Abstract
The ability to distinguish between Escherichia coli strains is critical for outbreak investigations. Binary typing, based on the presence or absence of genetic material, provides a high-throughput alternative to gel- and PCR-based typing techniques that generate complex banding patterns and lack uniform interpretation criteria. We developed, validated, and determined the discriminatory power of an E. coli binary typing method, probe hybridization array typing (PHAT). In PHAT, the absence or presence of genetic material is identified by using DNA hybridization to produce a reproducible and portable fingerprint for each genome. PHAT probes were generated from genome subtractive hybridization experiments. We PHAT typed the ECOR collection of strains from a variety of geographical locations, and 33 rectal E. coli strains selected from college-aged women with urinary tract infection. In the set of 33 human rectal strains, the discriminatory power of PHAT (98%) equaled that of multilocus sequence typing (MLST) and pulsed-field gel electrophoresis. However, for ECOR strains, which include nonhuman strains, the current set of PHAT probes was less discriminating than MLST, ribotyping, and enterobacterial repetitive intergenic consensus sequence PCR (80% versus 97, 92, and 97%, respectively). When we limited the analysis to ECOR strains of B2 and D lineage, which are associated with human infection, current PHAT probes were highly discriminatory (94%). PHAT can be applied in a high-throughput format (i.e., “library on a slide”), the discriminatory ability can be varied based on the probe set, and PHAT is readily adapted to other bacterial species with high variation in genetic content.
The ability to distinguish between Escherichia coli strains is critical for outbreak investigations; thus, the availability of rapid, reliable, valid, and high-throughput typing methods is desirable. Traditional serogroup- and phage-based typing methods have been increasingly replaced by more-rapid DNA fragment-based typing methods, including (i) repetitive sequence methods based on PCR such as enterobacterial repetitive intergenic consensus (ERIC) sequencing and randomly amplified polymorphic DNA (RAPD) detection (11, 16, 27), (ii) restriction digest and gel-based methods such as ribotyping and pulsed field gel electrophoresis (PFGE) (24), (iii) sequence-based methods such as multilocus sequence typing (MLST) (14, 24), (iv) whole-genome sequencing, and (v) single-nucleotide polymorphism (SNP) typing (10).
Most gel- and PCR-based techniques generate complex banding patterns that lack uniform interpretation criteria (17). Although PFGE can be highly reproducible when a standard protocol and equipment is used, problems remain (17). The interpretation of gel-based methods is most straightforward when additional information regarding the relationships between strains is available, such as when they are epidemiologically linked and when assays are conducted in a single laboratory (24).
DNA-based typing methods have the advantage of portability and reproducibility. MLST is based on direct sequencing of 400- to 500-bp regions of five to seven housekeeping genes (1, 14). Each strain is scored based on nucleotide substitutions observed and assigned to unique allelic profile sequence types. This method has a high discriminatory power but is labor-intensive, time-consuming, and still is impractical for high-throughput applications. SNP typing based on high-throughput sequencing of 13 SNPs from 11 genes used for MLST has been demonstrated for E. coli (10). Although SNP typing is less discriminatory than MLST (for the SNPs analyzed), when used for phylogeny the resulting groupings are similar to those found by using MLST.
Binary typing is an alternative DNA-based typing method to MLST and is suitable for organisms with a large variation in genetic content. In binary typing, each strain is assigned a signature based on the presence or absence of a set of defined DNA sequences rather than allelic profiles. Binary typing using comparative genomic hybridization, containing all of the open reading frames (ORFs) of a sequenced genome (genomotyping), has been demonstrated for typing clinical bacterial Campylobacter and Salmonella strains (13, 18). In this method, strains can be typed for the presence or absence of all the coding regions on the bacterial genome. Although genomotyping has high discriminatory power, it is time-consuming for typing large collections since it uses a large number of ORFs to type a few bacterial strains. Oligonucleotide-based arrays have also been used to type bacterial strains (10).
A binary typing method using probes generated from RAPD sequences has been validated for Staphylococcus aureus (25, 26, 29). We describe here the development and validation of a hybridization-based binary typing method for E. coli, probe hybridization array typing (PHAT), and compare it to other typing methods. By selecting probes with the most discriminating power, we demonstrate that a relatively small probe set can be used to type large numbers of diverse bacterial strains. Consecutive additions to the PHAT probe set can be used to adjust the discriminatory power of PHAT.
PHAT uses the genetic diversity of the genome for identification rather than the conserved sequences favored by MLST. The more diverse regions that are shared among a group of strains, the more likely the strains are closely related. By focusing on the presence or absence of genetic content rather than allelic variation in conserved genes, PHAT detects changes on a relatively short time scale. The presence or absence of genetic regions is identified by using DNA hybridization. The resulting string of zeros and ones, corresponding to the absence and presence of the chosen genetic regions, creates a reproducible and portable PHAT “type” that is easily compared across laboratories. PHAT has the advantage of an adjustable level of discrimination: increasing the number of probes in the probe set will increase the level of discrimination between strains. Further, collapsing to a smaller probe set has a clearer biological meaning than similarity based on gel band pattern, since the genetic content of specific bands is usually unknown. PHAT can be applied in a high-throughput “library-on-a-slide” (LOS) format (33) and is readily adapted to other bacterial species with high variation in genetic content.
MATERIALS AND METHODS
We selected potential genomic regions for inclusion in PHAT from a set of PCR probes generated from genome subtraction experiments (described below) using probes with a prevalence of 40 to 60% among rectal E. coli strains. Probe choice and optimal probe number was determined by probing a set of rectal E. coli strains and determining the statistical entropy (described below) of each probe, in all possible permutations of probe orders. We compared the discriminatory power of PHAT to those of other methods by use of Simpson's diversity index (12).
E. coli collections.
Subtraction PCR (sPCR) probes generated from genome subtraction experiments were used to probe three different E. coli collections: (i) the E. coli reference collection (ECOR), which is a collection of 72 strains isolated from a variety of hosts and geographical locations (http://foodsafe.msu.edu/Whittam/ecor/); (ii) a set of 33 E. coli strains for which PFGE was available, also selected from college women aged 18 to 39 years with urinary tract infections (UTI) (8); and (iii) a set of 106 rectal strains randomly selected from E. coli isolates collected from college women aged 18 to 39 years with their first diagnosed UTI (9). The UTI collections have previously been characterized for the presence or absence of genes encoding adhesin P-pili (pff) further divided by adhesin subgroup (papGAD, papGJ96, and prsGJ96), S fimbrial adhesin (sfa), aerobactin (aer), group II capsule (kpsMT), cytotoxic necrotizing factor (cnf1), Dr family of adhesins (drb), hemolysin (hly), outer membrane protease T (ompT), Irg homolog adhesin (iha), uropathogenic specific protein (usp), catechole siderophore receptor gene (iroNE. coli), and heat-resistant agglutinin (hra) as described previously (4, 15, 23).
sPCR fragment selection.
We generated a library of genomic sequences that are present on one bacterial strain (tester) but absent on another (driver) using sPCR. sPCR fragments from four different subtractions were used. These genomic subtraction experiments yielded sPCR fragments that were either uniquely present in a greater number of pathogenic UTI strains or more likely to be involved in shared strains between heterosexual partners or shared between bladder, vaginal, and rectal sites. The details of these subtractions are described elsewhere (3, 23, 28, 30, 32). sPCR fragments were cloned into commercial vectors (TOPO; Invitrogen, Inc.) and probed for presence or absence in UTI and non-UTI E. coli collections. Probes that were present in 40 to 60% of the screened study populations were selected as possible PHAT candidates. The magnitudes of the association between the different sPCR fragments were estimated by using the odds ratios and 95% confidence intervals, and the significance was tested by using the chi-square test. All analyses were done by using SAS v8.0.
Preparation of DNA probes.
sPCR fragments were prepared by PCR from the strains from which they were originally cloned by using M13R and T7 primers. PCR amplification was performed using the model PTC-100 programmable thermal cycler (MJ Research), and the conditions used were at 94°C for 1 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 68°C for 30 s, and extension at 74°C for 1 min. The PCR products were purified by using a commercial PCR purification kit (QIAGEN, Inc.) and stored at −20°C for long-term use.
Dot blot hybridizations for PHAT.
E. coli strains were probed by using dot blot hybridization with fluorescence-labeled PHAT probes. Briefly, bacterial DNA was prepared by growing strains overnight in LB medium in a 96-well deep-well plate (1 ml per well; Corning, Inc.). Bacterial cells were pelleted by centrifugation at 3,000 rpm in a Beckman desktop centrifuge and lysed with 800 μl of 0.4 N NaOH-10 mM EDTA at 70°C for 30 min. The bacterial lysate was arrayed on nylon membrane (Hybond H+; Amersham Pharmacia) using a BIO-dot microfiltration apparatus (Bio-Rad Laboratories). Nylon membranes were washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), dried, and fixed by using UV light. Fluorescently labeled gene fragments were hybridized to nylon membranes and detected by using the ALKPHOS fluorescein-based detection kit (Amersham) according to the manufacturer's instructions. Membranes were prehybridized with 20 ml of hybridization buffer for 30 min, followed by the addition of probe (200 ng). Hybridizations were carried out at 55°C overnight, and membranes were washed with primary and secondary wash buffers according to the manufacturer's protocol. Fluorescent signal was generated by using the ECF substrate provided in the kit. Hybridization intensities were detected by using Storm 860 PhosphorImager (Molecular Dynamics) and analyzed by using Image-QuaNT 5.0. The signal intensity of each spot was normalized to the intensity of each probe's positive control according to a previously published protocol (32). All strains were tested for the presence or absence of probe with a minimum of two independent membranes. Ambiguous results were retested on duplicate membranes and confirmed by Southern hybridization using previously described protocols (32). Sequencing of sPCR fragment DNA was performed at the University of Michigan Molecular Biology Core Facility using an Applied Biosystems model 373A automated sequencer.
MLST.
MLST was performed using the protocols listed on the EcMLST database (www.shigatox.net). Briefly, PCRs were performed to obtain ∼500-bp fragments for seven housekeeping genes, purified and sequenced at the University of Michigan Molecular Biology Core Facility in both the 3′ and the 5′ directions. A consensus sequence was obtained for each of the seven gene fragments in 33 strains of E. coli. Allele types were assigned to the PCR-amplified sequences after comparison with the EcMLST database for nucleotide substitutions. The combination of allele types for the seven housekeeping genes gave the sequence type (ST) for each strain.
PFGE.
PFGE was performed according to our previously published protocol (8). Briefly, NotI-digested DNA was electrophoresed in a Bio-Rad pulsed-field apparatus (Hercules, CA) in 1.3% SeaKem HGT agarose at 14°C with pause ramping from 10 to 22 s for 14 h and from 55 to 60 s for 8 h at field strength of 6 V/cm. Gels were stained with Vistra green (Amersham Biosciences) and scanned by using a Storm phosphorimager. The data was analyzed by using commercially available software (BioNumerics). The sequenced E. coli strain CFT-073 was used as the internal control for creating a dendrogram based on PFGE types.
ERIC-PCR and automated ribotyping (AR).
Ribotyping was performed by using the RiboPrinter microbial characterization system from Qualicon (Wilmington, DE). This automated typing system produces a RiboPrint pattern using an E. coli rRNA probe hybridized to restriction enzyme-digested chromosomal DNA. E. coli strains were digested using EcoRI enzyme based on the manufacturer's instructions. Ribotype groups were defined by the RiboPrinter system, which assigns ribogroups by comparing differences in band number, position, and signal intensity (19).
PCR amplifications of ERIC sequences were performed on E. coli strains using a modification of a protocol described previously (31). ERIC patterns were evaluated by using BioNumerics software from Applied Maths (Kortrijk, Belgium) (16, 31). Briefly, similarity matrices were constructed on the basis of Pearson correlation coefficient analysis of pairwise comparisons of ERIC patterns. We performed clustering analysis and constructed a dendrogram with the unweighted pair group method using arithmetic averages based on the similarity matrices. Strains with more than 90% similarity were placed in the same ERIC group.
Microarray LOS arraying and hybridizations.
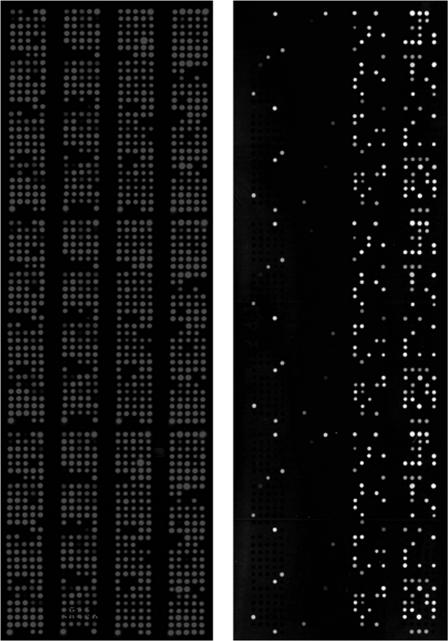
Genomic DNA (target) was purified from bacterial strains by using a QIAGEN genomic DNA purification kit according to the manufacturer's recommendations, sonicated, and centrifuged, and supernatants were arrayed and hybridized according to previously published protocols (33). Cy3 and Cy5 fluorescence- and biotin-labeled probes were generated from SJX206 and the 16S RNA housekeeping genes by using the BioPrime DNA labeling system (Invitrogen) and appropriate deoxynucleoside triphosphate mixtures. The probes were hybridized to glass slides that were previously arrayed with purified genomic DNA from 106 bacterial isolates in triplicate on Superamine glass slides (Telechem), and the hybridization signals were detected by using a Versarray Chipreader (Bio-Rad). The signal intensity of each spot was normalized to the signal intensity of the 16S RNA probe (housekeeping gene) to account for differences in genomic DNA concentrations at different spots and compared to the intensity of the positive control (sequence strain known to contain the gene probe) to determine the presence or absence of the sPCR fragment in different bacterial strains (see Fig. 6). Since LOS is a high-throughput microarray-based dot blot hybridization platform, we use the criteria established previously to determine probe positive cutoffs in dot blot hybridization to determine the positive cutoff points for LOS (32).
FIG. 6.
PHAT in an LOS microarray format.
Simpson's index of diversity.
We calculated an index of discrimination based on the probability that two unrelated strains sampled from the test population will be placed into distinct typing groups (12). This value can be calculated as Simpson's diversity index (D) by the following equation:
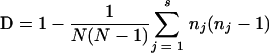 |
where N is the total number of strains in the sample population, s is the total number of types described, and nj is the number of strains belonging to the jth type.
Statistical entropy.
To determine the optimal number of probes required for PHAT typing, we calculated the entropy for the first probe and then calculated the entropy iteratively as more probes were added to the PHAT probe set. Entropy (E) is calculated as follows: E = p1 × log(p1) + p2 × log(p2) + … pk × log(pk), where pk is the contribution of the kth PHAT signature to the total entropy (22). A binary PHAT signature was generated by collating the presence or absence of different sPCR fragments (Table 1) . The occurrence of each unique PHAT signature in the collection was determined as a percentage of the total frequency of all PHAT signatures. This established the contribution of entropy of each unique PHAT signature to the total entropy for a given probe set. The total entropy calculation was repeated iteratively as additional PHAT probes were added to maximize the discrimination with a minimal number of probes for isolates in this collection.
TABLE 1.
PHAT probe candidates used for the calculation of Simpson's diversity index and entropy calculationsa
| Fragment | Prevalence (%) | No. of rectal isolatesb | Homology | Locus identifier |
|---|---|---|---|---|
| sJX198 | 43.7 | 547 | Putative C4-dicarboxylate-binding periplasmic protein in CFT073 | NP_757279 |
| sJX210 | 44.6 | 547 | Protein YjgK from CFT073 | NP_757200 |
| sSU32 | 40.0 | 88 | Hypothetical protein from CFT073 | NP_755106 |
| sJX76 | 49.0 | 547 | Hypothetical outer membrane usher protein precursor from CFT073 | NP_756076 |
| sJX83 | 50 | 88 | Putative iron compound receptor from CFT073 | NP_755646 |
| sJX150 | 53.0 | 547 | No known homology | |
| sRB19 | 54.3 | 313 | Conserved hypothetical protein from Salmonella enterica serovar Typhi strain CT18 | NP_458920 |
| sLZ13 | 42.7 | 350 | Usher protein | |
| sJX80 | 40.9 | 88 | Hypothetical protein YadM precursor from CFT073 | NP_752119 |
| sJX206 | 54.7 | 547 | Nucleoside-specific channel-forming protein TSX in CFT073 | NP_756748 |
| sJX208 | 57.8 | 547 | Putative conserved protein from CFT073 | NP_751977 |
Probes are listed in the order used for the entropy calculation.
Data analysis.
All analyses were done using SAS v8.0. Access (Microsoft, Redmond, WA) was used for data entry. Software packages from DNAStar (Madison, WI) were used for primer design, DNA sequence comparison, and analysis.
RESULTS
Selection of sPCR probes for PHAT analysis.
We identified 11 candidate probes from sPCR fragments generated from four genomic subtraction experiments with a uropathogenic E. coli strain as the tester and a nonuropathogenic E. coli strain as the driver. Genomic subtraction generates a library of candidate gene sequences that are present in one bacterial strain (tester) and absent in another (driver). Probes present in 40 to 60% of a preliminary sample of rectal E. coli strains from different study populations were considered candidates for PHAT typing, since these provide maximum discriminatory information between strains.
We did a pairwise comparison of the association between the prevalences of each probe in the rectal E. coli sample and all possible combinations of probes. If the association (as estimated by the odds ratio) between two probes exceeded 1.8 and was statistically significant by the chi-square test, the one with the higher prevalence was selected for inclusion in order to reduce redundancy among the probes selected for PHAT typing (data not shown). The final list of candidate probes is shown in Table 1.
Comparison of phylogenetic groupings based on PHAT, PFGE typing, ERIC-PCR typing, and AR.
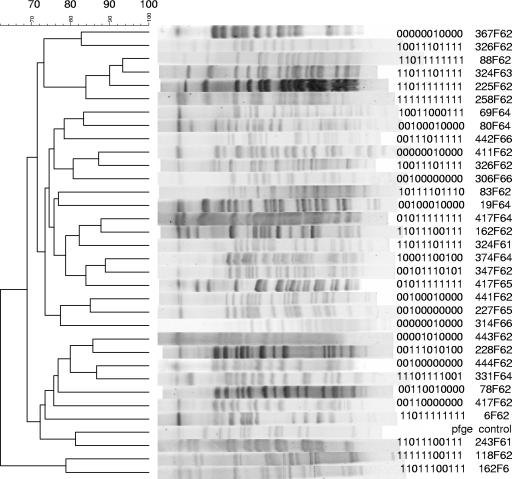
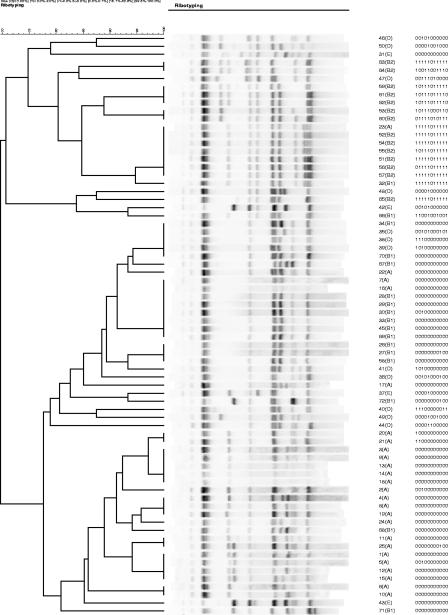
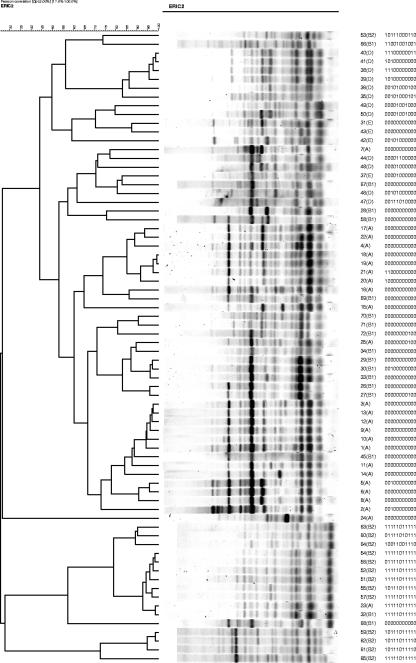
Thirty-three rectal strains from otherwise healthy women with UTI were typed by using PFGE (Fig. 1). We identified 25 pulsotypes (groups by PFGE) using 85% similarity as the cut-point. Note that some strains that are >90% similar by PFGE; for example, 88F62 and 324F63, the third and fourth strains from the top, have a single probe difference in PHAT signature. In contrast, 6F62 (fifth from the bottom of the dendrogram) has a PHAT type identical to that of 88F62, although it is considered quite distant from 88F62 by PFGE. The 72 ECOR strains were also typed by AR and ERIC-PCR (Fig. 2 and 3) and clustered based on their AR and ERIC-PCR types, respectively. A number of strains that were grouped similarly by PHAT and AR had the least resolved PHAT signature (00000000000). ERIC typing gave similar results; for example, ECOR strains 30 and 5, which are only one probe different by PHAT (00100000000), were determined to be more distant by ERIC (<70% similarity), whereas, in contrast, ECOR strains 20 and 21 are only one probe different by PHAT (11000000000 and 10000000000) and >90% similar by ERIC.
FIG. 1.
PFGE and PHAT analysis of 33 rectal E. coli strains. Clustering was constructed using PFGE data.
FIG. 2.
AR analysis of 72 strains from the E. coli reference collection (ECOR). The clustering dendrogram was constructed using AR data. PHAT signatures are shown adjacent to the ECOR strain names and phylogenetic groups.
FIG. 3.
ERIC-PCR analysis of 72 strains from the E. coli reference collection (ECOR). The clustering dendrogram was constructed using ERIC-PCR data. PHAT signatures are shown adjacent to the ECOR strain names and phylogenetic groups.
Discriminatory power of PHAT compared to those of MLST, AR, ERIC-PCR typing, and PFGE typing.
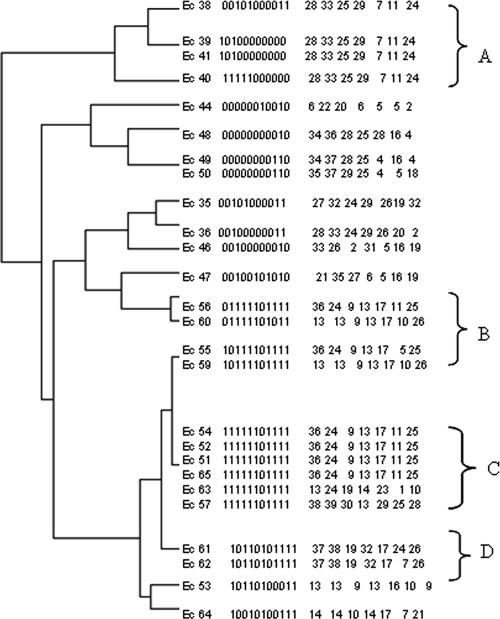
We compared the discriminatory power of PHAT to those of MLST, automatic ribotyping, ERIC-PCR, and PFGE, as expressed by the Simpson diversity index (Table 2). In the set of 33 human rectal strains that we examined, the discriminatory power of PHAT equaled that of MLST and PFGE (98%). PHAT typed the 33 rectal strains into 26 groups, while MLST and PFGE typed them into 25 and 26 groups, respectively (shown in Fig. 1). In the ECOR collection, which is a diverse set of E. coli strains from humans and nonhuman sources, PHAT was less discriminating than MLST, AR, and ERIC-PCR (80% versus 97, 92, and 97%, respectively). Since the PHAT probe set was identified from human E. coli, we reasoned that the lower discrimination resulted from the nonhuman strains found in ECOR. To test this hypothesis, we calculated Simpson's diversity index for ECOR strains belonging to B2 and D phylogenic groups, a lineage found frequently in human pathogenic E. coli (31). For this subset, PHAT had a discriminatory power similar to that of MLST (94% versus 95%). Figure 4 shows the PHAT typing of ECOR strains belonging to the B2 and D phylogenetic groups, along with their MLST sequence types. PHAT was able to subtype strains of the B2/D group with a similar or the same ST in some cases, as seen in clusters A, B and C. In contrast, PHAT grouped strains with the same or similar STs into one group, as seen in clusters C and D in Fig. 4.
TABLE 2.
Discriminatory power of PHAT, a binary typing method, compared to other genotyping techniques among the E. coli reference collection (ECOR) and a collection of human rectal isolates as determined by using Simpson's diversity index
| Collection (n)a | Typing method | Simpson diversity index (D)b | No. of groups | Avg no. of strains/group |
|---|---|---|---|---|
| ECORc (72) | PHAT | 80 | 25 | 3.1 |
| MLST | 97 | 42 | 1.5 | |
| AR | 92 | 36 | 2.0 | |
| ERIC-PCR | 97 | 43 | 1.6 | |
| ECOR isolates | PHAT | 94 | 17 | 1.4 |
| of B2 and D | MLST | 95 | 18 | 1.4 |
| lineage (26) | ||||
| Rectald (33) | MLST | 98 | 25 | 1.3 |
| PFGE | 98 | 26 | 1.2 | |
| PHAT | 98 | 26 | 1.2 |
n, number of strains in the study.
The Simpson's diversity index (D) was calculated as 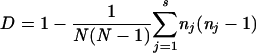 where N is the total number of strains in the sample population, s is the total number of types described, and nj is the number of strains belonging to the jth type (6).
where N is the total number of strains in the sample population, s is the total number of types described, and nj is the number of strains belonging to the jth type (6).
ECOR is a collection of 72 strains isolated from a variety of hosts and geographical locations (http://foodsafe.msu.edu/Whittam/ecor/). MLST data for ECOR isolates were obtained from www.mlst.net.
The rectal strains were randomly selected from E. coli collected from women aged 18 to 39 years with a first UTI, collected from the student health services of the University of Michigan.
FIG. 4.
PHAT analysis of 26 strains from the E. coli reference collection (ECOR) belonging to B2/D phylogenetic groups. The clustering dendrogram was constructed using PHAT signatures. MLST types are shown adjacent to the PHAT signatures.
Statistical entropy calculations.
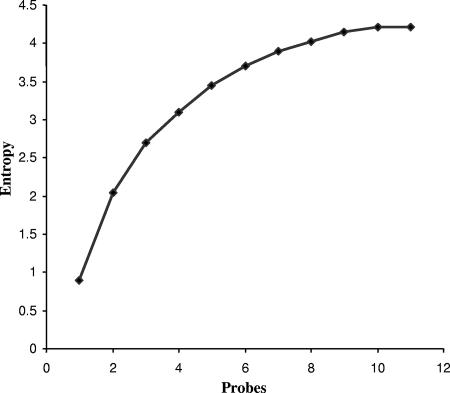
We determined the relationship between statistical entropy and the number of probes added for PHAT for the set of 106 rectal strains (Fig. 5). In Fig. 5, the probes were added in the order shown in Table 1. As the number of probes is increased, there is an initial linear gain in statistical entropy, followed by a gradual plateau. The change in entropy parallels the change in the Simpson's diversity index. Changing the order of probes resulted in a change in the calculated statistical entropy for each probe added but did not change the maximum entropy (data not shown). We observed a similar pattern when we repeated this calculation for PHAT of ECOR strains. For both the B2/D subset of ECOR strains and the rectal test set, entropy starts to level off around the fourth probe (diversity index values of 85 and 72% for the ECOR and rectal strains, respectively), indicating that the addition of further probes from this set will not substantially increase the discriminatory power.
FIG. 5.
Statistical entropy by number of probes used in PHAT in a collection of 106 rectal strains.
LOS microarray hybridizations.
To demonstrate the potential of using PHAT in a high-throughput format, we used PHAT probe sJX206 and housekeeping gene probe 16S RNA on replicate spots of ECOR (n = 72) and rectal (n = 106) strains using the LOS microarray format for DNA-DNA hybridizations (Fig. 6). The right side of the slide is arrayed with ECOR and rectal strains in randomized order. The spot intensities for the sJX206 probe were normalized to the 16S RNA probe to account for differences in DNA concentrations. These intensities were further normalized to the positive control (CFT073) to determine sJX206-positive and -negative strains. The normalized signal intensities for all strains are plotted between slides to determine probe-positive and probe-negative strains according to a previously published protocol (32).
DISCUSSION
PHAT, a binary typing method, offers a high-throughput alternative for bacterial strain typing. PHAT is based on using multiple genes as genetic markers, making it particularly suitable for determining relatedness between strains. On a set of 33 human E. coli strains, PHAT and MLST demonstrated similar discriminatory powers (Simpson's diversity index of 98%). By carefully selecting for probes, a high degree of discriminatory power can be obtained using a relatively small set of probes.
Sequence-based methods such as MLST use the variation within housekeeping loci to determine evolutionary relatedness within strains. Sequence variation in housekeeping genes is more likely to reflect phylogenetic descent than genes whose products are under selection. Thus, MLST is suitable for establishing evolutionary patterns in long-term global studies but less so for discriminating closely related strains (6) or strains involved in pathogenesis and antibiotic resistance. As for Streptococcus pneumoniae, invasive disease is rare for E. coli compared to the frequency of asymptomatic colonization, and MLST genotypes do not always correlate with virulence potential (5). Furthermore, even for MLST, the level of discrimination depends on the number of loci and the degree of allelic variation present in the population (6). For example, MLST lacks the discriminatory power required to distinguish between pathogenic strains of Listeria monocytogenes; in a recent study, more rapidly evolving virulence-associated genes were used to increase discriminatory power (34). Supplementing MLST by including sequence variation in multiple hypervariable loci also increases the discriminatory power of MLST (7, 20). In PHAT, many strains are screened for a few genes, and all strains are scored as 0/1 for each of the genes tested. By expanding the number of probes in the PHAT probe set, the discriminatory power of PHAT can be optimized to differentiate closely related strains.
PHAT resolution was at least as good as PFGE when we compared human rectal strains typed by both methods. However, the classifications of strains were different by the two systems. Strains that were determined to be similar by PFGE were not always classified in the same PHAT group and vice versa. Thus, the underlying genetic differences in the E. coli strains revealed by PHAT and PFGE are different. This is of critical importance in deciding which typing method to use. For example, integration of horizontally acquired genes will result in a change in the banding pattern obtained from PFGE but will be less likely to change the PHAT type, unless one or more of the newly integrated genes are included in the PHAT probe set. Analyzing the differences between closely related PHAT types provides more information about the genetic basis of differences between two strains than does PFGE; for example, we can determine whether strains are related by the loss or gain of mobile genetic element such as one conferring antimicrobial resistance.
A challenge of binary typing is determining the best candidate probe set to get maximum discriminatory power using the least number of probes. The minimum probe set is a function of the study population. For example, the PHAT probes in the present study were developed for human strains of E. coli. In that population discrimination was excellent (D = 94%); however, in the ECOR collection, which consists of E. coli strains from different organisms, serotypes, geographic regions, and phylogeny, discrimination was less (D = 80%). Adding additional probes specific to the diverse species found in ECOR would undoubtedly increase the discriminatory power for PHAT in ECOR.
The discriminatory power observed with PHAT is also influenced by the number of strains to be typed. In theory, an array consisting of ‘n’ probes can result in 2n signatures, but the number of strains and the nature of probes will dictate the actual number of observed signatures. As the number of strains increases, more “unique” PHAT signatures get populated, resulting in a bigger increase in discriminatory power. To maximize the discriminatory power attainable for a larger set of strains, additional probes may be added. The choice of probes is critical to increasing the discriminatory power of PHAT. Probes that appear frequently across strains in a small study and contribute minimally to the discriminatory power of PHAT may still prove to be useful in a more global epidemiologic setting. An analogy can be found in the coa and spa typing of methicillin-resistant S. aureus strains, where the less discriminating coa typing reveals the relatedness of clonal groups of methicillin-resistant S. aureus strains from temporally and geographically diverse locations (21).
Optimal PHAT probes provide unambiguous results (32). Some probes have a high degree of nonspecific binding and background signal, probably due to the degree of sequence homology with other ORFs. In such cases, probe-positive and probe-negative strains are hard to determine accurately; we excluded such probes from our PHAT set. One of the sPCR fragments initially included, sRB33, was later replaced due to high levels of cross-hybridization with other strains.
In conclusion, binary typing for bacterial strain classification, such as PHAT, provides a high-resolution, direct method that measures the presence or absence of genetic content, and the binary output can be easily formatted in large databases, allowing for data storage and portability. PHAT is a reproducible, cost-effective, and time-effective means for fine discrimination and for identifying short-term outbreaks and person-to-person transmission. Since PHAT relies on the presence or absence of genes determined by dot blot hybridization, it can be easily adapted to a high-throughput LOS microarray format wherein thousands of strains can be typed simultaneously (33). The efficiency gained through the implementation of the microarray dramatically increases the efficiency of the typing process, reducing the cost and time required to type large numbers of strains. When hypervariable loci are used as probes, PHAT complements the basic clonal assignments at a population level from MLST (1, 2). In the long term, PHAT in conjunction with MLST may lead to a more complete picture of strain variations within the context of a slowly evolving core genome.
Acknowledgments
We thank Patricia Tallman and Joan DeBusscher for the isolation and purification of E. coli strains and Richard Bauer for sRB19. We also thank Brian DeHaven for assisting with dot blot hybridizations, Sarah Jaden for providing the distribution data for probe sSU32, and Maneesh Dave for help with the MLST analysis.
This study was supported by an award from the National Institutes of Health (grant RO1 DK55496 to C.F.M.).
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Achtman, M. 2002. A phylogenetic perspective on molecular epidemiology, vol. 1. Academic Press, London, England.
- 2.Achtman, M., and G. Pluschke. 1986. Clonal analysis of descent and virulence among selected Escherichia coli. Annu. Rev. Microbiol. 40:185-210. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, R. J. 2003. Molecular epidemiologic discovery of uropathogenic Escherichia coli virulence and transmission factors. Ph.D. thesis. University of Michigan, Ann Arbor.
- 4.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 1521. Molecular epidemiology of three putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroN (E. coli). J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 5.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, J. E., and E. J. Feil. 2004. Multilocus sequence typing—what is resolved? Trends Microbiol. 12:373-377. [DOI] [PubMed] [Google Scholar]
- 7.Feavers, I. M., S. J. Gray, R. Urwin, J. E. Russell, J. A. Bygraves, E. B. Kaczmarski, and M. C. Maiden. 1999. Multilocus sequence typing and antigen gene sequencing in the investigation of a meningococcal disease outbreak. J. Clin. Microbiol. 37:3883-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foxman, B., S. D. Manning, P. Tallman, R. Bauer, L. Zhang, J. S. Koopman, B. Gillespie, J. D. Sobel, and C. F. Marrs. 2002. Uropathogenic Escherichia coli are more likely than commensal E. coli to be shared between heterosexual sex partners. Am. J. Epidemiol. 156:1133-1140. [DOI] [PubMed] [Google Scholar]
- 9.Foxman, B., L. Zhang, K. Palin, P. Tallman, and C. F. Marrs. 1995. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J. Infect. Dis. 171:1514-1521. [DOI] [PubMed] [Google Scholar]
- 10.Hommais, F., S. Pereira, C. Acquaviva, P. Escobar-Paramo, and E. Denamur. 2005. Single nucleotide polymorphism phylotyping of Escherichia coli. Appl. Environ. Microbiol. 71:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulton, C. S., C. F. Higgins, and P. M. Sharp. 1991. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol. Microbiol. 5:825-834. [DOI] [PubMed] [Google Scholar]
- 12.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard, E. E., T. Takata, M. J. Blaser, S. Falkow, L. S. Tompkins, and E. C. Gaynor. 2003. Use of an open-reading frame-specific Campylobacter jejuni DNA microarray as a new genotyping tool for studying epidemiologically related isolates. J. Infect. Dis. 187:691-694. [DOI] [PubMed] [Google Scholar]
- 14.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrs, C. F., L. Zhang, P. Tallman, S. D. Manning, P. Somsel, P. Raz, R. Colodner, M. E. Jantunen, A. Siitonen, H. Saxen, and B. Foxman. 2002. Variations in 10 putative uropathogen virulence genes among urinary, faecal, and peri-urethral Escherichia coli. J. Med. Microbiol. 51:138-142. [DOI] [PubMed] [Google Scholar]
- 16.Meacham, K. J., L. Zhang, B. Foxman, R. J. Bauer, and C. F. Marrs. 2003. Evaluation of genotyping large numbers of Escherichia coli isolates by enterobacterial repetitive intergenic consensus-PCR. J. Clin. Microbiol. 41:5224-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelludat, C., R. Prager, H. Tschape, W. Rabsch, J. Schuchhardt, and W. D. Hardt. 2005. Pilot study to evaluate microarray hybridization as a tool for Salmonella enterica serovar Typhimurium strain differentiation. J. Clin. Microbiol. 43:4092-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettigrew, M. M., B. Foxman, Z. Ecevit, C. F. Marrs, and J. Gilsdorf. 2002. Use of pulsed-field gel electrophoresis, enterobacterial repetitive intergenic consensus typing, and automated ribotyping to assess genomic variability among strains of nontypeable Haemophilus influenzae. J. Clin. Microbiol. 40:660-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson, D. A., and M. C. Enright. 2003. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3926-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shopsin, B., M. Gomez, M. Waddington, M. Riehman, and B. N. Kreiswirth. 2000. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 38:3453-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shumei, J., C. Tang, L. Zhang, A. Zhang, and M. Ramanathan. 2000. First SIAM International conference on data mining. http://www.cs.buffalo.edu/pub/WWW/DBGROUP/bioinformatics/papers/MaxEnt.pdf.
- 23.Srinivasan, U., B. Foxman, and C. F. Marrs. 2003. Identification of a gene encoding heat-resistant agglutinin in Escherichia coli as a putative virulence factor in urinary tract infection. J. Clin. Microbiol. 41:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Leeuwen, W., A. van Belkum, B. Kreiswirth, and H. Verbrugh. 1998. Genetic diversification of methicillin-resistant Staphylococcus aureus as a function of prolonged geographic dissemination and as measured by binary typing and other genotyping methods. Res. Microbiol. 149:497-507. (Erratum, 149:775.) [DOI] [PubMed] [Google Scholar]
- 26.van Leeuwen, W., H. Verbrugh, J. van der Velden, N. van Leeuwen, M. Heck, and A. van Belkum. 1999. Validation of binary typing for Staphylococcus aureus strains. J. Clin. Microbiol. 37:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel, L., E. van Oorschot, H. M. Maas, M. B., and L. Dijkshoorn. 2000. Epidemiologic typing of Escherichia coli using RAPD analysis, ribotyping and serotyping. Clin. Microbiol. Infect. 6:82-87. [DOI] [PubMed] [Google Scholar]
- 28.Xie, J., B. Foxman, L. Zhang, and C. F. Marrs. 2006. Molecular epidemiologic identification of Escherichia coli genes that are potentially involved in movement of the organism from the intestinal tract to the vagina and bladder. J. Clin. Microbiol. 44:2434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zadoks, R., W. van Leeuwen, H. Barkema, O. Sampimon, H. Verbrugh, Y. H. Schukken, and A. van Belkum. 2000. Application of pulsed-field gel electrophoresis and binary typing as tools in veterinary clinical microbiology and molecular epidemiologic analysis of bovine and human Staphylococcus aureus isolates. J. Clin. Microbiol. 38:1931-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, L., B. Foxman, S. D. Manning, P. Tallman, and C. F. Marrs. 2000. Molecular epidemiologic approaches to urinary tract infection gene discovery in uropathogenic Escherichia coli. Infect. Immun. 68:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, L., B. Foxman, and C. Marrs. 2002. Both urinary and rectal Escherichia coli isolates are dominated by strains of phylogenetic group B2. J. Clin. Microbiol. 40:3951-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, L., B. W. Gillespie, C. F. Marrs, and B. Foxman. 2001. Optimization of a fluorescent-based phosphor imaging dot blot DNA hybridization assay to assess Escherichia coli virulence gene profiles. J. Microbiol. Methods 44:225-233. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, L., U. Srinivasan, C. F. Marrs, D. Ghosh, J. R. Gilsdorf, and B. Foxman. 2004. Library on a slide for bacterial comparative genomics. BMC Microbiol. 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, W., B. M. Jayarao, and S. J. Knabel. 2004. Multi-virulence-locus sequence typing of Listeria monocytogenes. Appl. Environ. Microbiol. 70:913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]