Abstract
The yeast Saccharomyces cerevisiae utilizes rapidly responding mitogen-activated protein kinase (MAPK) signaling cascades to adapt efficiently to a changing environment. Here we report that phosphorylation of Cdc37p, an Hsp90 cochaperone, by casein kinase 2 controls the functionality of two MAPK cascades in yeast. These pathways, the high-osmolarity glycerol (HOG) pathway and the cell integrity (protein kinase C) MAPK pathway, mediate adaptive responses to high osmotic and cell wall stresses, respectively. Mutation of the phosphorylation site Ser14 in Cdc37p renders cells sensitive to osmotic stress and cell wall perturbation by calcofluor white. We found that levels of the MAPKs Hog1p and Slt2p (Mpk1p) in cells are reduced in a cdc37-S14A mutant, and consequently downstream responses mediated by Hog1p and Slt2p are compromised. Furthermore, we present evidence that Hog1p and Slt2p both interact in a complex with Cdc37p in vivo, something that has not been reported previously. The interaction of Hsp90, Slt2p, and Hog1p with Cdc37p depends on the phosphorylation status of Cdc37p. In fact, our biochemical data show that the osmosensitive phenotype of the cdc37-S14A mutant is due to the loss of the interaction between Cdc37p, Hog1p, and Hsp90. Likewise, during cell wall stress, the interaction of Slt2p with Cdc37p and Hsp90 is crucial for Slt2p-dependent downstream responses, such as the activation of the transcription factor Rlm1p. Interestingly, phosphorylated Slt2p, but not phosphorylated Hog1p, has an increased affinity for Cdc37p. Together these observations suggest that Cdc37p acts as a regulator of MAPK signaling.
Mitogen-activated protein kinase (MAPK) pathways in Saccharomyces cerevisiae are evolutionarily conserved signaling cascades that are triggered by changes in the extracellular environment and elicit physiological responses that allow the cell to adapt to these changes (21). The core of each MAPK pathway consists of three consecutive protein kinases. The first one (MAPK kinase kinase), after being activated by an external stimulus, phosphorylates the MAPK kinase, which in turn phosphorylates conserved threonine/tyrosine residues of the MAPK. In many cases, the activated MAP kinase then translocates from the cytosol into the nucleus, where it phosphorylates downstream effector proteins, such as transcription factors (21). For example, in response to high-osmotic-stress conditions, the MAP kinase Hog1p becomes phosphorylated and subsequently translocates into the nucleus, where it activates the transcription of target genes, e.g., genes involved in glycerol synthesis (10, 24). Another example is the protein kinase C (PKC) MAPK pathway, which is induced by stress conditions such as cell wall perturbation caused by cell wall-damaging agents such as calcofluor white (CFW). Upon induction of the PKC signaling cascade, the MAP kinase Slt2p (Mpk1p) becomes phosphorylated and mediates the expression of genes involved in cell wall metabolism via phosphorylation of the transcriptional regulator Rlm1p (27, 28, 63, 64).
The activation of many protein kinases involved in signal transduction relies on their association with other proteins, including Hsp90 and Cdc37p (3, 19, 53, 56, 62). The gene for Cdc37p was initially identified in a temperature-sensitive cell division cycle mutant in the budding yeast Saccharomyces cerevisiae (44) and was suggested to function as a “protein kinase accessory factor” for Hsp90 (56). Later, it was found that Cdc37p regulates the ATPase activity of Hsp90 (52) and physically interacts with Hsp90 clients; thus, Cdc37p was classified as a cochaperone. However, several studies suggest that Cdc37p can act as a chaperone, independently of Hsp90 (31, 32, 34, 59)—for example, the observation that a Cdc37p mutant deficient in an Hsp90 binding domain still maintains client protein kinase activity (32). The functionality of Cdc37p in S. cerevisiae is modulated by phosphorylation of the highly conserved Ser14 and Ser17 residues (2). For the mammalian homologue, p50, phosphorylation of Ser13 (equivalent to S. cerevisiae Ser14) is crucial for interaction with client kinases and the recruitment of Hsp90 to these kinase-Cdc37p complexes (40, 51). Biochemical and genetic analyses have revealed that this phosphorylation is carried out by casein kinase 2 (CK2) (2, 39, 40). CK2 activity is elevated in phases of the cell cycle when Cdc37p function appears to be essential as well, such as during the G1 and G2/M phases (2, 11, 18, 22).
Cdc37p is essential for cell viability in yeast as well as in several other species (7, 18, 29), and although yeast Cdc37p shares only 20% amino acid sequence identity with its mammalian counterpart, p50 (8), the first portion of the N-terminal domain, which contains the CK2 phosphorylation sites, is strongly conserved (19, 32). The roles of Cdc37p in cellular processes that mediate the stabilization and activation of many protein kinases appear to be similar for different species. For instance, in Drosophila melanogaster, a mutation on Cdc37p/p50 affects sevenless signaling, while in mammalian cells, the activation and stability of protein kinases such as Raf-1, Cdk4, and Akt depend on Cdc37p (3, 19, 53, 56). In yeast, Cdc37p is required for the activation of Cdc28p, Mps1p, Kin28p, and Cak1p (12, 18, 44, 48, 61). Involvement of Cdc37p in MAP kinase signaling in yeast is indicated by the fact that Cdc37p is required for Ste11p-mediated signaling of the pheromone MAP kinase route in S. cerevisiae (1) and for Spc1-mediated signaling of the stress-activated protein kinase (SAPK) pathway in Schizosaccharomyces pombe (57). We recently observed an involvement of Cdc37p in the adaptation of S. cerevisiae to osmotic stress via the filamentous-growth MAPK route (X. X. Yang, unpublished data). Since adaptation to osmotic stress is a complex process that involves different kinds of signaling pathways, Cdc37p may have roles at distinct levels in signaling pathways that operate parallel to each other or in a mutually exclusive manner in response to stress conditions.
To gain more insight into the exact role of Cdc37p in response to stress conditions in yeast, we have extended our investigation. In this study, we show that phosphorylation of Cdc37 at Ser14 is of prime importance in adaptation to stress conditions via the high-osmolarity glycerol (HOG) or PKC MAP kinase signaling cascade. We report on the interaction of Cdc37p with the MAP kinases Hog1p and Slt2p (Mpk1p) and on its importance in response to high osmotic and cell wall stresses, respectively.
MATERIALS AND METHODS
Strains and growth conditions.
The yeast strains used in this study are listed in Table 1. Yeast cells were cultured in 1% yeast extract-2% peptone-2% glucose (YPD) medium supplemented with 2% glucose or in YNB medium (0.67% yeast nitrogen base, 2% glucose) supplemented with the required amino acids. Cells were grown in liquid culture at 24°C on a rotational shaker (220 rpm). To analyze osmosensitivity and CFW sensitivity, growth was determined on a solid medium (YPD) supplemented with different concentrations of sorbitol and CFW, respectively, by spotting 5 μl of 10-fold serial dilutions of the cell suspension (starting optical density at 600 nm, ≈0.1). Cells were grown at 24°C for 3 days.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| YDH6 | MATaade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ura3-52 cka1-Δ1::HIS3 cka2-Δ1::TRP1 [pRS315 CKA2] | 22 |
| YDH8 | MATaade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ura3-52 cka1-Δ1::HIS3 cka2-Δ1::TRP1 [pRS315 cka2-8] | 22 |
| TM141 | MATaleu2 ura3 trp1 his3 | 10 |
| XX201 | TM141 cdc37Δ::HIS3 [Ycplac33 CDC37-GFP] | This study |
| PH101 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-34-HA] | This study |
| DH211 | TM141 cdc37Δ::HIS3 [Ycplac111 CDC37-HA] | This study |
| DH212 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-S14A-HA] | This study |
| DH213 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-S14E-HA] | This study |
| DH231 | TM141 cdc37Δ::HIS3 [Ycplac111 CDC37] | This study |
| DH232 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-S14A] | This study |
| DH233 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-S14E] | This study |
| DH234 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-S17A] | This study |
| DH235 | TM141 cdc37Δ::HIS3 [Ycplac111 cdc37-S17E] | This study |
| PH301 | DH231 YIL117c::LacZ::URA3 | This study |
| PH302 | DH232 YIL117c::LacZ::URA3 | This study |
| PH303 | DH233 YIL117c::LacZ::URA3 | This study |
| PH311 | DH231 STL1::LacZ::URA3 | This study |
| PH312 | DH232 STL1::LacZ::URA3 | This study |
| PH313 | DH233 STL1::LacZ::URA3 | This study |
| PH401 | TM141 hog1-13cmyc::KanMX6 cdc37Δ::HIS3 [Ycplac111 CDC37-HA] | This study |
| PH402 | TM141 hog1-13cmyc::KanMX6 cdc37Δ::HIS3 [Ycplac111 cdc37-S14A-HA] | This study |
| PH403 | TM141 hog1-13cmyc::KanMX6 cdc37Δ::HIS3 [Ycplac111 cdc37-S14E-HA] | This study |
| PH404 | TM141 hog1-13cmyc::KanMX6 cdc37Δ::HIS3 [Ycplac33 CDC37-GFP] | This study |
| PH500 | TM141 cdc37Δ::HIS3 [Ycplac111 CDC37-HA] slt2::TRP1 | This study |
| PH501 | TM141 cdc37Δ::HIS3 [Ycplac111 CDC37-HA] slt2::TRP1 [pUT36 SLT2-His6] | This study |
| PH502 | TM141 cdc37Δ::HIS3 [Ycplac111 CDC37-HA] slt2::TRP1 [pUT36 slt2-T190A,Y192F-His6] | This study |
Construction of CDC37 Ser14 phosphorylation mutants.
Site-directed mutation of the wild-type (Wt) CDC37 gene, in Yeplac112, was performed by means of the QuikChange II site-directed mutagenesis kit (Stratagene). Primers for mutation of serine 14 were 5′-G GAT AAA ATT GAA CTA GCA GAT GAT TCT GAT GTC GAG G-3′, to generate the cdc37-S14A mutant, and 5′-AAG TGG GAT AAA ATT GAA CTA GAG GAT GAT TCT GAT GTC GAG GTG C-3′, to generate the cdc37-S14E mutant. To confirm that only the desired mutations were introduced, the 0.6-kb NotI/PstI mutant fragments were subcloned into pRS305 for DNA sequencing. For expression of the mutant proteins in yeast, each mutated NotI/PstI fragment was inserted into the wild-type CDC37 gene present in the single-copy vector YCplac111. In order to allow isolation of Cdc37p, a hemagglutinin (HA) tag was inserted at the C-terminal NotI site of CDC37 in YCplac111-CDC37-NotI (Yang, unpublished). Strains containing the cdc37-S14A or cdc37-S14E mutation as the sole allele were generated by plasmid shuffling. Ycplac111 carrying either the HA-tagged cdc37 gene or an untagged, mutant cdc37 gene was introduced into cdc37 deletion strain XX201 containing wild-type Cdc37p-green fluorescent protein from YCplac33. Transformants were subsequently grown on a solid medium containing 5-fluoroorotic acid, resulting in loss of the Ycplac33 plasmid. Control strains expressing wild-type tagged and untagged Cdc37p were constructed in the same manner.
To tag the HOG1 gene with the 13cmyc tag at the C-terminal chromosomal locus, PCR-based homologous recombination was performed using pFA6a-13cMyc-kanMX6 as a template, as described previously (33).
The SLT2 gene in strain DH211, expressing wild-type Cdc37p-HA, was knocked out with a TRP1 disruption cassette by using a PCR-based technique as described previously (4), to produce strain PH500. Strains PH501 and PH502 were generated by transforming a URA3-based single-copy vector containing the gene for His6-tagged wild-type Slt2p and a nonphosphorylatable mutant of Slt2p, respectively (38).
Western blotting and antibody staining.
Total-protein extracts were prepared as described previously (9). Protein concentrations were determined using the Bio-Rad protein assay, and 40 μg of total protein was separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher & Schuell). Dually phosphorylated Hog1p and Slt2p (Mpk1) were visualized using a mouse monoclonal antibody against phospho-p38 MAPK (Thr180/Tyr182) (Cell Signaling) and anti-phospho-p42/44 MAPK (New England Biolabs), respectively. In order to detect Hog1p and Slt2p, we used the Yc20 antibodies (Santa Cruz Biotechnology) that recognize the C termini of the respective proteins. A mouse monoclonal anti-HA antibody and a mouse monoclonal anti-cmyc antibody were used to detect Cdc37p-HA and Hog1-cmyc, respectively (Roche). For detection of Hsp90, we used a polyclonal antiserum kindly provided by D. Picard (University of Geneva). Glucose-6-phosphate dehydrogenase (G6PDH) was detected using an anti-G6PDH antibody (Sigma). For detection of His6-tagged Slt2p, a mouse monoclonal anti-His antibody was used (QIAGEN). Antibody binding was visualized using the ECL (Amersham) kit after binding of a horseradish peroxidase-conjugated secondary antibody.
Immunoprecipitation.
Cells were grown in YPD medium to an optical density at 600 nm of ≈0.6. Cultures were cooled rapidly on ice, and cells were harvested by centrifugation, washed once with ice-cold water, and resuspended in ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 5% glycerol) containing Complete protease inhibitor mix (Boehringer) and phosphatase inhibitors (50 mM NaF, 5 mM sodium pyrophosphate, and 1 mM sodium orthovanadate). The cells were lysed by vortexing five times with glass beads for 1 min each time. The cell extract was prepared by centrifugation using a Megafuge 1.0 at 4,000 rpm for 5 min at 4°C. The supernatant was then centrifuged again using an Eppendorf centrifuge at 14,000 rpm for 10 min. The protein concentration was determined using the Bio-Rad protein assay kit. The protein extract (2.5 mg total protein) was mixed with anti-HA agarose beads (Sigma), and the mixture was incubated for 1 h at 4°C with rotation. The beads were washed three times with 1 ml of lysis buffer. HA-bound proteins were eluted by the addition of twice-concentrated SDS sample buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 200 mM β-mercaptoethanol, 20% glycerol, 0.1% bromphenol blue) and heating at 95°C for 5 min. Elution of HA-bound proteins with SDS sample buffer resulted in the coelution of the heavy chain of the anti-HA antibody (Mw, ∼50,000). This results in overlapping of (phosphorylated) Hog1p and the heavy chain of the anti-HA antibody on SDS-polyacrylamide gels and Western blots. To avoid coelution of the anti-HA heavy chain, HA-bound proteins were eluted by incubating the beads with 100 μg/ml HA peptide (Sigma) for 5 min at room temperature. Proteins were separated by SDS-polyacrylamide gel electrophoresis and detected by Western blotting, as described above.
LacZ reporter gene constructs.
The Rlm1p-regulated YIL117C::LacZ reporter gene was constructed using primers Rlm1UAS-a (5′-AAT TCA GTC GAC GTT CTA AAA ATA AAA TGG ACG TTC TAA AAA TAA AAT GGC-3′) and Rlm1UAS1-b (5′-TCG AGC CAT TTT ATT TTT AGA ACG TCC ATT TTA TTT TTA GAA CGT CGA CTG-3′) to amplify the Rlm1-regulated promoter (YIL117C) from genomic DNA (28). The resulting EcoRI/XhoI fragment was inserted into the integrative vector pLacZi (Invitrogen), which had been digested with EcoRI and XhoI. For the STL1::LacZ reporter construct, the STL1 promoter was obtained by PCR using primers Stl1-a (5′-AAG AAT TCA GTC GAC GGC CAA GAT AGA ATT AAA GGG-3′) and Stl2-b (5′-AAG GAT CCG GTC ATG GTC TAA AAC TTT CTA TGT TC-3′) with the STL1::LacZ reporter construct PEN05 (a generous gift from F. Posas [10]) as a template. The resulting fragment was transferred to pLacZi using the BamHI and EcoRI restriction sites. The reporter gene construct YIL117c-LacZ or STL1-LacZ was integrated into the genomes of DH231, DH232, and DH233, resulting in strains PH301, PH302, and PH303 or strains PH311, PH312, and PH313, respectively. Single-copy integrants were identified by PCR.
β-Galactosidase assays.
Cells were grown to mid-log phase and then treated with 1 M sorbitol or 20 μg/ml CFW for 2 h (10, 13, 28). Cell extracts were prepared by suspending the cells in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4 [pH 7.0]) and vortexing with glass beads for 4 min at 4°C. The samples were centrifuged in an Eppendorf centrifuge for 10 min at 14,000 rpm and 4°C. The protein concentration of the supernatant was determined using the bicinchoninic acid protein assay kit (Pierce). β-Galactosidase assays were performed as described previously (46). Specific activities are given in nanomoles of o-nitrophenyl-β-d-galactopyranoside (ONPG) converted per minute per milligram of protein. The measurements were performed in duplicate for two independent transformants of the same strain.
qPCR.
Total RNA was extracted as described elsewhere (54) and treated with DNase I (Ambion). cDNA synthesis and quantitative PCR (qPCR) were carried out using the DyNAmo SYBR green 2-Step qRT-PCR kit (Finnzymes). Samples were run on an ABI PRISM 7900 HT sequence detection system (Applied Biosystems). Relative transcript quantities were determined according to the ΔΔCT method using HTA1 as a reference gene (55).
RESULTS
Phosphorylation of Cdc37p at Ser14 is crucial for adaptation to high osmolarity and cell wall stress.
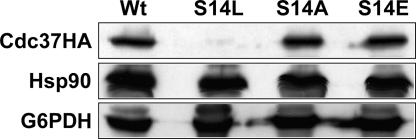
We reported recently that the cdc37-S14L mutant strain is sensitive to high osmotic stress, suggesting that Cdc37p is required for adaptation to this condition (66). Since this mutation maps to the highly conserved Ser14 residue, which has been shown to be phosphorylated in vivo (2), we investigated the functional relationship of this phosphorylation and the osmosensitive phenotype. We therefore constructed two additional mutant alleles in which the codon for Ser14 was changed to either alanine or glutamate. The CDC37 wild-type gene and the cdc37-S14L, -S14A, and -S14E mutant alleles were all fitted with a C-terminal HA tag and introduced on a single-copy plasmid carrying a LEU2 nutritional marker. Next, a cdc37 deletion strain with wild-type CDC37 on a URA3-based single-copy vector was used for transformation of these plasmids (X. X. Yang, unpublished). Plasmid shuffling on a medium containing 5-fluoroorotic acid resulted in the strains carrying the various CDC37 mutants. To verify proper expression of the tagged proteins, Western blot analysis of the cell extracts using an anti-HA antibody was performed. As can be seen in Fig. 1 (upper panel), the levels of the S14A and S14E mutant proteins were essentially the same as that of the wild-type control. However, Cdc37pS14L was almost undetectable, suggesting that the S14L mutation, but not the S14A or S14E mutation, causes the protein to become metabolically unstable (14). Hsp90 protein levels were not affected in any of the cdc37-S14 mutants (Fig. 1, center panel).
FIG. 1.
Western blot of Cdc37pWt and Cdc37pS14 mutant proteins. Cells expressing HA-tagged versions of wild-type Cdc37p or one of the three mutant forms carrying a replacement of S14 by Leu, Ala, or Glu were grown in YPD at 24°C to mid-log phase. Total-cell extracts were analyzed by Western blotting using an anti-HA antibody. After being stripped, the blots were reprobed with anti-Hsp90, stripped again, and finally probed with anti-G6PDH to assess loading.
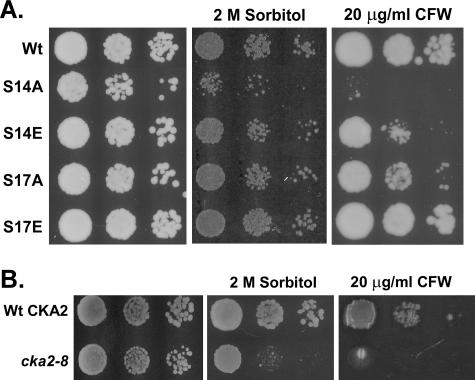
To determine the functional importance of Ser14 phosphorylation, we analyzed the growth of the cdc37-S14A and cdc37-S14E strains in the presence of either 2 M sorbitol or CFW, a fluorescent dye that destabilizes the cell wall by binding to nascent chitin chains (30, 45). As can be seen in Fig. 2A (center panel), the cdc37-S14A mutant was osmosensitive, while growth of the cdc37-S14E mutant was not affected by sorbitol. In addition, the cdc37-S14A mutant was hypersensitive to 20 μg/ml CFW, whereas growth of the cdc37-S14E mutant was only slightly reduced (Fig. 2A, right panel). These data strengthen the argument for an involvement of Cdc37p in the main MAPK signaling routes responsive to osmotic and cell wall stresses—the HOG and PKC pathways, respectively. Furthermore, the results reveal that phosphorylation of Cdc37p at Ser14 is important for proper response to high osmotic challenge and cell wall stress, because replacement of Ser14 by Glu, which structurally mimics a phosphoserine, had a much less dramatic negative effect on the ability of the cells to cope with such stress conditions than did replacement with a nonphosphorylatable Ala residue.
FIG. 2.
Phenotypic analysis of the cdc37 and cka2 mutants. (A) Sensitivity of cdc37 mutants to osmotic and cell wall stress. Cells expressing wild-type Cdc37p or its S14A, S14E, S17A, or S17E mutant version (strain DH231, DH232, DH233, DH234, or DH235, respectively) were grown on YPD plates containing no additive, 2 M sorbitol, or 20 μg/ml CFW. (B) Sensitivity of the cka2 mutant to osmotic and cell wall stresses. Strains YDH6 and YDH8, containing the wild-type CKA2 gene and the cka2-8 mutant allele, respectively, were grown on YPD plates containing either no additive, 2 M sorbitol, or 20 μg/ml CFW.
To determine the importance of the second major phosphorylation site of Cdc37p (2), Ser17 was replaced with either Ala (cdc37-S17A) or Glu (cdc37-S17E) and the growth of the mutant cells was analyzed in the presence of either sorbitol or CFW (Fig. 2A). At a concentration of 2 M sorbitol, the growth of neither mutant appeared to be affected. Similarly, cell wall stress had no effect on the cdc37-S17E mutant, whereas, in clear contrast to its cdc37-S14A counterpart, the cdc37-S17A mutant was only slightly sensitive to 20 μg/ml CFW. These observations demonstrate that Ser14 is the more important of the two phosphorylation sites for Cdc37p functionality. Because phosphorylation of Cdc37p at Ser14 is carried out by casein kinase 2 (2), we investigated the phenotype of the cka2-8 mutant strain (22). The cka2-8 mutant is temperature sensitive, and cell extracts of this mutant display very low levels of CKA2p activity (5). As shown in Fig. 2B, the cka2-8 strain showed an osmosensitive and CFW-sensitive phenotype, comparable to that of the cdc37-S14A mutant. This result supports our conclusion that phosphorylation of Cdc37p at Ser14 is essential for adaptation to osmotic and cell wall stresses.
Phosphorylation of Cdc37p at Ser14 is required for its association with Hsp90 in vivo.
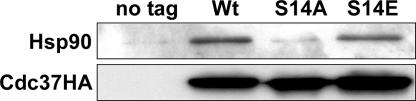
Cdc37p is a cochaperone that targets client proteins to the Hsp90 chaperone complex and interacts physically with Hsp90 (19, 23, 31, 43, 49). Hence, we wondered whether the S14A and S14E mutations would affect the association of Cdc37p with Hsp90 in vivo. To analyze these interactions, protein extracts of the CDC37-Wt strain and the cdc37-S14A and -S14E mutant strains were prepared, and the HA-tagged proteins were isolated using an anti-HA agarose resin. Analysis of the proteins retained by the resin revealed that the S14E mutation did not affect coimmunoprecipitation of Hsp90 but that the S14A mutation reduced the amount of coprecipitated Hsp90 to virtually the level of the control (Fig. 3). Thus, replacement of Ser14 with the nonphosphorylatable Ala almost abolishes the ability of Cdc37p to associate with Hsp90, whereas replacement with the phosphoserine-mimicking Glu residue has little or no effect. From these data we conclude that phosphorylation of Ser14 is a requirement for efficient interaction with Hsp90 in vivo. Although Ser14 is located outside the middle domain of Cdc37p to which Hsp90-binding activity is attributed (47, 50), it should be noted that it resides in a highly conserved portion of the client-binding domain of the protein.
FIG. 3.
Analysis of Hsp90 coimmunoprecipitation with Cdc37pS14 mutant proteins. Yeast strains expressing plasmid-encoded, HA-tagged versions of wild-type Cdc37p (DH211), Cdc37pS14A (DH212), or Cdc37S14E (DH213) were grown in YPD at 24°C to mid-log phase. Cell extracts were prepared, and an aliquot of cell-free proteins was loaded onto an anti-HA agarose column. Bound protein was eluted, and 15% of the eluate was subjected to Western blot analysis using an anti-HA or anti-Hsp90 antibody. The wild-type strain TM141, expressing untagged Cdc37p, was used as a control.
HOG pathway activation is reduced in a cdc37-S14A mutant.
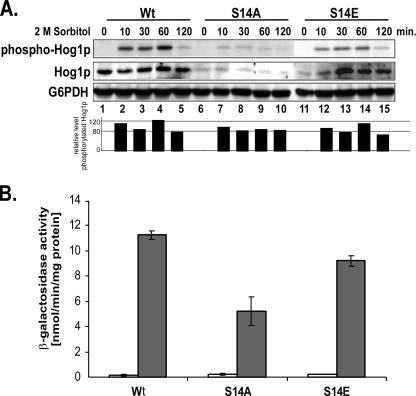
To learn more about the molecular reason for the osmosensitive phenotype of the cdc37-S14A mutant, we determined whether the presence of a nonphosphorylatable residue at position 14 generates a defect in the osmoresponsive signaling of the HOG MAP kinase pathway. To that end, we assessed the degree of phosphorylation of Hog1p after exposure of the mutant and wild-type strains to high osmolarity. As shown in Fig. 4A (center panel), the cellular Hog1p level was strongly reduced in the unstressed cdc37-S14A and -S14E mutants compared to that in the control (compare lanes 6 and 11 to lane 1). However, whereas in the S14A mutant this level did not change appreciably upon application of osmotic stress (Fig. 4A, lanes 7 to 10), in the S14E mutant the Hog1p level increased over a period of 30 min to about wild-type levels (lanes 11 to 15). Quantification of phosphorylated Hog1p levels and total Hog1p levels from the Western blot shown in Fig. 4A revealed that despite the low level of Hog1p in the S14A mutant, the amount of phosphorylated Hog1p relative to the total amount of Hog1p was about 80% of the values observed for the wild-type control (lanes 6 to 10 and bar graph). The S14E mutant also showed only a slight reduction (to about 85%) in the level of phosphorylated Hog1p relative to the total amount of Hog1p (lanes 11 to 15 and bar graph). Neither mutation had a significant effect on the kinetics of Hog1p phosphorylation.
FIG. 4.
Analysis of HOG pathway activation in the cdc37 S14 mutants during osmotic shock. (A) Analysis of Hog1p activation in the cdc37-S14 mutant strains. Strains expressing wild-type Cdc37p (DH231) or its S14A (DH232) or S14E (DH233) mutant version were grown to mid-log phase in YPD medium and then given an osmotic shock by the addition of 2 M sorbitol. Aliquots were taken at the indicated time points, and cell extracts were prepared and subsequently analyzed by Western blotting using anti-phospho-p38 MAPK and an anti-Hog1p antibody. Loading was assessed by probing with an anti-G6PDH antibody. The same blot was used for all antibody analyses. Bar graphs represent quantification (using Quantity One software from Bio-Rad) of the level of phosphorylated Hog1p relative to the total amount of Hog1p. To avoid interference with quantification analysis due to different blot backgrounds, a Quantity One tool was selected that subtracts the image background from each selected signal. (B) Analysis of STL1-LacZ reporter gene expression in cdc37-S14 mutant strains. Strains PH311, PH312, and PH313, which contain the STL1-LacZ reporter construct and express wild-type Cdc37 and the S14A and S14E mutant versions, respectively, were grown to mid-log phase in YPD medium. Osmotic stress was induced by the addition of 1 M sorbitol, and cells were allowed to grow for an additional 2 h. β-Galactosidase activity in cell extracts was determined (filled bars). Extracts from cells grown in the absence of sorbitol were used as a control (open bars). Error bars, standard deviations from the means of four experiments.
To determine whether these effects in the cdc37-S14 mutants would be reflected in a similarly reduced activation of Hog1p-mediated downstream effectors, we monitored Hog1p-mediated activation of gene expression after exposure to sorbitol by measuring β-galactosidase activity by using a STL1-LacZ reporter gene construct (10). STL1 encodes a putative sugar transporter and is one of the most strongly induced genes in response to osmotic stress, since it is under the exclusive control of Hog1p. Since β-galactosidase is a very stable protein, the β-galactosidase activity measured 2 h after exposure to high osmolarity reflects total cumulative Hog1p-mediated gene expression. As expected, based on the observed reduced cellular level of phosphorylated Hog1p, expression of the STL1-LacZ reporter in the cdc37-S14A mutant was significantly reduced, to about 55% of the level shown by the wild-type control. In the cdc37-S14E mutant, on the other hand, we observed only about a 10% reduction in expression (Fig. 4B), reflecting the slightly reduced relative amount of phosphorylated Hog1p in this mutant.
Thus, permanent phosphorylation of Cdc37p, as mimicked by the presence of Glu instead of Ser at position 14, has a dual effect on the HOG signaling pathway. Besides its effect on the phosphorylation of Hog1p, this mutation causes a reduction in the level of Hog1p in unstressed cells, which is compensated for upon exposure to high osmolarity (Fig. 4A, lanes 11 to 15). The reduced Hog1p protein level in the cdc37-S14A mutant and the initial low Hog1p protein level in the cdc37-S14E mutant could be due either to reduced HOG1 transcription or to the instability of the protein itself. In order to distinguish between these two possibilities, mRNA was isolated from unstressed CDC37-Wt, cdc37-S14A, and cdc37-S14E cells, and hog1 mRNA levels were determined by qPCR. Although Hog1p protein levels were significantly decreased in the cdc37-S14A mutant, hog1 mRNA levels in both the cdc37-S14A and the cdc37-S14E mutant were essentially the same as that in the wild-type control (data not shown). This result demonstrates that phosphorylation of Cdc37p is crucial for the stability, and hence for the efficient activation, of Hog1p.
Phosphorylation of Ser14 is relevant for the interaction of Cdc37p with Hog1p.
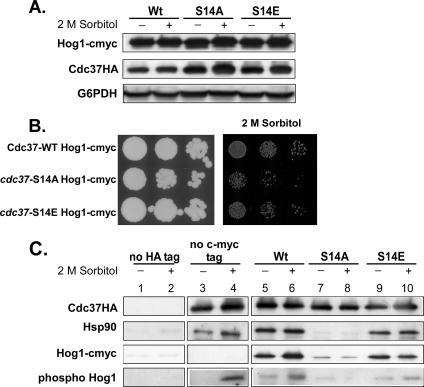
The osmosensitive phenotype and the reduced levels of Hog1p in the cdc37-S14A mutant may indicate that the interaction between Cdc37p and the Hog1p MAP kinase is affected. To test this hypothesis, CDC37-Wt and cdc37-S14 mutant cells containing a 13cmyc-tagged HOG1 gene at the C-terminal chromosomal locus were constructed. Protein extracts were prepared from cells grown at 24°C (unstressed) and cells that had been treated with 2 M sorbitol for 30 min. Interestingly, the 13cmyc tag appeared to have a positive effect on the stability of Hog1p, even in the cdc37-S14A mutant: Hog1p-cmyc levels in the S14A mutant were essentially the same as in the wild type or the S14E mutant (Fig. 5A). To determine whether Hog1p-cmyc might have an effect on the osmosensitive phenotype of the S14A mutant, the growth of the cdc37-S14A and cdc37-S14E mutants expressing 13cmyc-tagged Hog1p in the presence of 2 M sorbitol was analyzed. As can be seen in Fig. 5B, Hog1p-cmyc does not suppress the osmosensitive phenotype of the cdc37-S14A mutant.
FIG. 5.
Effects of high osmotic stress on the interaction of Cdc37p with Hsp90 and Hog1p. (A) A C-terminal 13cmyc tag stabilizes Hog1p in the cdc37-S14A mutant. Strains PH401, PH402, and PH403, expressing HA-tagged wild-type Cdc37p and the S14A and S14E mutant versions, respectively, and 13cmyc epitope-tagged Hog1p were grown to mid-log phase in YPD medium and treated with 2 M sorbitol for 30 min. Cell extracts were prepared, and each extract was analyzed by Western blotting using anti-HA and an anti-cmyc antibody. An anti-G6PDH antibody was used as a loading control. (B) Hog1p-cmyc does not suppress the osmosensitive phenotype of the cdc37-S14A strain. Cells expressing wild-type Cdc37p or its S14A or S14E mutant version and containing 13cmyc epitope-tagged Hog1p (strains PH401, PH402, and PH403, respectively) were grown on YPD plates containing no additive or 2 M sorbitol. (C) Coimmunoprecipitation of Hsp90 and Hog1p with wild-type and mutant Cdc37p before and during osmotic stress. An aliquot of the same cell-free protein extracts analyzed in panel A was loaded onto an anti-HA agarose column (lanes 5 to 10). Cell extracts of strain PH404, containing 13cmyc-tagged Hog1 but no HA-tagged Cdc37 (lanes 1 and 2), and strain DH211, containing HA-tagged Cdc37Wt but no 13cmyc-tagged Hog1 (lanes 3 and 4), were used as controls. Bound protein was eluted by 2× SDS sample buffer. To avoid coelution of the heavy chain of the anti-HA antibody, which results in overlapping protein bands of endogenously phosphorylated Hog1 with the anti-HA heavy chain on a Western blot, bound protein of the control sample, containing endogenous Hog1, was eluted with 100 μg/ml HA peptide (see Materials and Methods). Fifteen percent of the total eluate was subjected to Western blot analysis using an anti-HA, anti-Hsp90, anti-phospho-Hog1p, or anti-cmyc antibody. The same blot was used for all analyses.
The stabilizing effect of the 13cmyc tag was used to analyze the interaction between Hog1p and the wild-type and mutant Cdc37p proteins. Aliquots of the above-mentioned protein extracts were loaded onto an anti-HA resin. Anti-HA-retained proteins of these extracts were analyzed by Western blotting for the presence of Cdc37p, total and phosphorylated Hog1p-cmyc, and Hsp90. The data showed that Hog1p-cmyc interacts with Cdc37p-Wt and Cdc37pS14E in its nonphosphorylated and phosphorylated states (Fig. 5C, compare lanes 5 and 6 to lanes 9 and 10). A very striking result of this analysis was the significantly decreased binding of Hog1p-cmyc to Cdc37pS14A in the presence or absence of 2 M sorbitol (Fig. 5C, lanes 7 and 8). Retention of Hsp90 by Cdc37pS14A was abolished in the stressed as well as the unstressed cells (see also Fig. 3). The cdc37-S14E mutation had only a minor effect on the retention of Hsp90, Hog1-cmyc, or phospho-Hog1-cmyc (Fig. 5C, lanes 9 and 10). Thus, the reduced phospho-Hog1-cmyc binding to Cdc37pS14E seems to account for the slightly reduced expression of the STL1 reporter in this mutant, as shown in Fig. 4. These results suggest that phosphorylation of Ser14 of Cdc37p is essential for an interaction between Cdc37p, Hsp90, and Hog1p. It seems likely that this interaction is required for Hog1p stability and crucial for Hog1p-mediated downstream responses. Taken together, our observations indicate that the osmosensitive phenotype of the cdc37-S14A mutant is due to the strongly decreased interaction of Cdc37pS14A with Hog1p. The fact that the stabilized Hog1p-cmyc in the S14A mutant does not have an effect on the osmosensitive phenotype of this mutant may also indicate that Cdc37p is required for Hog1p kinase activity.
Cdc37p function is important for Slt2p-mediated activation of Rlm1p.
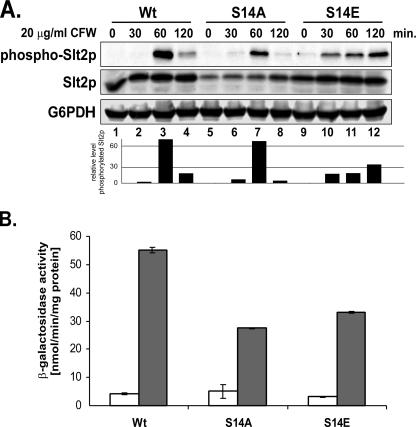
To obtain insight into the cause of the CFW-sensitive phenotype of the cdc37-S14A and -S14E mutants, we analyzed the functionality of the PKC MAPK route by monitoring the phosphorylation of the MAP kinase Slt2p after exposure of cells to 20 μg/ml CFW. As shown in Fig. 6A (top panel, lanes 5 to 8), phosphorylation of Slt2p in the cdc37-S14A mutant followed wild-type kinetics, reaching a peak 60 min after induction and then dropping off sharply over the next hour. Also, the amount of phosphorylated Slt2p relative to the total amount of Slt2p, as calculated from the quotient of phosphorylated and total Slt2p, showed only a negligible decrease compared to the ratio for the wild type (Fig. 6A, bar graph). The initial level of Slt2p in the S14A mutant cells was lower than that in the wild-type control and the S14E mutant but showed a steady increase after the addition of CFW, ultimately reaching almost wild-type levels. However, a striking difference between the S14A and S14E mutants is the Slt2p phosphorylation kinetics. While the S14A mutant displayed kinetics very similar to that of the wild-type control, in the S14E mutant a substantial amount of phospho-Slt2p could already be detected 30 min after the addition of CFW, i.e., much earlier than in wild-type cells (Fig. 6A, top panel, compare lane 10 to lane 2; bar graph). Moreover, rather than showing a clear maximum followed by a sharp decrease, the amount of phosphorylated Slt2p relative to the total amount of Slt2p in the S14E mutant slowly increased over the next 1.5 h, finally reaching about 40% of the maximum level for the cdc37-S14A mutant or the wild-type control (Fig. 6A, bar graph). However, the cdc37-S14A mutant is more sensitive to CFW than its cdc37-S14E counterpart (Fig. 2).
FIG. 6.
Analysis of PKC pathway activation in cdc37-S14 mutants during CFW stress. (A) Slt2p activation in cdc37-S14 mutant strains. Strains expressing wild-type Cdc37p (DH231) or the S14A (DH232) or S14E (DH233) mutant version were grown to mid-log phase in YPD medium and exposed to cell wall stress by the addition of CFW (20 μg/ml). Aliquots were taken at the indicated time points, and cell extracts were prepared and analyzed by Western blotting, using an anti-phospho-p42/44 MAPK antibody to detect phosphorylated Slt2p. The total amount of Slt2p was determined by using an anti-Slt2p antibody. Loading was assessed by means of an anti-G6PDH antibody. The same blot was used for all analyses. Bar graphs represent quantification of the level of phosphorylated Slt2p relative to total Slt2p. (B) Analysis of YIL117c-LacZ reporter gene expression in cdc37-S14 mutant strains. Strains PH301, PH302, and PH303, containing the Rlm1p-responsive YIL117c-LacZ reporter gene constructs and expressing wild-type Cdc37p and the S14A and S14E mutant versions, respectively, were grown to mid-log phase in YPD at 24°C and subsequently exposed to 20 μg/ml CFW in YPD for 2 h. Cell extracts were prepared, and β-galactosidase activity was measured (filled bars). Extracts from cells grown in the absence of CFW were used as a control (open bars). Error bars, standard deviations from the means of four experiments.
To investigate the reason for this apparent discrepancy, we investigated the activation of Rlm1p, a target of phosphorylated Slt2p, after exposure of the cells to CFW. Rlm1p is a transcription factor that regulates the expression of genes implicated in cell wall biogenesis (27, 63). Activation of Rlm1p was monitored by measuring the β-galactosidase activity of a LacZ reporter construct that contains the Rlm1p-responsive promoter of the YIL117c gene (28). As can be seen in Fig. 6B, the expression level of the reporter gene was reduced to about 50% of the wild-type control level both in cdc37-S14A cells and in cdc37-S14E cells (Fig. 6B). This indicates, on the one hand, that the lack of a phosphorylatable residue at position 14 of Cdc37p in some way compromises the activation of Rlm1p by Slt2p, even though phosphorylation of Slt2p itself is not affected. On the other hand, the similarly reduced expression of the reporter gene in the S14E mutant suggests that a decreased fraction of phosphorylated Slt2p is available for Rlm1p activation, resulting in the observed lower expression level of the reporter gene. It should be noted that timely dephosphorylation of Cdc37p Ser14 may affect the activation kinetics of Slt2p, thereby contributing to a less efficient activation of Rlm1p.
Phosphorylation of Ser14 is required for interaction of Cdc37p with Slt2p.
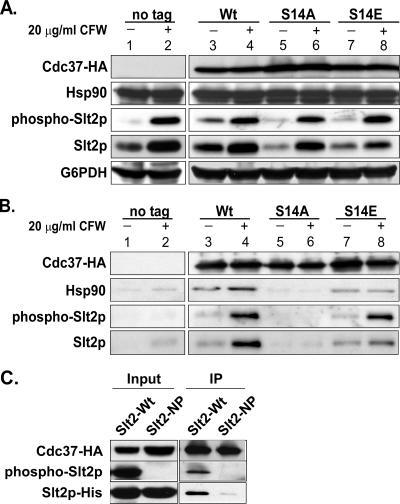
Our data so far show that activation of Rlm1p by Slt2p in response to treatment of yeast cells with CFW is compromised to about the same extent in both the cdc37-S14A and cdc37-S14E mutants but that the sensitivities of the two mutant strains to CFW differ substantially. To investigate the cause of this apparent discrepancy, we analyzed the interaction of the Cdc37pS14 mutant proteins with Slt2p and Hsp90 in the presence and absence of cell wall stress induced by treatment with 20 μg/ml CFW for 1 h. Western blot analysis of extracts prepared from stressed and unstressed cells showed that the levels of HA-tagged wild-type or mutant Cdc37p were not affected by exposure to CFW. Neither were the levels of Hsp90 affected (Fig. 7A, upper two panels). However, the amounts of Slt2p in the unstressed S14A and S14E mutants were smaller than that in the wild-type control (Fig. 7A, compare lanes 5 and 7 to lane 3). In contrast, Slt2p reached wild-type levels in the S14E mutant and wild-type levels in the S14A mutant upon treatment with CFW (compare lanes 6 and 8 to lane 4; see also Fig. 6A).
FIG. 7.
Effects of CFW stress on the interaction of Cdc37p with Hsp90 and Slt2p. (A) Analysis of protein levels of Hsp90, Slt2p, and Cdc37p during CFW stress in cdc37-S14 mutant strains. Strains DH211, DH212, and DH213, expressing HA-tagged wild-type Cdc37p and its S14A and S14E mutant versions, respectively, were grown to mid-log phase in YPD at 24°C and treated with 20 μg/ml CFW for 1 h. Cell extracts were prepared and analyzed by Western blotting using an anti-HA antibody, an anti-Hsp90 antibody, an anti-phospho-p42/44 MAPK antibody to detect phosphorylated Slt2p, and an anti-Slt2p antibody. Loading was assessed by means of an anti-G6PDH antibody. Untreated cells from each strain, as well as CFW-treated and untreated cells from strain TM141 expressing untagged Cdc37p, were used as controls. (B) Coimmunoprecipitation of Hsp90 and Slt2p with wild-type and mutant Cdc37p before and during CFW stress. An aliquot of the same cell-free protein extracts analyzed in panel A was loaded onto an anti-HA agarose column. Bound protein was eluted, and 15% of the total eluate was subjected to Western blot analysis using an anti-HA, anti-phospho-p42/44 MAPK, or anti-Slt2p antibody. (C) Coimmunoprecipitation of phosphorylated Slt2pHis-Wt with wild-type Cdc37p. Cell cultures of strain PH501 or PH502, expressing wild-type Slt2pHis or a nonphosphorylatable Slt2pHis mutant (T190A Y192F), respectively, were treated with 20 μg/ml CFW for 1 h. Total-cell extracts (lanes labeled “Input”) and anti-HA-agarose-retained protein (lanes labeled “IP” [immunoprecipitation]) were analyzed by Western blotting using an anti-HA antibody to detect Cdc37-HA, an anti-His antibody to detect Slt2His, and anti-phospho-p42/44 MAPK to detect phosphorylated Slt2p.
To investigate the interactions of Cdc37p with other proteins, aliquots of the above-mentioned cell extracts were loaded onto an anti-HA resin and the bound fraction was analyzed by Western blotting for the presence of Cdc37p, Hsp90, and both total and phosphorylated Slt2p (Fig. 7B). The most interesting result of this analysis was the virtually complete absence not only of Hsp90 (as shown in Fig. 3) but also of phosphorylated and nonphosphorylated Slt2p in both stressed and unstressed cdc37-S14A cell extracts (Fig. 7B, lanes 5 and 6). In contrast, there was little or no effect on the retention of Hsp90, Slt2p, or phospho-Slt2p by Cdc37pS14E in extracts prepared from either untreated or CFW-treated cells compared to retention in the wild-type controls (Fig. 7B, compare lanes 7 and 8 to lanes 3 and 4). Interestingly, retention of phosphorylated Slt2p by wild-type (phosphorylated) Cdc37p and the S14E mutant protein was substantially more efficient than retention of its unphosphorylated counterpart (Fig. 7B, compare lanes 4 and 8 and lanes 3 and 7, respectively). In fact, the low-intensity signals seen in Fig. 7B, lanes 3 and 7, probably correspond to the small fraction of phosphorylated Slt2p present even under nonstress conditions (Fig. 7A). The latter observation stands in contrast to the data obtained for the retention of Hog1p by Cdc37p, which suggest that Hog1p, even in the nonphosphorylated form, is in a complex with Cdc37p.
To further support the conclusion that the phosphorylated form of Slt2p is bound more efficiently by Cdc37p, we used a C-terminally His6-tagged wild-type Slt2p (Slt2pHis-Wt) and a His6-tagged Slt2p mutant protein carrying the T190A and Y192F mutations, which abolish Slt2p phosphorylation (Slt2pHis-NP) (a generous gift from P. W. Piper, Sheffield, United Kingdom) (38). These constructs were used to transform an SLT2 deletion strain expressing Cdc37p-WtHA. Cells were grown at 24°C and treated with 20 μg/ml CFW for 1 h to induce the PKC pathway and obtain phosphorylated Slt2p. Cell extracts from stressed cells expressing Slt2pHis-Wt or Slt2pHis-NP as their sole Slt2p source were loaded onto an anti-HA agarose resin, and retained proteins were analyzed by Western blotting. The presence of Cdc37pHA, Slt2pHis, and phosphorylated Slt2p was determined. As can be seen in Fig. 7C (right panel, labeled “IP”), only the phosphorylated form of Slt2p (Slt2His-Wt) was coisolated with Cdc37p, while association of the nonphosphorylatable Slt2p mutant (Slt2pHis-NP) with Cdc37p was abolished. Thus, it appears that phosphorylation of Ser14 of Cdc37p is crucial for the interaction with Slt2p and that phosphorylation of Slt2p increases the affinity for Cdc37p.
The data show that phosphorylation of Ser14 of Cdc37p is crucial for its interaction with phosphorylated Slt2p, and so the observed cell wall phenotype of the cdc37-S14A mutant could well be due to the inability of Cdc37pS14A to interact with Hsp90 and Slt2p. In addition, the altered kinetics of Slt2p phosphorylation, in combination with a Cdc37p Ser14 residue that cannot be dephosphorylated (S14E), leads to a similar effect on the level of Rlm1p activation. This suggests that dephosphorylation of Cdc37p may be an important feature to allow efficient Rlm1p activation by phosphorylated Slt2p. The observation that the S14E mutant displays a less severe CFW-sensitive phenotype than the S14A mutant can be explained by the fact that the slightly higher activation level of Rlm1p in the S14E mutant may be just sufficient for survival.
DISCUSSION
In this study we present genetic and biochemical evidence that both the HOG and the PKC MAP kinase signaling cascade are dependent on Cdc37p. We show that phosphorylation of the Ser14 residue of Cdc37p by CK2 is crucial for adaptation to stress conditions due to high osmolarity or cell wall perturbation that induce the HOG or PKC signaling cascade, respectively.
Loss of phosphorylation of Cdc37 at Ser14, either by mutating this residue to Ala or by inactivating CK2, affects the HOG MAP kinase signaling output in response to osmotic stress, resulting in an osmosensitive phenotype. The observation that the amount of Hog1p is significantly decreased but the mRNA level is not altered in the cdc37-S14A mutant strain indicates that Cdc37p has a role in stabilizing Hog1p. The inability of Cdc37pS14A to interact with Hog1p and hence to facilitate the transcription of downstream osmoregulatory genes, as demonstrated by the reduced transcription of the STL1-LacZ reporter gene during osmotic stress, appears to form the basis for the osmosensitive phenotype of the cdc37-S14A mutant. When the phosphorylation of Cdc37p Ser14 was mimicked by replacing Ser14 with Glu, only a minor effect on HOG MAP kinase signaling was observed. The slightly reduced interaction of Cdc37pS14E with Hog1p causes the weak osmosensitive phenotype of the cdc37-S14E mutant. Interestingly, a previous study with S. pombe showed that Cdc37p plays a role in the regulation of Spc1p activity, the MAPK analogue of Hog1p in fission yeast (57).
In addition to the observed role of Cdc37p in HOG MAPK signaling, we show that the protein also has an effect on another MAPK signaling route, the PKC MAPK pathway, which becomes activated upon exposure to cell wall stress. The role of Cdc37p in this pathway appears to be somewhat different from what was observed for the HOG pathway. In cells containing the Cdc37pS14A mutant protein, the MAP kinase Slt2p of the PKC pathway is activated normally upon exposure to cell wall stress. However, Cdc37pS14A does not associate with phosphorylated Slt2p, as does wild-type Cdc37p, and consequently Slt2p-mediated activation of its downstream target, Rlm1p, is less efficient, which could explain the CFW-sensitive phenotype of the S14A mutant. However, Cdc37pS14E, which mimics a permanently phosphorylated protein, also compromises the activation of Rlm1p in response to CFW stress to about the same extent as Cdc37pS14A, despite the fact that Cdc37pS14E is fully able to associate with activated Slt2p. Our data show that both Slt2p and Cdc37p have to be phosphorylated in order to interact and enable the activation of Rlm1p-mediated gene expression. However, since the S14E mutant also displays low Rlm1p activity in response to CFW stress, dephosphorylation of Cdc37p may be essential as well. Possibly, an additional factor might depend on functional Cdc37p for the activation of Rlm1p, such as Knr4p, which has been reported to interact with Slt2p in order to allow proper Slt2p-mediated downstream signaling (36). Moreover, we cannot exclude the possibility that reduced activation of factors other than Rlm1p may account for the CFW-sensitive phenotype, which is more severe in the S14A than in the S14E mutant. The more severe phenotype of the cdc37-S14A mutant could also be a consequence of reduced activation of the Slt2p-regulated downstream target, the Swi4/6 cell cycle box binding factor, a transcriptional regulator that also controls the expression of cell wall genes (20, 25, 35). SBF consists of the DNA-binding subunit Swi4p and the regulatory subunit Swi6p (42). Both subunits interact with Slt2p and are phosphorylated by the MAP kinase. Since the sets of cell wall genes that are controlled by Rlm1p and SBF seem to overlap (25-27), it will be interesting to investigate the role of Cdc37p in SBF-regulative processes.
A classic phenotype of cell integrity mutants is the ability to remediate their temperature sensitivity via growth on osmotic medium plates. We have observed that the cdc37-S14A mutant, as well as the cka2-8 mutant, did not display an osmo-remedial phenotype (i.e., the temperature sensitivity of these mutants was not rescued by the presence of sorbitol at 37°C), while the S14E mutant did (data not shown). The inability of the cdc37-S14A mutant to grow in the presence of sorbitol at 37°C may be due to defective HOG pathway signaling. However, the growth difference between the cdc37-S14A and cdc37-S14E mutants indicates that reversible phosphorylation of Cdc37p may be needed in the HOG and PKC MAPK pathways.
Like the interaction of Cdc37p with Hog1p and Slt2p, the association of Cdc37p with Hsp90 is regulated by phosphorylation of the Ser residue at position 14. Whereas replacement of Ser14 by Glu does not affect the ability of Cdc37p to associate with Hsp90, replacement by alanine disrupts the recruitment of Cdc37p to both Hsp90 and Slt2p or Hog1p. It is interesting that in mammalian cells the binding of kinases to nonphosphorylatable Cdc37p mutants is reduced, while binding of these Cdc37p mutants to Hsp90 is unaffected (40). Hence, it may be that in yeast Cdc37p, the second phosphorylation site at Ser17 (which is absent in the mammalian p50 protein) contributes to efficient association of Cdc37p with Hsp90. Although there seems to be a difference between yeast and mammalian cells, association of a client kinase with Cdc37p is disrupted by a nonphosphorylatable Cdc37p Ser14 residue. This further corroborates the view that Cdc37p is needed to facilitate the interaction of a client kinase with Hsp90 (56).
Though Hsp90 is part of the Cdc37p-MAP kinase complex in yeast, the specific role of Hsp90 in these complexes is as yet unknown. In fact, Cdc37p has been reported to function independently from Hsp90 in the stress-responsive (SAPK) pathway in fission yeast (57). A study by Citri et al. claimed that MAP kinases such as p38 may not be clients of Hsp90 and that Cdc37p may function independently of Hsp90 at the level of the p38-like MAP kinase. Also, the human analogue of Slt2p, extracellular signal-regulated kinase 5 (ERK5), has been considered to function in an Hsp90-independent manner (6). However, in another study, where a two-hybrid screen in yeast was used, an interaction of Hsp90 with the MAP kinases Hog1p and Slt2p was shown (38). The authors further showed that only phosphorylated Slt2p interacts physically with Hsp90. Interestingly, Truman and colleagues have demonstrated that in yeast, ERK5 not only compensates for the loss of Slt2p but also is associated with Hsp90 in its phosphorylated form (58), a scenario similar to what we report here for Stl2p. The recent work of Vaughan et al. has shown that a kinase can be found in a complex with Cdc37pp50 alone (62). The work further revealed that a kinase first binds to Cdc37pp50 in a kinase-(Cdc37pp50)2 complex and may subsequently be loaded to an Hsp90 dimer, resulting in an (Hsp90)2-Cdc37pp50-kinase complex (62). It is intriguing that in our hands, one kinase client when phosphorylated (Slt2p) shows an increased affinity for the Cdc37p complex, whereas the other (Hog1p) does not show that effect. Perhaps interaction of the MAP kinases with Cdc37p alone may reflect regulatory aspects. Interestingly, the HOG and PKC MAPK pathways seem to be mutually exclusive in osmolarity-induced signaling (9, 65). One signaling pathway responds to increased osmolarity (the HOG pathway), whereas the other (the PKC pathway) responds to decreased osmolarity. Therefore, since simultaneous activation of both signaling pathways is undesirable, a regulatory factor would be needed to prevent cross talk. It may hence be that Cdc37p regulates MAPK signaling by differential interaction with its client proteins.
Considering the fact that Cdc37p and/or Hsp90 is required for the function of Slt2p in the PKC MAPK pathway and at least two components of the HOG MAPK pathway (Hog1p and Ste11p), both these molecular chaperones may have a regulatory function as an active scaffold checkpoint in MAPK signaling routes, guaranteeing proper functioning of these signaling cascades. The scaffold checkpoint function of Cdc37p might be regulated by CK2. In fact, Cdc37p appears to be required for the functionality of a wide range of protein kinases involved in cell cycle and signal transduction, such as Cdc28p and Cak1p (12, 18), v-src (1, 11, 41), Mps1p (48), Kin28p (61), Cdk4p, and Raf-1 (19, 53, 56). In some cases, a scaffold function for Hsp90 and Cdc37p has been suggested, since they appeared to facilitate interactions between a protein kinase and its downstream factor, such as the Akt-endothelial nitric oxide synthase interaction (15) or the Akt-PDK1 interaction (3). Interaction between Slt2p and one of its downstream factors might be controlled by an Hsp90-Cdc37p complex in a similar way. Interestingly, like Slt2p, phosphorylated Akt has been found in a complex with Cdc37p and Hsp90 (3), which might prevent the kinase from transferring a signal to a downstream target when not required. In some aspects, the function of an Hsp90-Cdc37p complex reminds us of the 14-3-3 proteins, which interact as homo- or heterodimers with protein kinases involved in signal transduction pathways such as Raf-1. Most interestingly, 14-3-3 proteins have been found to be required for Raf-1 activation (16, 17, 37, 60), functioning as scaffold proteins that facilitate interaction between two consecutive kinases. In a similar way, a role for the Hsp90-Cdc37p complex as scaffold proteins involved in the regulation of MAP kinase signaling in general becomes more and more attractive.
Acknowledgments
We thank Didier Picard for providing the anti-Hsp90 antibody, Francesco Posas and Peter Piper for plasmid DNA, and Claiborne Glover for yeast strains. We thank Dick Raué for critical reading of the manuscript.
This study was supported by The Netherlands Organization for Scientific Research, project 700.99.012.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Abbas-Terki, T., O. Donze, and D. Picard. 2000. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 467:111-116. [DOI] [PubMed] [Google Scholar]
- 2.Bandhakavi, S., R. O. McCann, D. E. Hanna, and C. V. Glover. 2003. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J. Biol. Chem. 278:2829-2836. [DOI] [PubMed] [Google Scholar]
- 3.Basso, A. D., D. B. Solit, G. Chiosis, B. Giri, P. Tsichlis, and N. Rosen. 2002. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 277:39858-39866. [DOI] [PubMed] [Google Scholar]
- 4.Berben, G., J. Dumont, V. Gilliquet, P. A. Bolle, and F. Hilger. 1991. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7:475-477. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas, M. E., R. Walter, D. Hanna, and S. M. Gasser. 1993. Casein kinase II copurifies with yeast DNA topoisomerase II and re-activates the dephosphorylated enzyme. J. Cell Sci. 104:533-543. [DOI] [PubMed] [Google Scholar]
- 6.Citri, A., D. Harari, G. Shohat, P. Ramakrishnan, J. Gan, S. Lavi, M. Eisenstein, A. Kimchi, D. Wallach, S. Pietrokovski, and Y. Yarden. 2006. Hsp90 recognizes a common surface on client kinases. J. Biol. Chem. 281:14361-14369. [DOI] [PubMed] [Google Scholar]
- 7.Cutforth, T., and G. M. Rubin. 1994. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77:1027-1036. [DOI] [PubMed] [Google Scholar]
- 8.Dai, K., R. Kobayashi, and D. Beach. 1996. Physical interaction of mammalian CDC37 with CDK4. J. Biol. Chem. 271:22030-22034. [DOI] [PubMed] [Google Scholar]
- 9.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 10.de Nadal, E., L. Casadome, and F. Posas. 2003. Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell. Biol. 23:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey, B., J. J. Lightbody, and F. Boschelli. 1996. CDC37 is required for p60v-src activity in yeast. Mol. Biol. Cell 7:1405-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell, A., and D. O. Morgan. 2000. Cdc37 promotes the stability of protein kinases Cdc28 and Cak1. Mol. Cell. Biol. 20:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan, C. A., and J. Thorner. 1992. Purification and characterization of a soluble phosphatidylinositol 4-kinase from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 267:24117-24125. [PubMed] [Google Scholar]
- 14.Fliss, A. E., Y. Fang, F. Boschelli, and A. J. Caplan. 1997. Differential in vivo regulation of steroid hormone receptor activation by Cdc37p. Mol. Biol. Cell 8:2501-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana, J., D. Fulton, Y. Chen, T. A. Fairchild, T. J. McCabe, N. Fujita, T. Tsuruo, and W. C. Sessa. 2002. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 90:866-873. [DOI] [PubMed] [Google Scholar]
- 16.Freed, E., M. Symons, S. G. Macdonald, F. McCormick, and R. Ruggieri. 1994. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science 265:1713-1716. [DOI] [PubMed] [Google Scholar]
- 17.Fu, H., K. Xia, D. C. Pallas, C. Cui, K. Conroy, R. P. Narsimhan, H. Mamon, R. J. Collier, and T. M. Roberts. 1994. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science 266:126-129. [DOI] [PubMed] [Google Scholar]
- 18.Gerber, M. R., A. Farrell, R. J. Deshaies, I. Herskowitz, and D. O. Morgan. 1995. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc. Natl. Acad. Sci. USA 92:4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grammatikakis, N., J. H. Lin, A. Grammatikakis, P. N. Tsichlis, and B. H. Cochran. 1999. p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell. Biol. 19:1661-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray, J. V., J. P. Ogas, Y. Kamada, M. Stone, D. E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna, D. E., A. Rethinaswamy, and C. V. Glover. 1995. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J. Biol. Chem. 270:25905-25914. [DOI] [PubMed] [Google Scholar]
- 23.Hartson, S. D., A. D. Irwin, J. Shao, B. T. Scroggins, L. Volk, W. Huang, and R. L. Matts. 2000. p50cdc37 is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules. Biochemistry 39:7631-7644. [DOI] [PubMed] [Google Scholar]
- 24.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igual, J. C., A. L. Johnson, and L. H. Johnston. 1996. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15:5001-5013. [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer, V. R., C. E. Horak, C. S. Scafe, D. Botstein, M. Snyder, and P. O. Brown. 2001. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409:533-538. [DOI] [PubMed] [Google Scholar]
- 27.Jung, U. S., and D. E. Levin. 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34:1049-1057. [DOI] [PubMed] [Google Scholar]
- 28.Jung, U. S., A. K. Sobering, M. J. Romeo, and D. E. Levin. 2002. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 46:781-789. [DOI] [PubMed] [Google Scholar]
- 29.Kamath, R. S., and J. Ahringer. 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30:313-321. [DOI] [PubMed] [Google Scholar]
- 30.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura, Y., S. L. Rutherford, Y. Miyata, I. Yahara, B. C. Freeman, L. Yue, R. I. Morimoto, and S. Lindquist. 1997. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 11:1775-1785. [DOI] [PubMed] [Google Scholar]
- 32.Lee, P., J. Rao, A. Fliss, E. Yang, S. Garrett, and A. J. Caplan. 2002. The Cdc37 protein kinase-binding domain is sufficient for protein kinase activity and cell viability. J. Cell Biol. 159:1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 34.MacLean, M., and D. Picard. 2003. Cdc37 goes beyond Hsp90 and kinases. Cell Stress Chaperones 8:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Yken, H., A. Dagkessamanskaia, F. Basmaji, A. Lagorce, and J. Francois. 2003. The interaction of Slt2 MAP kinase with Knr4 is necessary for signalling through the cell wall integrity pathway in Saccharomyces cerevisiae. Mol. Microbiol. 49:23-35. [DOI] [PubMed] [Google Scholar]
- 37.Michaud, N. R., J. R. Fabian, K. D. Mathes, and D. K. Morrison. 1995. 14-3-3 is not essential for Raf-1 function: identification of Raf-1 proteins that are biologically activated in a 14-3-3- and Ras-independent manner. Mol. Cell. Biol. 15:3390-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millson, S. H., A. W. Truman, V. King, C. Prodromou, L. H. Pearl, and P. W. Piper. 2005. A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p). Eukaryot. Cell 4:849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyata, Y. 2005. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 11:1131-1138. [DOI] [PubMed] [Google Scholar]
- 40.Miyata, Y., and E. Nishida. 2004. CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol. Cell. Biol. 24:4065-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perdew, G. H., H. Wiegand, J. P. Vanden Heuvel, C. Mitchell, and S. S. Singh. 1997. A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry 36:3600-3607. [DOI] [PubMed] [Google Scholar]
- 42.Primig, M., S. Sockanathan, H. Auer, and K. Nasmyth. 1992. Anatomy of a transcription factor important for the start of the cell cycle in Saccharomyces cerevisiae. Nature 358:593-597. [DOI] [PubMed] [Google Scholar]
- 43.Rao, J., P. Lee, S. Benzeno, C. Cardozo, J. Albertus, D. M. Robins, and A. J. Caplan. 2001. Functional interaction of human Cdc37 with the androgen receptor but not with the glucocorticoid receptor. J. Biol. Chem. 276:5814-5820. [DOI] [PubMed] [Google Scholar]
- 44.Reed, S. I. 1980. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 95:561-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roncero, C., and A. Duran. 1985. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J. Bacteriol. 163:1180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose, M. 1990. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Scholz, G. M., K. Cartledge, and N. E. Hall. 2001. Identification and characterization of Harc, a novel Hsp90-associating relative of Cdc37. J. Biol. Chem. 276:30971-30979. [DOI] [PubMed] [Google Scholar]
- 48.Schutz, A. R., T. H. Giddings, Jr., E. Steiner, and M. Winey. 1997. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J. Cell Biol. 136:969-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao, J., N. Grammatikakis, B. T. Scroggins, S. Uma, W. Huang, J. J. Chen, S. D. Hartson, and R. L. Matts. 2001. Hsp90 regulates p50cdc37 function during the biogenesis of the active conformation of the heme-regulated eIF2α kinase. J. Biol. Chem. 276:206-214. [DOI] [PubMed] [Google Scholar]
- 50.Shao, J., A. Irwin, S. D. Hartson, and R. L. Matts. 2003. Functional dissection of cdc37: characterization of domain structure and amino acid residues critical for protein kinase binding. Biochemistry 42:12577-12588. [DOI] [PubMed] [Google Scholar]
- 51.Shao, J., T. Prince, S. D. Hartson, and R. L. Matts. 2003. Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J. Biol. Chem. 278:38117-38120. [DOI] [PubMed] [Google Scholar]
- 52.Siligardi, G., B. Panaretou, P. Meyer, S. Singh, D. N. Woolfson, P. W. Piper, L. H. Pearl, and C. Prodromou. 2002. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277:20151-20159. [DOI] [PubMed] [Google Scholar]
- 53.Silverstein, A. M., N. Grammatikakis, B. H. Cochran, M. Chinkers, and W. B. Pratt. 1998. p50cdc37 binds directly to the catalytic domain of Raf as well as to a site on hsp90 that is topologically adjacent to the tetratricopeptide repeat binding site. J. Biol. Chem. 273:20090-20095. [DOI] [PubMed] [Google Scholar]
- 54.Spellman, P. T., G. Sherlock, M. Q. Zhang, V. R. Iyer, K. Anders, M. B. Eisen, P. O. Brown, D. Botstein, and B. Futcher. 1998. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9:3273-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spijker, S., S. W. Houtzager, M. C. De Gunst, W. P. De Boer, A. N. Schoffelmeer, and A. B. Smit. 2004. Morphine exposure and abstinence define specific stages of gene expression in the rat nucleus accumbens. FASEB J. 18:848-850. [DOI] [PubMed] [Google Scholar]
- 56.Stepanova, L., X. Leng, S. B. Parker, and J. W. Harper. 1996. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10:1491-1502. [DOI] [PubMed] [Google Scholar]
- 57.Tatebe, H., and K. Shiozaki. 2003. Identification of Cdc37 as a novel regulator of the stress-responsive mitogen-activated protein kinase. Mol. Cell. Biol. 23:5132-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truman, A. W., S. H. Millson, J. M. Nuttall, V. King, M. Mollapour, C. Prodromou, L. H. Pearl, and P. W. Piper. 2006. Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Eukaryot. Cell 5:1914-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnbull, E. L., I. V. Martin, and P. A. Fantes. 2005. Cdc37 maintains cellular viability in Schizosaccharomyces pombe independently of interactions with heat-shock protein 90. FEBS J. 272:4129-4140. [DOI] [PubMed] [Google Scholar]
- 60.Tzivion, G., Z. Luo, and J. Avruch. 1998. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature 394:88-92. [DOI] [PubMed] [Google Scholar]
- 61.Valay, J. G., M. Simon, M. F. Dubois, O. Bensaude, C. Facca, and G. Faye. 1995. The KIN28 gene is required both for RNA polymerase II mediated transcription and phosphorylation of the Rpb1p CTD. J. Mol. Biol. 249:535-544. [DOI] [PubMed] [Google Scholar]
- 62.Vaughan, C. K., U. Gohlke, F. Sobott, V. M. Good, M. M. Ali, C. Prodromou, C. V. Robinson, H. R. Saibil, and L. H. Pearl. 2006. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol. Cell 23:697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe, Y., K. Irie, and K. Matsumoto. 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15:5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe, Y., G. Takaesu, M. Hagiwara, K. Irie, and K. Matsumoto. 1997. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wojda, I., R. Alonso-Monge, J. P. Bebelman, W. H. Mager, and M. Siderius. 2003. Response to high osmotic conditions and elevated temperature in Saccharomyces cerevisiae is controlled by intracellular glycerol and involves coordinate activity of MAP kinase pathways. Microbiology 149:1193-1204. [DOI] [PubMed] [Google Scholar]
- 66.Yang, X. X., K. C. T. Maurer, M. Molanus, W. H. Mager, M. Siderius, and S. M. van der Vies. 2006. The molecular chaperone Hsp90 is required for high osmotic stress response in Saccharomyces cerevisiae. FEMS Yeast Res. 6:195-204. [DOI] [PubMed] [Google Scholar]