Abstract
At present, point-of-care (POC) diagnostics typically provide a binary indication of health status (e.g., home pregnancy test strip). Before anticipatory use of diagnostics for assessment of complex diseases becomes widespread, development of sophisticated bioassays capable of quantitatively measuring disease biomarkers is necessary. Successful translation of new bioassays into clinical settings demands the ability to monitor both the onset and progression of disease. Here we report on a clinical POC diagnostic that enables rapid quantitation of an oral disease biomarker in human saliva by using a monolithic disposable cartridge designed to operate in a compact analytical instrument. Our microfluidic method facilitates hands-free saliva analysis by integrating sample pretreatment (filtering, enrichment, mixing) with electrophoretic immunoassays to quickly measure analyte concentrations in minimally pretreated saliva samples. Using 20 μl of saliva, we demonstrate rapid (<10 min) measurement of the collagen-cleaving enzyme matrix metalloproteinase-8 (MMP-8) in saliva from healthy and periodontally diseased subjects. In addition to physiologically measurable indicators of periodontal disease, conventional measurements of salivary MMP-8 were used to validate the microfluidic assays described in this proof-of-principle study. The microchip-based POC diagnostic demonstrated is applicable to rapid, reliable measurement of proteinaceous disease biomarkers in biological fluids.
Keywords: lab-on-a-chip, point-of-care, electrophoresis, periodontal disease, micro total analysis system
Nascent diagnostic technologies capable of ready measurement of putative disease biomarkers in physiological fluids promise to greatly advance clinical medicine. Quantitative, multianalyte biochemical assays housed within compact, easy-to-use instrumentation improve the prospects for routine use of molecular biomarkers to augment clinically observed symptoms, frequent monitoring of disease progression (especially as pertinent to episodic disease progression), and near-continuous assessment of therapeutic efficacy. Such robust analyses could enable early, possibly even presymptomatic, diagnosis of a disease.
Over the last decade, the incorporation of microfluidic methods in bioassays of blood has reduced sample and reagent consumption and decreased assay times. Such performance enhancements open the door to rapid molecular diagnosis of disease at the point-of-care (POC) (1). State-of-the-art bioanalytical methods employing microfluidic manipulation surmount shortcomings associated with bulky, complex instrumentation (2). Following the lead of promising blood analysis techniques (3), recent reports illustrate the potential for combining microsystem-based diagnostics with use of saliva (4, 5). Reports have established the likelihood that clinically relevant analyte concentrations in saliva mirror tissue fluid levels (6–10). Further, recent studies have recognized the relevance of oral fluid protein content to the development and progression of oral, as well as systemic disease (11–15). Finally, unlike blood collection, saliva collection is straightforward and noninvasive. Consequently, these attributes make saliva attractive as a diagnostic fluid, especially for use at the clinical POC.
Miniaturization of bioanalytical methods enables exceptionally rapid analyses with sensitivity and specificity rivaling that of conventional analytical techniques. Here, we demonstrate lab-on-a-chip technology for measurement of a putative biomarker of periodontal disease in saliva. Periodontal disease (periodontitis) is initiated by tooth-associated pathogenic bacterial plaque biofilms that induce a host immune response. Periodontitis leads to chronic tissue injury, loss of connective tissue attachment, and alveolar bone loss, making early diagnosis relevant to improving clinical outcomes (16). The implications of biochemical diagnosis of periodontitis are potentially far-reaching (17). Periodontal disease has recently been implicated as a cofactor for initiation of cardiovascular disease (18, 19), pulmonary disease (20), and preterm delivery (21). Periodontal disease progresses episodically, making accurate assessment of disease progression difficult. Together, these factors indicate that measurement of molecular biomarkers correlated with periodontal disease would permit rapid accurate diagnoses, dynamic monitoring of disease activity, and potentially more effective treatment. Matrix metalloproteinase-8 (MMP-8) has been identified as a major tissue-destructive enzyme in periodontal disease (22–24). Consequently, MMP-8 is a promising candidate for diagnosing and, possibly more importantly, assessing the progression of this episodic disease (23, 25–28).
This work details significant advances in our efforts to simplify assay operation and improve assay sensitivity by integrating saliva pretreatment (mixing, incubation, and enrichment) with subsequent quantitative immunoassays. Exploiting advantages inherent to microfluidic formats, we demonstrate proof-of-principle for our diagnostic by measuring the concentration of endogenous MMP-8 in saliva. MMP-8 levels were measured in a cohort of patients classified a priori (based on physiological markers) as clinically healthy or periodontally diseased. Analysis of periodontally diseased patients allowed comparison of novel biochemical measurements (i.e., concentrations of MMP-8) to measurable well accepted physiological indicators of periodontal disease (i.e., tissue destruction). The microchip electrophoretic immunoassay (μCEI) core of the diagnostic instrument relies on photolithographically fabricated molecular sieving gels to enrich sample and subsequently resolve fluorescent antibody from MMP-8 complex (MMP-8 antigen bound to αMMP-8*) under native electrophoresis conditions. This approach obviates both the need for matched antibody pairs and surface immobilization of capture antibodies. The method allows direct, automated measurement of the total salivary MMP-8 content and shows good agreement with gold-standard measurements. The diagnostic is designed for chair-side use and, as such, relies on a compact reader instrument used in conjunction with single-use disposable fluidic cartridges (29). Our results suggest that the μCEI assays can measure proteinaceous biomarkers quickly, reliably, and with low sample volume requirements, thereby providing diagnostic tool attributes relevant to a wide range of diseases.
Results and Discussion
μCEI Characterization.
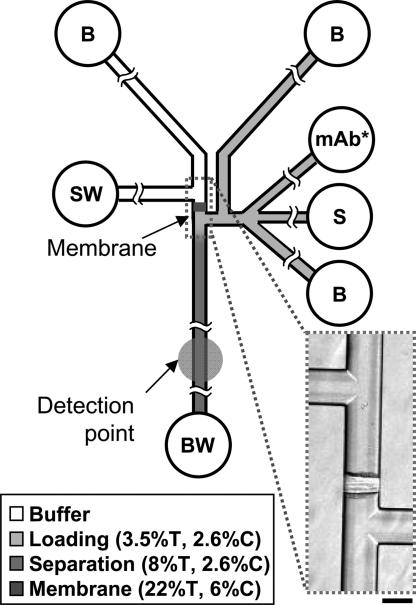
To integrate sample preparation and subsequent electrophoretic immunoassays, polymeric elements with specific physical properties were photopatterned in glass microfluidic devices. The microfluidic devices consisted of channels tailored for specific functions, including: (i) sample loading, (ii) sample enrichment, (iii) rapid diffusive mixing of sample with fluorescently labeled monoclonal antibody (αMMP-8*), and (iv) subsequent rapid native gel electrophoretic separations of αMMP-8* from MMP-8 complex. A schematic of the μCEI chip is shown in Fig. 1, and details of the fabrication process are given in Materials and Methods. Three specific functional regions were fabricated: (i) a size-exclusion membrane, (ii) a small pore-size separation gel, and (iii) a larger pore-size loading gel.
Fig. 1.
μCEI device layout. Fluid wells are labeled according to contents as follows: S, sample; B, buffer; SW, sample waste; BW, buffer waste; mAb*, fluorescently labeled monoclonal antibody to MMP-8. Polyacrylamide gel composition is indicated by grayscale shading (%T and %C are percentage of total acrylamide and bis-acrylamide cross-linker, respectively). Inset shows a 40× bright-field image of the size-exclusion membrane. (Scale bar, 100 μm.)
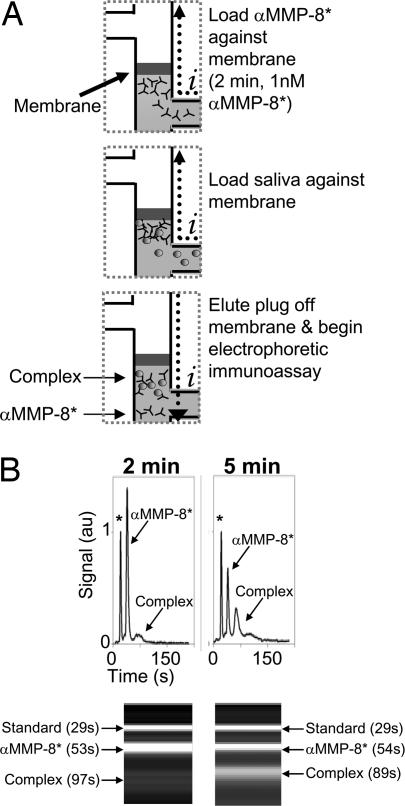
Fig. 2A depicts sequential sample processing and analysis steps implemented in the μCEI device. During loading, sample and detection mixture (1 nM αMMP-8* spiked with the 1 nM BSA* protein standard) were electrophoretically introduced into the device through a large pore-size loading gel. BSA* was used to normalize peak areas and migration times for each experiment. The loading gel enabled rapid sample loading with minimized analyte mobility bias. The polyacrylamide gel present in all channels eliminated bulk flow; thus, electrophoretic transport was used for analyte mobilization. During enrichment, saliva was electrophoretically enriched against a size-exclusion membrane for a fixed duration. The size-exclusion membrane composition (22%T, 6%C) yielded pore sizes small enough to allow buffer ion transport and current flow through the membrane, yet exclude a wide range of proteins (>10 kDa) (30). Photopatterning of the size-exclusion membrane adjacent to the separation gel enabled a zero dead-volume integration of the protein enrichment and electrophoretic separation functions. To further streamline the MMP-8 assay, αMMP-8* and saliva were mixed near the size-exclusion membrane during the saliva loading step. Our previous studies showed that analytes are confined to a region having an axial extent of ≈50 μm during enrichment at a similar membrane (30). Confinement of αMMP-8* and saliva samples in this small volume resulted in exceptionally efficient mixing and rapid incubation of αMMP-8* with MMP-8. We observed no differences between assays performed with on-chip mixing during the saliva loading step and assays performed with a 15-min off-chip incubation before analysis (data not shown).
Fig. 2.
On-chip sample enrichment. (A) Schematic depicting operation of the μCEI device. After a buffer priming step (not depicted), the detection mixture is loaded against the size-exclusion membrane. Saliva sample is then loaded, resulting in coenrichment of saliva and αMMP-8* at the size-exclusion membrane. An electric potential is applied across the membrane, causing the enriched species to elute into the separation channel, thus initiating the electrophoretic immunoassay. Subsequently, the electric potential is switched to omit the membrane from the current path. Current flow is indicated by i. (B) Electropherograms and gel-like plots show that, with all other conditions held fixed, substantial complex is observed after a 5-min enrichment. Protein internal standard is marked with an asterisk on the electropherograms.
After enrichment and on-chip mixing, the applied electric potentials were adjusted such that the enriched excess αMMP-8*, MMP-8 complex, and BSA* protein standard eluted away from the membrane and into the separation channel. An intermediate pore size polyacrylamide gel (8%T, 2.6%C) was used in the separation channel. The gel composition allows high separation resolution through enhanced size-based sieving of analytes exhibiting little difference in charge-to-mass characteristics (31), as is the case with αMMP-8* and MMP-8 complex. In previous work, the effects of concentration polarization (i.e., decreasing current over time, irreproducible protein migration behavior) were observed when electric fields were applied across the size-exclusion membrane for the duration of the separation (30). As described in that work, two steps were taken to lessen concentration polarization effects: the loading gel was only polymerized on the separation side of the membrane, and the size-exclusion membrane was omitted from the current path during the separation step. Migrating species were detected as they moved past a single-point laser-induced fluorescence (LIF) detection system located near the end of the separation channel.
Development of Quantitative μCEI.
μCEI assays shown in Fig. 2B allow comparison of two different sample enrichment conditions with all other conditions held constant. The assays generally exhibit three analyte peaks corresponding to the high-mobility BSA* protein standard, excess αMMP-8*, and the lower-mobility MMP-8 complex. Increased saliva enrichment times resulted in increased complex peak areas as the local concentration of MMP-8 at the size-exclusion membrane increased. The elevated local MMP-8 concentration changed not only the amount of MMP-8 present, but also the amount of MMP-8 bound to the detection antibodies. Consequently, the sensitivity and dynamic range of the μCEI assay can be adjusted by varying either the duration of sample enrichment at the membrane or the magnitude of the electric potential applied during the enrichment step. Although potentially confounding “inert” background species are also enriched at the membrane, the specificity of the assay minimizes significant increases in the background signal, as the detection antibody showed no appreciable cross-reactivity with background species (see Fig. 2B). The electrophoretic immunoassay baseline resolves MMP-8 complex from excess αMMP-8* in <2 min.
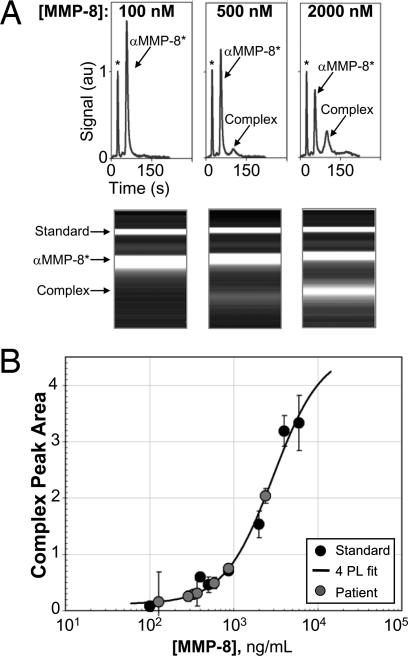
The ability to quantitate MMP-8 may aid in the monitoring of dynamic disease activity, as MMP-8 is primarily derived from polymorphonuclear leukocytes during active stages of periodontitis (32). To generate a calibration curve, as well as to verify specificity and assign an identity to each resolved peak, increasing amounts of recombinant MMP-8 were spiked into diluted saliva pooled from four healthy control patients. Representative data from the standards analysis are shown in Fig. 3A. Complex peak areas were extracted from the μCEI analysis of saliva standards. In samples having a high MMP-8 content a second complex peak was detected. In these cases the major and minor complex peak areas were summed, as would be the case in ELISA analysis using the same detection antibody. The complex peak areas were normalized by the peak area of the BSA* internal standard for each run and used to generate the MMP-8 calibration curve shown in Fig. 3B. A nonlinear least-squares fit with a four-parameter logistic model was used to establish the relationship between MMP-8 concentration and measured normalized peak areas of the complex (β1 = 12, β2 = 4.63, β3 = 2926, and β4 = 1.5).
Fig. 3.
Calibration of the MMP-8 μCEI using pooled saliva from healthy controls. (A) Electropherograms and corresponding gel-like plots for MMP-8 standard sample analyses demonstrate detection of recombinant human MMP-8 spiked into saliva at known concentrations. (B) MMP-8 dose–response curve generated by μCEI. The MMP-8 complex peak areas of the standard samples (black filled symbols) are plotted as a function of known MMP-8 concentration. A four-parameter logistic model (4PL, solid line) of the form y = β2 + (β1 − β2)/(1 + (x/β3)β4) was fit by the method of nonlinear least-squares to the standard samples. The 4PL fit was used to calculate the concentration of endogenous MMP-8 in the patient saliva samples (gray filled symbols).
To estimate the lower limit of quantitation (LLOQ) for the MMP-8 μCEI method repeated measurements of a blank sample (fivefold dilution of saliva pooled from four healthy patients) were made. The normalized complex peak area was measured for each replicate and used to determine an average and standard deviation (SD) for the response. The mean blank value was added to 10 times the SD of the response to yield an LLOQ estimate of 130 ng/ml. In addition to the affinity of the antibody for the desired antigen, optimization of the extent of sample enrichment adjusts the final upper and lower limits of quantitation.
μCEI for Quantitation of MMP-8 in Saliva from Healthy and Periodontally Diseased Individuals.
A conventional colorimetric sandwich ELISA was used to measure concentrations of MMP-8 in saliva from the patient population. The average MMP-8 concentration in the saliva of the healthy population (64.6 ± 16.4 ng/ml) was significantly lower than that of the periodontally diseased patients (623.8 ± 204.0 ng/ml, P < 0.05). Elevated MMP-8 levels in saliva have been reported for patients identified as having periodontal disease (28, 33). MMP-8 concentration exhibited substantial variability in saliva, even among the periodontally diseased group, and is hypothesized to reflect dynamic disease activity (32).
To assess disease severity by clinical examination, measurements of the clinical attachment level, pocket depth, bleeding upon probing, and radiographic bone loss were made at the time of saliva collection. Mean pocket depth and clinical attachment loss were significantly different between the two populations [see supporting information (SI) Experimental Procedures]. Although pocket depth alone does not necessarily indicate active disease, significant correlation was identified between the measured MMP-8 concentration in saliva and the periodontal pocket depth of the population (r = 0.884 where r is the Pearson product-moment correlation coefficient; one outlying patient was excluded from the analysis). Correlation between MMP-8 concentration and each of the following measurements was noted: mean clinical attachment loss of >3 mm (r = 0.8223), clinical attachment loss (r = −0.653), and degree of radiographic bone loss (r = 0.548). No correlation between MMP-8 concentration and bleeding on probing (r = 0.033) was observed. Strong correlation between MMP-8 and clinical measures of bone and tissue loss, such as those described here, support the assertion that MMP-8 is a biochemical indicator of periodontal disease severity and may relate to disease activity. An indicator of periodontal disease activity could improve timing of MMP inhibitor therapy, especially if measured during the active phase associated with collagen degradation. Treatment of patients with MMP inhibitors has been shown to reduce periodontal disease activity (34).
Validation of μCEI Using Conventional ELISA.
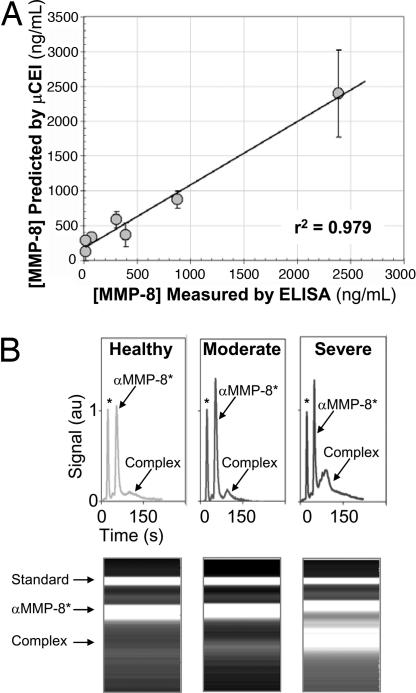
The MMP-8 values measured in the patient population by using μCEI were compared with values measured by benchmark ELISAs for total MMP-8 concentration. As shown in Fig. 4A, linear regression was performed to determine the correlation between the two measurement techniques and shows that μCEI can be used to quantify MMP-8 concentrations in saliva (r2 = 0.979). Fig. 4B shows gel-like plots from μCEI analyses of three patients that span the MMP-8 levels observed across the range of disease states analyzed. Comparison of the MMP-8 complex peak intensity and area reveals substantially higher MMP-8 concentrations in the severely diseased periodontitis patient when compared with the healthy control patient. MMP-8 was also quantifiably higher in the moderately diseased patient when compared with the healthy control. The MMP-8 level in saliva from the moderately diseased patient was markedly less than that of the severely diseased patient. In contrast to measurement of MMP-8 levels in localized fluid such as gingival crevicular fluid (25), the possibility for rapid measurement of MMP-8 in saliva is especially promising. Analysis of saliva may provide an overall appraisal of a patient's periodontal disease state and severity.
Fig. 4.
μCEI measurement of endogenous MMP-8 in saliva. (A) Linear regression analysis for the MMP-8 concentrations measured by μCEI and commercial ELISA. A linear relationship (y = 0.91x + 181.7, r2 = 0.979) was observed over the operating range investigated. (B) μCEI MMP-8 measurements from a healthy patient and patients clinically diagnosed as having moderate to severe periodontitis reveal differences in MMP-8 levels.
Although putative biomarkers of periodontal disease have been proposed in saliva, this work presents a rapid means amenable to POC use for measuring salivary biomarkers to aid in diagnosis and monitoring of periodontal disease that has not been described previously. The rapid nature and specificity of the μCEI bioassay, combined with the use of saliva as a diagnostic fluid, makes this technology attractive as a means for clinical quantitation of protein biomarkers associated with periodontal disease. The clinical applicability of the assay method is demonstrated through the quantitation of minimally pretreated human saliva for an enzyme implicated in an important oral disease. The operating range of the assay has clinical relevance to a low-abundance endogenous protein, MMP-8. Several important differences exist between the μCEI technique introduced in this study and commercially available ELISAs. Namely, the μCEI assays described here obviate the need for numerous, time-consuming reaction and washing steps required to complete an ELISA. The self-contained, electronically controlled operation of the μCEI assay is well suited for automation. Unlike ELISAs, the μCEI assay requires a single antibody, therefore broadening the range of applicable antigens. In addition, the bioassay described in this work requires <20 μl of patient sample and αMMP-8* to perform literally hundreds of analyses. This is especially significant when working with reagents available in limited quantities such as costly monoclonal antibodies or precious clinical or forensic samples.
The results and methods presented in this study demonstrate μCEI as a means to identify periodontitis populations, providing a potentially significant advantage for diagnosis and monitoring of disease progression. The results further indicate the importance of additional investigations regarding MMP-8 as a biomarker for periodontal disease, preferably as a component of a biomarker panel. Longitudinal studies regarding the ability of MMP-8 to predict disease progression, as well as a means to monitor therapeutic efficacy are in progress. The core microfluidic techniques demonstrated in this work form the basis for rapid, quantitative analysis of protein levels in saliva. Adaptation of the described methods should allow for use of other diagnostic fluids and may be especially relevant to diagnostic fluids localized to a disease site and available in only limited volumes [e.g., breast cancer and nipple aspirate fluid (35, 36)]. Active development is needed for this diagnostic method as a core technology adaptable to monitoring and/or diagnosis of a broad range of local and systemic diseases, rapid analysis of promising diagnostic fluids only available in minute volumes, and simultaneous analysis of panels of protein biomarkers in a single patient sample.
Materials and Methods
μCEI Bioinstrumentation.
The μCEI assay and disposable cartridge were developed for use in a portable analytical reader described previously (37). An 8-bit Rabbit 2000 microprocessor (Rabbit Semiconductor, Davis, CA) was the heart of a system board that provided a menu-based user interface, high-voltage power supply connections, software control, data acquisition, and integration of instrument communications. Miniature power supplies (3.8 × 1.5 × 2.0 cm) designed and fabricated in-house provided high voltage via DC-to-DC converters (EMCO, Sutter Creek, CA) and an electronics board for voltage regulation and current monitoring. Microdevices were designed in-house and fabricated in glass by Caliper Life Sciences (Hopkinton, MA) using standard wet etching protocols. Channels were ≈25 μm deep and ≈100 μm wide. Before thermal bonding of the glass substrate and lid, 2-mm wells were drilled in the top layer of glass to allow fluid access to the channels. Reusable fluidic manifolds (37) allowed sample and reagent storage and optical access to the separation channels.
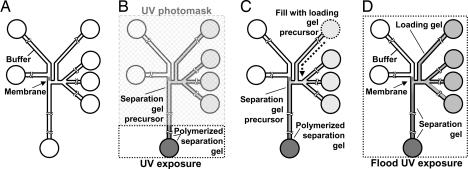
Fig. 5 details the most important steps of the fabrication protocol. The size-exclusion (or enrichment) membrane was fabricated by using laser-photopolymerization of a solution of acrylamide monomer, cross-linker, and photoinitiator. This solution of degassed 22% (15.7:1) acrylamide/bis-acrylamide containing 0.2% (wt/vol) VA-086 photoinitiator was introduced into the channels by pressure-driven flow and equilibrated for 30 min to eliminate disruptive bulk flow. A 15-s exposure to a 355-nm laser sheet resulted in formation of a well defined size-exclusion membrane in the microchannels (Fig. 5A). Unpolymerized solution was vacuumed from the channels.
Fig. 5.
Multistep photopolymerization process enables fabrication of μCEI device. (A) μCEI chip layout with size-exclusion membrane. (B) Separation gel precursor solution loaded into channels. UV photomasking is used to define an 8% total acrylamide plug near the end of the separation channel. (C) Loading gel precursor solution is used to define the loading gel (3.5%) and the smaller pore-size separation gel. (D) Flood UV exposure polymerizes the separation and loading gels.
To define and localize the separation gel in the separation channel, all channels were rinsed with buffer and gently pressure-loaded with the separation gel precursor solution. The separation gel precursor solution consisted of degassed 8% (37.5:1) acrylamide/bis-acrylamide containing 0.2% (wt/vol) VA-086 in 1X Tris/glycine buffer. An intermediate porosity gel plug was fabricated at the terminus of the separation channel via photomasking and a 10 min flood UV exposure using a fan-cooled 100-W lamp (Fig. 5B). Fabrication of the plug resulted in (i) a separation channel filled with the separation gel precursor solution and (ii) elimination of bulk flow in the separation channel. Next, the larger pore size loading gel was defined by gently pressure-filling the loading channels with the loading gel precursor solution (Fig. 5C). The loading gel precursor solution consisted of degassed 3.5% (37.5:1) acrylamide/bis-acrylamide containing 0.2% (wt/vol) VA-086 in 1X Tris/glycine buffer. The loading gel precursor solution could not enter into the separation channel except by diffusion, as bulk flow was eliminated in the plug-capped separation channel. Polymerization of the loading and separation gels was conducted by means of a 15-min photopolymerization of the unmasked chip using a 100-W UV lamp (Fig. 5D). Chips were stored filled and submerged in buffer at 4°C when not in use. The yield of fabricated membrane, loading, and separation gels was ≈90%. Once successfully fabricated, chip usage lifetimes varied, but typically were >40 hours of usage over several weeks.
Reagents and Saliva.
Solutions of 40% acrylamide and 30% (37.5:1) acrylamide/bis-acrylamide were purchased from Sigma (St. Louis, MO). Premixed 10X Tris/glycine (25 mM Tris, pH 8.3, 192 mM glycine) electrophoresis buffer was purchased from Bio-Rad (Hercules, CA). The Tris/glycine was adjusted by addition of NaOH to a final pH of 8.9. The water-soluble photoinitiator 2,2′-Azobis[2-methyl-N-(2-hydroxyethyl)propionamide] (VA-086) was purchased from Wako Chemicals (Richmond, VA). Alexa Fluor 647 (Invitrogen, Carlsbad, CA) was used to fluorescently label the monoclonal antibody to MMP-8 (R&D Systems, Minneapolis, MN). The monoclonal antibodies to MMP-8 recognized pro and active forms of MMP-8. BSA labeled with AF647 (BSA*) was spiked into the αMMP-8* solution at 1 nM and used as a standard. Final concentrations of the fluorescently labeled proteins were measured by using an absorbance method outlined by Invitrogen.
The study was approved by Sandia and the University of Michigan's Institutional Review Boards, and informed consent was obtained from each patient before participation. A total of 23 human subjects [14 classified as having periodontitis according to standard clinical criteria (39) and 9 healthy controls] were selected for study (see SI Experimental Procedures). Unstimulated whole saliva was collected from each patient at the Michigan Center for Oral Health Research in a manner similar to that described by Mandel (7) (see SI Experimental Procedures).
Benchmark Analysis.
Quantitative sandwich ELISAs for total MMP-8 (R&D Systems) were conducted in triplicate to provide a benchmark measure of the total MMP-8 concentration present in saliva per manufacturer's instructions.
Data Acquisition and Analysis.
LIF was used to obtain electropherograms (see SI Experimental Procedures). Peak areas were measured by using PeakFit4 (Systat Software Inc., Point Richmond, CA). Electropherograms were converted into gel-like plots by using Matlab (The Math Works, Inc., Natick, MA). Each “lane” of the gel-like plot was generated from a single electropherogram, with the vertical dimension indicating the elapsed separation time and, thus, the apparent mobility of each analyte. The gel-like plots were formatted to display gray scale intensity information in a manner similar to data collected from standard slab-gels (e.g., Western blots). Intensities were normalized to a standard maximum defined for all plots in a group, and apparent mobilities were normalized to the BSA* protein standard band from each analysis. A statistical analysis software package (JMP; SAS Institute, Inc., Cary, NC) was used to generate estimates of significance using Student's t test, linear correlations, and the Pearson product-moment correlation coefficient (a measure of the strength of the linear relationship between two variables, where −1 ≤ r ≤ 1).
Supporting Information.
Details regarding the human subject inclusion criteria, off-chip saliva collection and preparation, and the LIF detection system are included as SI Experimental Procedures. MMP-8 concentrations from ELISA, as well as mean clinical measurement levels, are included as SI Fig. 6.
Supplementary Material
Acknowledgments
We thank V. VanderNoot, R. Renzi, and J. Stamps at Sandia National Laboratories. The authors thank M. McReynolds, K. Ghandi, and D. Degrasse at Caliper Life Sciences. At the University of Michigan, the authors thank J. Kinney, C. Ramseier, L. Rayburn, and J. Sugai. This work was supported by National Institute of Dental and Craniofacial Research Grant NIDCR U01-DE014961 (A.K.S. as PI). Sandia is a multiprogram laboratory operated by Sandia Corp., a Lockheed Martin Co., for the U.S. Department of Energy under Contract DE-AC04-94AL85000.
Abbreviations
- POC
point-of-care
- μCEI
microchip electrophoretic immunoassay
- MMP-8
matrix metalloproteinase-8
- αMMP-8*
fluorescently labeled mAb to MMP-8
- BSA*
fluorescently labeled BSA
- LIF
laser-induced fluorescence.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607254104/DC1.
References
- 1.Kricka LJ. Clin Chem. 1998;44:2008–2014. [PubMed] [Google Scholar]
- 2.Schulte TH, Bardell RL, Weigl BH. Clin Chim Acta. 2002;321:1–10. doi: 10.1016/s0009-8981(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 3.Toner M, Irimia D. Annu Rev Biomed Eng. 2005;7:77–103. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, Ali MF, Bernard B, Romanovicz D, Anslyn E, Fox PC, McDevitt JT. Lab Chip. 2005;5:261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- 5.Yang CY, Brooks E, Li Y, Denny P, Ho CM, Qi FX, Shi WY, Wolinsky L, Wu B, Wong DTW, Montemagno CD. Lab Chip. 2005;5:1017–1023. doi: 10.1039/b504737d. [DOI] [PubMed] [Google Scholar]
- 6.Malamud D. Br Med J. 1992;305:207–208. doi: 10.1136/bmj.305.6847.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandel ID. J Oral Path. 1990;19:119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 8.Mandel ID. Ann NY Acad Sci. 1993;694:1–10. doi: 10.1111/j.1749-6632.1993.tb18336.x. [DOI] [PubMed] [Google Scholar]
- 9.Schramm W, Smith RH, Craig PA, Kidwell DA. J Anal Toxi. 1992;16:1–9. doi: 10.1093/jat/16.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Streckfus CF, Bigler LR. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez PM, Torres AR, de Lejarazu PO, Montoya A, Martin JF, Eiros JM. J Clin Microbiol. 1999;37:1100–1106. doi: 10.1128/jcm.37.4.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streckfus CF, Bigler L, Dellinger T, Kuhn M, Chouinard N, Dai XL. J Oral Path. 2004;33:595–600. doi: 10.1111/j.1600-0714.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence HP. J Can Dent Assoc. 2002;68:170–174. [PubMed] [Google Scholar]
- 14.Malamud D. Am J Med. 1997;102:9–14. doi: 10.1016/s0002-9343(97)00032-6. [DOI] [PubMed] [Google Scholar]
- 15.Donovan BJ, Rublein JC, Leone PA, Pilcher CD. Ann Pharmacother. 2004;38:670–676. doi: 10.1345/aph.1D314. [DOI] [PubMed] [Google Scholar]
- 16.Genco R. J Periodontol. 1992;63:338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman E, Lamster IB. J Clin Periodontol. 2000;27:453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- 18.Beck JD, Offenbacher S. J Periodontol. 2005;76:2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 19.Al-Zahrani MS, Kayal RA, Bissada NF. Quintessence Int. 2006;37:11–18. [PubMed] [Google Scholar]
- 20.Scannapieco FA, Bush RB, Paju S. Ann Periodontol. 2003;8:54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- 21.Tucker R. J R Soc Health. 2006;126:24–27. doi: 10.1177/1466424006061170. [DOI] [PubMed] [Google Scholar]
- 22.Sorsa T, Tjaderhane L, Salo T. Oral Dis. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorsa T, Mantyla P, Ronka H, Kallio P, Kallis GB, Lundqvist C, Kinane DF, Salo T, Golub LM, Teronen O, et al. Ann NY Acad Sci. 1999:130–140. doi: 10.1111/j.1749-6632.1999.tb07679.x. [DOI] [PubMed] [Google Scholar]
- 24.Mantyla P, Stenman M, Kinane DF, Tikanoja S, Luoto H, Salo T, Sorsa T. J Periodont Res. 2003;38:436–439. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- 25.Ingman T, Tervahartiala T, Ding Y, Tschesche H, Haerian A, Kinane D, Konttinen Y, Sorsa T. J Clin Periodontol. 1996;23:1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 26.Kiili M, Cox SW, Chen HW, Wahlgren J, Maisi P, Eley BM, Salo T, Sorsa T. J Clin Periodontol. 2002;29:224–232. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 27.Wong DT. J Am Dent Assoc. 2006;137:313–321. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 28.Miller CS, King CP, Langub C, Kryscio RJ, Thomas MV. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 29.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 30.Hatch AV, Herr AE, Throckmorton DJ, Brennan JS, Singh AK. Anal Chem. 2006;78:4976–4984. doi: 10.1021/ac0600454. [DOI] [PubMed] [Google Scholar]
- 31.Herr AE, Throckmorton DJ, Davenport AA, Singh AK. Anal Chem. 2005;77:585–590. doi: 10.1021/ac0489768. [DOI] [PubMed] [Google Scholar]
- 32.Sodek J, Overall CM. Matrix Suppl. 1992;1:352–362. [PubMed] [Google Scholar]
- 33.Miller CS, King CP, Langub MC, Kryscio RJ, Thromas MV. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 34.Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 35.Sauter ER, Lininger J, Magklara A, Hewett JE, Diamandis EP. Int J Cancer. 2004;108:588–591. doi: 10.1002/ijc.11607. [DOI] [PubMed] [Google Scholar]
- 36.Alexander H, Stegner AL, Wagner-Mann C, Du Bois GC, Alexander S, Sauter ER. Clin Cancer Res. 2004;10:7500–7510. doi: 10.1158/1078-0432.CCR-04-1002. [DOI] [PubMed] [Google Scholar]
- 37.Renzi RF, Stamps J, Horn BA, Ferko S, VanderNoot VA, West JAA, Crocker R, Wiedenman B, Yee D, Fruetel JA. Anal Chem. 2005;77:435–441. doi: 10.1021/ac049214f. [DOI] [PubMed] [Google Scholar]
- 38.Song S, Singh AK, Kirby BJ. Anal Chem. 2004;76:4589–4592. doi: 10.1021/ac0497151. [DOI] [PubMed] [Google Scholar]
- 39.Armitage GC. J Periodontol. 2003;74:1237–1247. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.