parkinson’s disease in postmenopausal women. Clin. Neuropharm. 1999;22:93–97. doi: 10.1097/00002826-199903000-00005. [DOI] [PubMed] [Google Scholar]
- [122].Sun M, Alkon D. Differential gender-related vulnerability to depression induction and converging antidepressant responses in rats. J. Pharmacol. Exp. Ther. 2006;316:926–932. doi: 10.1124/jpet.105.093948. [DOI] [PubMed] [Google Scholar]
- [123].Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross K. 17beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J. Neurochem. 2004;89:660–673. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- [124].Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J. Neurosci. 2005;25:430–435. doi: 10.1523/JNEUROSCI.3170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tang M-X, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- [126].Thanos S, Bahr M, Barde Y-A, Vanselow J. Survival and axonal elongation of adult rat retinal ganglion cells In vitro effects of lesioned sciatic nerve and brain derived neurotrophic factor. Eur. J. Neurosci. 1989;1:19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- [127].Theoharides T, Dimitriadou V, Letourneau R, Rozniecki J, Vliagoftis H, Boucher W. Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain myelin changes resembling early stages of demyelination. Neurosci. 1993;57:861–871. doi: 10.1016/0306-4522(93)90030-j. [DOI] [PubMed] [Google Scholar]
- [128].Thorogood M, Hannaford P. The influence of oral contraceptives on the risk of multiple sclerosis. Br. J. Obstet. Gynaecol. 1998;105:1296–1299. doi: 10.1111/j.1471-0528.1998.tb10008.x. [DOI] [PubMed] [Google Scholar]
- [129].Vanderboom R, Sheffield L. Estrogen enhances epidermal growth factor-induced DNA synthesis in mammary epithelial cells. J. Cell. Physiol. 1993;156:367–372. doi: 10.1002/jcp.1041560220. [DOI] [PubMed] [Google Scholar]
- [130].Viant M, Millam J, Delany M, Fry D. Regulation of brain-derived neurotrophic factor messenger RNA levels in avian hypothalamic slice cultures. Neurosci. 2000;99:373–380. doi: 10.1016/s0306-4522(00)00167-6. [DOI] [PubMed] [Google Scholar]
- [131].Wang Q, Zheng J. cAMP-mediated regulation of neurotrophin-induced collapse of nerve growth cones. J. Neurosci. 1998;18 doi: 10.1523/JNEUROSCI.18-13-04973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Watson F, Heerssen H, Moheban D, Lin M, Sauvageot C, Bhattacharyya A, Pomeroy S, Segal R. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J. Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].West A, Chen W, Dalva M, Dolmetsch R, Kornhauser J, Shaywitz A, Takasu M, Tao X, Greenberg M. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. USA. 2001;98 doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp. Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- [135].Wetmore C, Cao Y, Pettersson R, Olson L. Brain-derived neurotrophic factor: subcellular compartmentalization and interneuronal transfer as visualized with anti-peptide antibodies. Proc. Natl. Acad. Sci. USA. 1991;88:9843–9847. doi: 10.1073/pnas.88.21.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid E, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J. Cell. Sci. 1994;107:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- [137].Wong V, Arriaga R, Ip N, Lindsay R. The neurotrophins BDNF, NT-3, and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur. J. Neurosci. 1993;5:466–474. doi: 10.1111/j.1460-9568.1993.tb00513.x. [DOI] [PubMed] [Google Scholar]
- [138].Wu H, Friedman W, Dreyfus C. Differential regulation of neurotrophin expression in basal forebrain astroctyes by neuronal signals. J. Neurosci. 2004;76:76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- [139].Xu X, Guenard V, Kleitman N, Aebischer P, Bunge M. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- [140].Yamamoto H, Gurney M. Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 1990;10:3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Yan Q, Elliott J, Snider W. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360:753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- [142].Yan Q, Zheng S, Yan S. Prenatal cocaine exposure decreases brain-derived neurotrophic factor proteins in the rat brain. Brain Res. 2004;1009:228–233. doi: 10.1016/j.brainres.2004.02.052. [DOI] [PubMed] [Google Scholar]
- [143].Ye J-H, Houle J. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp. Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- [144].Yu I, Lee S, Lee Y, Son H. Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro. Biochem. Biophys. Res. Comm. 2004;317:484–490. doi: 10.1016/j.bbrc.2004.03.071. [DOI] [PubMed] [Google Scholar]
- [145].Yu L, Liao P. Estrogen and progesterone distinctively modulate methamphetamine-induced dopamine and serotonin depletions in C57BL/J6 mice. J. Neural Transm. 2000;107:1139–1147. doi: 10.1007/s007020070027. [DOI] [PubMed] [Google Scholar]
- [146].Zandi P, Carlson M, Plassman B, Welsh-Bohmer K, Mayer L, Steffens D, Breitner J. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- [147].Zhou J, Zhang H, Cohen R, Pandey S. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinol. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ziylan Y, Lefauconnier J, Bernard G, Bourre J. Blood-brain barrier permeability: regional alterations after acute and chronic administration of ethinyl estradiol. Neurosci. Lett. 1990;118:181–184. doi: 10.1016/0304-3940(90)90621-f. [DOI] [PubMed] [Google Scholar]