Abstract
Two polyomaviruses, BK virus (BKV) and JC virus (JCV), are ubiquitous in the human population, generally infecting children asymptomatically and then persisting in renal tissue. It is generally thought that reactivation leads to productive infection for both viruses, with progeny shed in the urine. Several studies have shown that the rate of JC viruria increases with the age of the host, but a systematic approach to examine the shedding of BKV has not been developed. To elucidate the relationship between BK viruria and host age, we obtained urine from donors (healthy volunteers or nonimmunocompromised patients) who were divided into nine age groups, each containing 50 members. A high-sensitivity PCR was used to detect BKV and JCV DNA from urinary samples, and the specificity of amplification was confirmed by sequencing or restriction analysis of the amplified fragments. The rate of BK viruria was relatively low in subjects aged <30 years but gradually increased with age in subjects aged ≥30 years. However, BK viruria was less frequent than JC viruria in adults. The detected BKV isolates were classified into subtypes, and detection rates for individual subtypes were compared among age groups; this analysis showed that viruria of subtypes I (the most prevalent subtype) and IV (the second most prevalent subtype) occurred more frequently in older subjects. Therefore, our results reveal new aspects of BK viruria in nonimmunocompromised individuals.
Humans are infected with two polyomaviruses, JC virus (JCV) and BK virus (BKV), and serological surveys have shown that both viruses are ubiquitous in the human population, generally infecting children asymptomatically (18) and then persisting in renal tissue (4, 7). Both viruses are usually nonpathogenic for nonimmunocompromised individuals, but they cause clinically significant diseases in immunocompromised patients. Thus, BKV causes BKV-associated nephropathy in organ transplant patients (e.g., renal transplant patients) (8), while JCV causes progressive multifocal leukoencephalopathy, typically in patients infected with human immunodeficiency virus (2).
Renal JCV and BKV in nonimmunocompromised individuals are not latent but replicate frequently, excreting progeny viruses in urine. The incidence of JC viruria is known to increase with age and reaches nearly 70% at 80 to 89 years old (12, 13); in contrast, the shedding of BKV has not been studied systematically (15), although it is likely that BK viruria is also age dependent. To establish the relationship between BK viruria and host age, we collected urine samples from nonimmunocompromised individuals (healthy volunteers and general patients, all of whom were Japanese). The urine donors were divided into nine groups based on age (0 to 9, 10 to 19, 20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, 70 to 79, and 80 to 89 years old), with each group having 50 members. A high-sensitivity PCR was used to detect BKV DNA from urinary samples, and the specificity of amplification was confirmed by sequencing of the amplified fragments. In parallel, we analyzed the relationship between JC viruria and host age in the same subjects, and we classified the detected BKV isolates into subtypes by using a phylogenetic analysis based on nucleotide sequences. Our results provide several new insights into the reactivation of BKV in humans.
MATERIALS AND METHODS
Urine samples.
Urine samples were collected in 6 mM EDTA (pH 8.0) from 351 outpatients who attended Nagareyama Central Hospital and Yasuda Children's Clinic as well as from 99 healthy volunteers (all Japanese). Urine samples were consecutively collected from outpatients attending the hospitals. The drop-out rate was about 10% at Nagareyama Central Hospital and about 50% at Yasuda Children's Clinic. None of the patients were receiving immunosuppressive or anticancer drug therapy. The period of urine collection was from April 2005 to April 2006. Urine donors were divided into nine groups based on decades of age. The numbers of males and females and the mean age, median age, and standard deviation (SD) for each group are given in Table 1. This study was approved by the medical ethics committee of the University of Tokyo Hospital, and informed consent was obtained from each patient.
TABLE 1.
Age groups in this study
| Age group (yr) | No. of males | No. of females | Age distribution
|
|
|---|---|---|---|---|
| Mean ± SD (yr) | Median (yr) | |||
| 0-9 | 25 | 25 | 6.0 ± 2.1 | 6.0 |
| 10-19 | 26 | 24 | 14.6 ± 3.3 | 15.0 |
| 20-29 | 24 | 26 | 24.7 ± 2.9 | 25.0 |
| 30-39 | 37 | 13 | 34.2 ± 2.9 | 34.0 |
| 40-49 | 34 | 16 | 45.0 ± 3.1 | 46.0 |
| 50-59 | 26 | 24 | 55.5 ± 2.6 | 56.0 |
| 60-69 | 23 | 27 | 64.9 ± 3.0 | 65.0 |
| 70-79 | 31 | 19 | 74.3 ± 2.8 | 74.0 |
| 80-89 | 27 | 23 | 84.0 ± 2.8 | 83.5 |
| Total | 253 | 197 | 44.8 ± 25.7 | 46.0 |
DNA extraction.
Urinary DNA was extracted from the virion fraction of urine as described previously (12). In brief, after the removal of urinary sediment, urine samples were subjected to high-speed centrifugation at 100,000 × g for 3 h to pellet the virions. DNA was extracted from the resulting pellets by digestion with proteinase K and subsequent phenol treatment. The DNA was then concentrated by ethanol precipitation and dissolved in 80 μl of sterilized water.
PCR amplification.
The 287-bp typing region of BKV DNA (10, 21) and the 610-bp intergenic (IG) region of JCV DNA (1) were amplified from urinary DNA (see above) by using HotStar Taq DNA polymerase (QIAGEN GmbH, Hilden, Germany) and primers described previously (16, 21). A total reaction mixture volume of 50 μl contained 2.5 μl of urinary DNA, 1.25 units of HotStar DNA polymerase, 200 μM of each deoxynucleoside triphosphate, 0.5 μM of the primers, and a PCR buffer supplied by the manufacturer. After enzyme activation at 95°C for 15 min, amplification was performed for 50 cycles. The cycle profile was 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min. PCR products were electrophoresed on 1% agarose gels stained with ethidium bromide and photographed under a UV light. Amplification of the typing region of BKV DNA and the IG region of JCV DNA was repeated three times for each sample. The amplified fragments were purified with a Montage PCR centrifugal filter device (Millipore Corporation, Bedford, MA) and subjected to DNA sequencing (for BKV) or restriction fragment length polymorphism (RFLP) analysis (for JCV).
DNA sequencing.
Amplified typing region fragments of BKV were subjected to a cycle sequencing reaction using a BigDye Terminator cycle sequencing kit, v. 3.1 (Applied Biosystems, Foster City, CA). The primers used for amplification (327-1PST and 327-2HIN) (21) were also used for the sequencing reaction. Sequencing was carried out with an automated DNA sequencer (3130 Genetic Analyzer; Applied Biosystems).
Phylogenetic analysis.
DNA sequences were aligned using the CLUSTAL W program (22), and phylogenetic relationships among DNA sequences were evaluated using the neighbor-joining (NJ) method (20). CLUSTAL W with Kimura's correction (11) was used for analysis with the NJ method, and the phylogenetic tree was visualized using the NJ plot program (19). To assess the confidence level of the phylogenetic tree, bootstrap probabilities (BPs) were estimated with 1,000 bootstrap replicates (5).
RFLP analysis.
An RFLP analysis with NlaIII (New England Biolabs, Inc., Ipswich, MA) was performed to distinguish various JCV genotypes prevalent in Japan (14).
Determination of PCR sensitivity.
The sensitivity of the PCR was determined using 10-fold serial dilutions of the plasmids pRYU-2 and pRYU-3 (belonging to subtypes I and IV, respectively) (17) and pCY and pMY (representing genotypes CY and MY, respectively) (23). Each dilution was prepared in distilled water containing 1 ng/μl of λ phage DNA. PCR using 10-fold serial dilutions of each plasmid was performed in the presence of urinary DNA lacking the target viral DNAs, and the amplified products were analyzed by agarose gel electrophoresis with visualization by ethidium bromide staining.
Statistical analysis.
Statistical analysis was performed using the chi-square test with Yates' correction and Fisher's exact test, using the statistical software package SPSS. All statistical analyses were performed with numbers rather than with percentages. The significance level was set at 5%.
Nucleotide sequence accession numbers.
The sequences of the BKV isolates described in this article have been deposited in the EMBL/GenBank/DDBJ data library and have been assigned accession numbers AB268362 to AB268480.
RESULTS
Assay validation.
To determine PCR sensitivity, we used recombinant plasmids containing full-length viral DNAs representing the major subtypes of BKV or the major genotypes of JCV prevalent in Japan (17, 23). Using various amounts of these viral DNAs as the template, PCR amplification of the typing region of BKV or the IG region of JCV (see Materials and Methods for detailed conditions) was carried out in the presence or absence of urinary DNAs lacking the target viral DNA. PCR was repeated three times for each viral DNA.
In the absence of urinary DNA, both the BKV typing region and the JCV IG region were amplified in two or three of the three trials at 2.5 genome equivalents of the viral DNA and in all three trials at 25 and 250 genome equivalents. In the presence of urinary DNA, these regions were amplified in one to three of the three trials at 2.5 genome equivalents of the BKV DNA and in all three trials at 25 and 250 genome equivalents. These results indicate that (i) urinary DNA exhibited no significant inhibitory effect on amplification and (ii) at least three PCR trials are needed to detect a small amount of BKV DNA or JCV DNA (i.e., about 2 genome equivalents/ml of urine). We also attempted to amplify the noncoding control regions of BKV and JCV but found that the efficiency with which these regions were amplified was lower than the efficiency with which the BKV typing region or the JCV IG region was amplified (data not shown).
Detection rates for BKV and JCV DNA in various age groups.
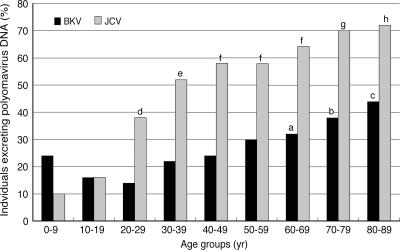
We attempted to detect BKV and JCV DNA from urine samples collected from 50 subjects (healthy volunteers or nonimmunocompromised patients) for each of the nine age groups (see Materials and Methods). PCR was repeated three times for each sample to detect the BKV typing region or the JCV IG region at the highest sensitivity (see above). Specific amplification was confirmed by DNA sequencing of amplicons (for the BKV typing region) or by RFLP analysis (for the JCV IG region). The results are shown in Table 2 and Fig. 1 and are summarized in the following paragraphs.
TABLE 2.
Amplification of BKV and JCV DNA from urine samples
| Age group (yr)a | No. (%) of subjects from which BKV DNA was amplified inb:
|
No. (%) of subjects from which JCV DNA was amplified inb:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 trial | 2 trials | 3 trials | Total | 1 trial | 2 trials | 3 trials | Total | |
| 0-9 | 3 | 3 | 6 | 12 (24) | 0 | 0 | 5 | 5 (10) |
| 10-19 | 1 | 3 | 4 | 8 (16) | 0 | 0 | 8 | 8 (16) |
| 20-29 | 1 | 1 | 5 | 7 (14) | 0 | 0 | 19 | 19 (38) |
| 30-39 | 1 | 5 | 5 | 11 (22) | 0 | 0 | 26 | 26 (52) |
| 40-49 | 2 | 3 | 7 | 12 (24) | 0 | 0 | 29 | 29 (58) |
| 50-59 | 2 | 2 | 11 | 15 (30) | 1 | 0 | 28 | 29 (58) |
| 60-69 | 1 | 2 | 13 | 16 (32) | 0 | 1 | 32 | 33 (66) |
| 70-79 | 1 | 4 | 14 | 19 (38) | 0 | 1 | 34 | 35 (70) |
| 80-89 | 1 | 5 | 16 | 22 (44) | 0 | 0 | 36 | 36 (72) |
| Total | 13 | 28 | 81 | 122 (27) | 1 | 2 | 217 | 220 (49) |
Each group contained 50 subjects, and each subject gave a single urine sample.
Amplification of the typing region of BKV DNA and the IG region of JCV DNA was repeated three times for each sample.
FIG. 1.
Incidence of urinary excretion of polyomavirus DNA in various age groups. There were 50 individuals in each age group. a, P < 0.05 versus the 20- to 29-year-old group; b, P < 0.05 versus the 10- to 19-year-old group and P < 0.01 versus the 20- to 29-year-old group; c, P < 0.01 versus the 0- to 9-, 10- to 19-, 30- to 39-, and 40- to 49-year-old groups and P < 0.01 versus the 20- to 29-year-old group; d, P < 0.01 versus the 0- to 9-year-old group and P < 0.05 versus the 10- to 19-year-old group; e, P < 0.01 versus the 0- to 9- and 10- to 19-year-old groups; f, P < 0.01 versus the 0- to 9- and 10- to 19-year-old groups and P < 0.05 versus the 20- to 29-year-old group; g, P < 0.01 versus the 0- to 9-, 10- to 19-, and 20- to 29-year-old groups; h, P < 0.01 versus the 0- to 9-, 10- to 19-, and 20- to 29-year-old groups and P < 0.05 versus the 30- to 39-year-old group.
The BKV typing region was detected at rates that varied depending on subject age. Thus, the detection rate for the BKV typing region was relatively high (24%) in the 0- to 9-year-old group but declined in the 10- to 19- and 20- to 29-year-old groups. The detection rate then increased gradually with age, reaching the highest level (44%) in the 80- to 89-year-old group. Differences in detection rates among age groups were examined statistically: the detection rates in older age groups (i.e., the 60- to 69-, 70- to 79-, and 80- to 89-year-old groups) were significantly higher than in younger age groups (Fig. 1); however, there were no significant differences in detection rates among the younger age groups (i.e., the 0- to 9-, 10- to 19-, and 20- to 29-year-old groups).
The JCV IG region was detected at the lowest rate (10%) in the youngest age group (0- to 9-year-old group), and the detection rate then increased with age before reaching a plateau in the two oldest groups (70- to 79- and 80- to 89-year-old groups). A statistical examination showed that the detection rates for the IG region were significantly higher in older age groups than in younger age groups (Fig. 1).
In the two youngest age groups (0- to 9- and 10- to 19-year-old groups), the detection rate for the BKV typing region did not differ significantly from that for the JCV IG region. However, the former detection rate was significantly lower than the latter in the older age groups (P < 0.01). Detection rates for BKV or JCV DNA did not differ significantly between males and females in each age group (data not shown).
BKV subtypes detected in various age groups.
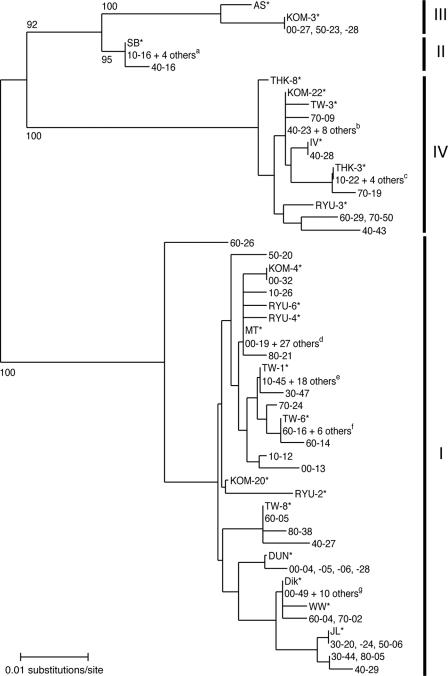
We unambiguously sequenced BKV typing regions (n = 119) (Table 3) amplified from most subjects in the nine age groups, excluding three isolates (00-47, 60-06, and 60-07) that contained multiple BKV DNA sequences. An NJ phylogenetic tree (20) was constructed from the 119 sequences and from sequences previously identified as belonging to individual subtypes of BKV (10, 21). As shown in the tree (Fig. 2), four major clusters with high BPs (95% to 100%) were identified. The cluster that contained the largest number of isolates was judged to correspond to subtype I, since 13 reference isolates (Dik, DUN, JL, KOM-4, KOM-20, MT, RYU-2, RYU-4, RYU-6, TW-1, TW-6, TW-8, and WW) for subtype I (10, 21) fell within this cluster. The cluster containing the second largest number of isolates was judged to correspond to subtype IV, since six reference isolates (isolate IV, KOM-22, RYU-3, THK-3, THK-8, and TW-3) (10, 21) were found in this cluster. In addition, two minor clusters were identified: one was judged to correspond to subtype II and the other to subtype III, since certain reference sequences (SB for subtype II and AS and KOM-3 for subtype III [10, 21]) were found in the respective clusters. Excluding isolates from three subjects (00-47, 60-06, and 60-07), all isolates obtained in the current study were unequivocally classified as belonging to subtype I, II, III, or IV.
TABLE 3.
BKV isolates detected in individual age groups
| Age group (yr) | Subtype | No. of isolates | Isolate(s) detected |
|---|---|---|---|
| 0-9 | I | 10 | 00-04, 00-05, 00-06, 00-08, 00-13, 00-19, 00-28, 00-32, 00-49, 00-50 |
| III | 1 | 00-27 | |
| UCa | 1 | 00-47 | |
| 10-19 | I | 6 | 10-12, 10-14, 10-19, 10-26, 10-35, 10-45 |
| II | 1 | 10-16 | |
| IV | 1 | 10-22 | |
| 20-29 | I | 5 | 20-08, 20-32, 20-35, 20-38, 20-46 |
| II | 2 | 20-36, 20-40 | |
| 30-39 | I | 9 | 30-05, 30-06, 30-19, 30-20, 30-24, 30-38, 30-44, 30-47, 30-49 |
| II | 2 | 30-30, 30-33 | |
| 40-49 | I | 8 | 40-01, 40-08, 40-27, 40-29, 40-40, 40-46, 40-47, 40-50 |
| II | 1 | 40-16 | |
| IV | 3 | 40-23, 40-28, 40-43 | |
| 50-59 | I | 11 | 50-06, 50-09, 50-11, 50-13, 50-16, 50-20, 50-29, 50-35, 50-43, 50-44, 50-48 |
| III | 2 | 50-23, 50-28 | |
| IV | 2 | 50-04, 50-37 | |
| 60-69 | I | 12 | 60-04, 60-05, 60-13, 60-14, 60-16, 60-18, 60-23, 60-26, 60-32, 60-34, 60-46, 60-50 |
| IV | 2 | 60-29, 60-37 | |
| UC | 2 | 60-06, 60-07 | |
| 70-79 | I | 12 | 70-02, 70-08, 70-20, 70-23, 70-24, 70-25, 70-31, 70-32, 70-34, 70-42, 70-45, 70-46 |
| IV | 7 | 70-09, 70-10, 70-11, 70-12, 70-19, 70-47, 70-50 | |
| 80-89 | I | 17 | 80-01, 80-05, 80-08, 80-16, 80-18, 80-20, 80-21, 80-22, 80-25, 80-33, 80-34, 80-35, 80-36, 80-37, 80-38, 80-41, 80-43 |
| IV | 5 | 80-07, 80-26, 80-29, 80-40, 80-45 |
UC, unclassified.
FIG. 2.
NJ phylogenetic tree classifying 119 BKV isolates into subtypes. The 287-bp typing sequences detected in the current study plus 22 reference sequences (indicated by asterisks) (10, 21) were used to reconstruct the NJ phylogenetic tree. The phylogenetic tree was visualized using the NJ plot program. Subtypes are indicated to the right of the tree. The numbers at the nodes are the BPs (%) obtained for 1,000 replicates (shown only for major nodes). a, 20-36, 20-40, 30-30, and 30-33; b, 50-04, 60-37, 70-10, 70-11, 70-12, 80-26, 80-29, and 80-40; c, 50-37, 70-47, 80-07, and 80-45; d, 10-35, 20-32, 30-05, 30-06, 40-08, 40-47, 50-09, 50-11, 50-13, 50-16, 50-35, 50-43, 50-44, 50-48, 60-34, 60-46, 70-20, 70-25, 70-32, 70-34, 70-45, 80-01, 80-18, 80-20, 80-25, 80-36, and 80-43; e, 10-14, 20-35, 20-38, 20-46, 30-19, 40-40, 40-46, 40-50, 60-32, 70-08, 70-23, 70-42, 70-46, 80-16, 80-22, 80-33, 80-34, and 80-35; f, 40-01, 60-13, 60-18, 60-23, 60-50, and 70-31; g, 00-08, 00-50, 10-19, 20-08, 30-38, 30-49, 50-29, 80-08, 80-37, and 80-41.
The numbers of isolates classified as belonging to each subtype are shown for individual age groups in Table 3. For each BKV subtype, only a small number of isolates were detected in the nine age groups, and therefore, the subtype analysis was performed using three larger age groups, the 0- to 29-, 30- to 59-, and 60- to 89-year-old groups (Table 4). The findings can be summarized as follows: (i) subtype I was prevalent in all age groups, with proportions ranging from 71% to 77% of the detected isolates; (ii) subtype IV occurred with the second highest frequency in two age groups (30- to 59- and 60- to 89-year-old groups), with proportions of 13% and 25%, and subtypes II and III occurred only rarely in all age groups; and (iii) the incidence of subtype I was significantly higher in the 60- to 89-year-old group than in the 0- to 29-year-old group, and that of subtype IV was significantly higher in the 60- to 89-year-old group than in the 0- to 29- and 30- to 59-year-old groups (P < 0.01 and P < 0.05, respectively).
TABLE 4.
Incidence of BKV subtypes in various age groupsa
| Age group (yr) | Total no. of isolates | No. (%) of isolates classified as belonging to the indicated subtype
|
||||
|---|---|---|---|---|---|---|
| I | II | III | IV | UCb | ||
| 0-29 | 27 | 21 (77) | 3 (11) | 1 (4) | 1 (4) | 1 (4) |
| 30-59 | 38 | 28 (74) | 3 (8) | 2 (5) | 5 (13) | 0 (0) |
| 60-89 | 57c | 41 (71)d | 0 (0) | 0 (0) | 14 (25)c | 2 (4) |
| Total | 122 | 90 (74) | 6 (5) | 3 (2) | 20 (16) | 3 (2) |
Based on the data in Table 3.
UC, unclassified.
P < 0.01 versus the 0- to 29-year-old group and P < 0.05 versus the 30- to 59-year-old group.
P < 0.01 versus the 0- to 29-year-old group.
Effect of clinical status on detection rates for BKV and JCV DNA.
Subjects were excluded from the study if they were receiving immunosuppressants or anticancer drugs. However, it is possible that BK or JC viruria occurred more frequently in subjects who were immunosuppressed due to other causes, such as pregnancy, renal failure, diabetes mellitus, and infection. To examine this possibility, we selected subjects aged more than 30 years from Nagareyama Central Hospital. These subjects were classified into four groups: one group (A) comprised subjects who did not seem to be immunosuppressed, and the other three groups (B to D) included subjects in whom mild immunosuppression appeared to be possible. The number of subjects, clinical status, and age distribution in each group are given in Table 5, and the rates of BK and JC viruria are shown in Table 6. The rates of BK or JC viruria did not differ significantly among the groups (P > 0.05 by the chi-square test) (Table 6); therefore, we concluded that a mild difference in immune activity has little effect on the rate of BK viruria.
TABLE 5.
Groups of subjects for comparison of the rates of BK and JC viruriaa
| Group | Clinical status (no. of subjects) | No. of subjects (males/females) | Age distribution
|
|
|---|---|---|---|---|
| Mean ± SD (yr) | Median (yr) | |||
| A | Healthy (16), hypertension (12), benign prostatic hyperplasia (8), bone fracture (5), urinary calculus (3), hyperlipidemia (2), vertigo (2), cholecystolithiasis (1), constipation (1), colon polyp (1), emphysema (1), gout (1), hyperuricemia (1) | 54 (31/23) | 60.9 ± 14.9 | 62.0 |
| B | Diabetes mellitus (HbA1Cb ≥ 6.5%) (66) | 66 (35/31) | 66.9 ± 13.5 | 63.5 |
| C | Infection (25), cancer (7), allergic diathesis (2), rheumatoid arthritis (3), thyroid disorder (2), chronic renal failure (1), Sjogren's syndrome (1) | 41 (19/22) | 60.6 ± 18.8 | 59.0 |
| D | Cerebral infarction (26), angina pectoris (6), myocardial infarction (3), cardiac insufficiency (3), cerebral hemorrhage (3), arrhythmia (2), subarachnoidal hemorrhage (2) | 45 (23/22) | 75.0 ± 9.9 | 78.0 |
See Table 6.
HbAIC, level of glycated hemoglobin.
TABLE 6.
Rates of BK and JC viruria in different groups of subjectsa
| Group | Total no. of subjects | No. (%) of subjects with:
|
|
|---|---|---|---|
| BK viruria | JC viruria | ||
| A | 54 | 17 (31.5) | 31 (57.4) |
| B | 66 | 28 (42.4)b | 46 (69.7)b |
| C | 41 | 11 (26.8)c | 23 (56.1)c |
| D | 45 | 16 (35.5)d | 30 (66.7)d |
See Table 5.
P > 0.05 versus group A, C, or D.
P > 0.05 versus group A, B, or D.
P > 0.05 versus group A, B, or C.
DISCUSSION
Our results suggest several new aspects of BK viruria in immunocompetent individuals. Thus, we showed that (i) BK viruria occurs at low but significant rates in all age groups; (ii) in adults, the incidence of BK viruria increases with subject age; (iii) both subtype I and IV viruria occurs at higher rates in elderly individuals; (iv) the incidence of BK viruria in adults is about half that of JC viruria; and (v) a mild change in immune activity has little effect on the rate of BK viruria. These findings provide basic information required for an understanding of the interaction between BKV and humans.
Following primary infection, BKV persists frequently in the kidneys (4, 7). It is thought that renal BKV exists in a latent form, and it is often stated that latent BKV is reactivated upon immunosuppression, excreting progeny viruses in urine (15). However, this statement does not seem valid, since we found significant rates of BK viruria in nonimmunosuppressed individuals in this study. Thus, it can be hypothesized that productive infection of BKV occurs in the kidneys of a substantial proportion of immunocompetent individuals; this hypothesis predicts that the detection rate for BKV DNA in renal tissue should be essentially identical to that in urine if age-matched groups are compared. Therefore, to test the above hypothesis, we are currently examining the detection rate of BKV DNA in renal tissue samples from nonimmunosuppressed cadavers.
We observed a higher level of BK viruria in children than in younger adults (20- to 29-year-old group), although we were unable to provide statistical support for this result, probably due to the low number of subjects used for comparison. We assume that BK viruria in children is frequently correlated with primary infection, and this may explain our data; this assumption is based on a report that BK viruria was detected in a 15-year-old renal transplant recipient who showed seroconversion following allograft transplantation, probably due to primary infection (6). BKV DNA in urine eventually became undetectable in this patient, and Ginevri et al. ascribe the clearance of BKV to the establishment of humoral and cellular immunity against BKV (6). Thus, it appears that primary infection by BKV often accompanies BK viruria.
Since it has been shown that JCV has coevolved with human populations (24), we recently started a series of studies to elucidate relationships between BKV lineages and human populations (3, 9, 25). In some of these studies, we used BKV DNA from urine samples collected from nonimmunocompromised individuals. The present study shows that BKV DNA of the two major subtypes (I and IV) is detected at higher rates in urine from elderly individuals; therefore, nonimmunosuppressed elderly subjects may be the most appropriate population in which to study the evolution of BKV.
In summary, our results show new aspects of BK viruria in nonimmunocompromised individuals. However, current practice guidelines recommend routine screening of serum for BK virus in all kidney transplantation recipients, and further study is required to determine how many subjects with BK viruria also have BK viremia and whether subjects without BK viruria can develop BK viremia.
Acknowledgments
We are grateful to all of the urine donors.
This study was supported in part by grants from the Ministry of Health, Labor and Welfare, Japan.
Footnotes
Published ahead of print on 8 November 2006.
REFERENCES
- 1.Ault, G. S., and G. L. Stoner. 1992. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J. Gen. Virol. 73:2669-2678. [DOI] [PubMed] [Google Scholar]
- 2.Berger, J. R., and S. Houff. 2006. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol. Res. 28:299-305. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Q., H.-Y. Zheng, S. Zhong, H. Ikegaya, H. X. He, W. Wei, Y. Y. He, N. Kobayashi, T. Honjo, T. Takasaka, S. Takahashi, T. Kitamura, and Y. Yogo. 2006. Subtype IV of the BK polyomavirus is prevalent in East Asia. Arch. Virol. 151:2419-2429. [DOI] [PubMed]
- 4.Chesters, P. M., J. Heritage, and D. J. McCance. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676-684. [DOI] [PubMed] [Google Scholar]
- 5.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 6.Ginevri, F., N. Pastorino, R. de Santis, I. Fontana, A. Sementa, G. Losurdo, A. Santopietro, F. Perfumo, F. Locatelli, R. Maccario, A. Azzi, and P. Comoli. 2003. Retransplantation after kidney graft loss due to polyoma BK virus nephropathy: successful outcome without original allograft nephrectomy. Am. J. Kidney Dis. 42:821-825. [DOI] [PubMed] [Google Scholar]
- 7.Heritage, J., P. M. Chesters, and D. J. McCance. 1981. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J. Med. Virol. 8:143-150. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79:1277-1286. [DOI] [PubMed] [Google Scholar]
- 9.Ikegaya, H., P. J. Saukko, R. Tertti, K. P. Metsärinne, M. J. Carr, B. Crowley, K. Sakurada, H.-Z. Zheng, T. Kitamura, and Y. Yogo. 2006. Identification of a genomic subgroup of BK virus spread in European populations. J. Gen. Virol. 87:3201-3208. [DOI] [PubMed]
- 10.Jin, L., P. E. Gibson, J. C. Booth, and J. P. Clewley. 1993. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J. Med. Virol. 41:11-17. [DOI] [PubMed] [Google Scholar]
- 11.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura, T., Y. Aso, N. Kuniyoshi, K. Hara, and Y. Yogo. 1990. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J. Infect. Dis. 161:1128-1133. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura, T., T. Kunitake, J. Guo, T. Tominaga, K. Kawabe, and Y. Yogo. 1994. Transmission of the human polyomavirus JC virus occurs both within the family and outside the family. J. Clin. Microbiol. 32:2359-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura, T., C. Sugimoto, H. Ebihara, A. Kato, J. Guo, F. Taguchi, T. Tominaga, Y. Ogawa, N. Ohta, N. Kizu, K. Imamura, H. Funaki, T. Kurosawa, S. Ichikawa, T. Suzuki, K. Chiba, K. Nagashima, S. Yasumoto, and Y. Yogo. 1998. Peopling of Japan as revealed by genotyping of urinary JC virus DNA. Anthropol. Sci. 106:311-325. [Google Scholar]
- 15.Knowles, W. A. 2001. The epidemiology of BK virus and the occurrence of antigenic and genomic subtypes, p. 527-559. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives, John Wiley & Sons, New York, NY.
- 16.Kunitake, T., T. Kitamura, J. Guo, F. Taguchi, K. Kawabe, and Y. Yogo. 1995. Parent-to-child transmission is relatively common in the spread of the human polyomavirus JC virus. J. Clin. Microbiol. 33:1448-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimoto, Y., T. Takasaka, M. Hasegawa, H.-Y. Zheng, Q. Chen, C. Sugimoto, T. Kitamura, and Y. Yogo. 2006. Evolution of BK virus based on complete genome data. J. Mol. Evol. 63:341-352. [DOI] [PubMed] [Google Scholar]
- 18.Padgett, B. L., and D. L. Walker. 1973. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 127:467-470. [DOI] [PubMed] [Google Scholar]
- 19.Perrière, G., and M. Gouy. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 20.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 21.Takasaka, T., N. Goya, T. Tokumoto, K. Tanabe, H. Toma, Y. Ogawa, S. Hokama, A. Momose, T. Funyu, T. Fujioka, S. Omori, H. Akiyama, Q. Chen, H.-Y. Zheng, N. Ohta, T. Kitamura, and Y. Yogo. 2004. Subtypes of BK virus prevalent in Japan and variation in their transcriptional control region. J. Gen. Virol. 85:2821-2827. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yogo, Y., T. Kitamura, C. Sugimoto, T. Ueki, Y. Aso, K. Hara, and F. Taguchi. 1990. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J. Virol. 64:3139-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yogo, Y., C. Sugimoto, H.-Y. Zheng, H. Ikegaya, T. Takasaka, and T. Kitamura. 2004. JC virus genotyping offers a new paradigm in the study of human populations. Rev. Med. Virol. 14:179-191. [DOI] [PubMed] [Google Scholar]
- 25.Zheng, H.-Y., Y. Nishimoto, Q. Chen, M. Hasegawa, S. Zhong, H. Ikegaya, N. Ohno, C. Sugimoto, T. Takasaka, T. Kitamura, and Y. Yogo. Relationships between BK virus lineages and human populations. Microbes Infect., in press. [DOI] [PubMed]