Coccidioidomycosis was first described in Argentina in 1892 by Alexandro Posadas (4). Robert Wernecke confirmed the syndrome in the same year and, together with Posadas, discerned that the etiologic agent was infectious. Rixford and Gilchrist formally described the organism in California, and thinking the organism to be a coccidian protozoan, they named it Coccidioides immitis Rixford and Gilchrist 1896. In 1900, Öphuls and Moffitt recognized it to be a fungus (4).
MYCOLOGY
The Coccidioides genus is considered dimorphic. Dimorphism is characterized by production of filamentous (mycelial) forms by certain fungi during their saprophytic phase in the environment or when incubated at lower temperatures on media. Conversion to yeast-like cellular forms occurs during their parasitic phase when invading an animal host or when incubated at elevated temperatures (11). Dimorphism in the genera Blastomyces, Histoplasma, Paracoccidioides, and Sporothrix, as well as in a single species of Penicillium (P. marneffei), is temperature dependent (thermal dimorphic). Dimorphism in Coccidioides is also characterized by the production of septate hyphae and thick-walled arthroconidia (2 by 4 μm) that are formed along the length of the hyphae (enteroarthric development) during the saprophytic phase (6). The arthroconidia are commonly separated by empty, thin-walled, brittle cells (disjunctors) formed by autolysis of alternate conidia along the hyphae. Thus, arthroconidia are easily released into the air by soil disruption and wind. Upon inhalation into lungs or on rare occasion after percutaneous implantation into tissue, each arthroconidium transforms into a new multinucleated, spherical structure called the spherule. This structure increases in size and forms a thick outer wall. As it grows, the spherule begins to divide internally by invagination and formation of cleavage furrows, culminating in the production of numerous uninucleated endospores. Spherules vary from 60 to over 100 μm in diameter, while endospores remain 2 to 5 μm in size. Mature spherules may contain 800 to 1,000 endospores, which are released into tissue upon the spherule's rupture. In its parasitic phase, each endospore grows into a new spherule (1, 11). This phase of Coccidioides is influenced by more complex factors, including presence of phagocytic cells and increase in CO2, rather than temperature (10).
Until the 1990s, the genus Coccidioides was thought to have only one species. No teleomorph (sexual form) has been described. Molecular phylogenetic methods have since shown that there are definite differences between isolates originating from diverse geographic locations, such as California (CA), Arizona (AZ), Texas (TX), Mexico, and South America (SA). The strains fall into two phylogenetic clades representing separate evolutionary species. One group (Group II) contains isolates originating from CA, while a second group (Group I) contains isolates from outside of CA (non-CA), inclusive of those from AZ, TX, Mexico, and SA. These clades have now been given species rank (4). The CA group (Group II) retained the species name of C. immitis, while the non-CA group (Group I) was named C. posadasii Fisher, Koenig, White, et Taylor, sp. nov. (2, 4).
EPIDEMIOLOGY
Coccidioides spp. are found in the hot, dry regions of the southwestern United States, where winters are relatively mild and the soil is alkaline (6, 11). They have been associated with the Lower Sonora Life zone. Recent studies have found a wider diversity of habitat characteristics in areas associated with the fungus. The presence of fine sand and silt in the soil is the single characteristic common in all areas in which the organism is found (F. Fisher, M. Bultman, S. Johnson, D. Pappagianis, and E. Zabovsky, Abstr. 6th Int. Symp. Coccidioidomycosis, abstr. 17, 2006). Coccidioides spp. are most highly concentrated in the San Joaquin Valley of CA (Kern County) and in south-central AZ. The major burden of coccidioidomycosis falls on AZ and CA, with greater than 95% of all cases reported being from those two states. The organism is also found in smaller numbers in southern New Mexico, TX, northern Mexico, and areas of SA. It has been reported sporadically in areas of Utah and Nevada (6).
The incidence of coccidioidomycosis is increasing. Progressively greater numbers of cases are being reported to the Arizona Department of Health Services (ADHS); 2,695 cases were reported in 2003, and 3,515 were reported in 2005. Preliminary data indicate that 3,036 cases were reported to the ADHS in the first 6 months of 2006 (ADHS, personal communication).
Clinical infection can occur in any age group, most commonly 30- to 75-year-olds in AZ. Increased risk of infection is associated with outdoor activity. Incidence may be dependent on interspersed seasonal precipitation, severity of wind and dust storms, continued regional influx of susceptible hosts, and disruption and aerosolization of the desert surface by construction, wildfires, and earthquakes (6, 11). Commonly, two peak periods of activity (spring and end of summer) occur in AZ, and one (end of summer) occurs in CA. Factors governing the recent increases in coccidioidomycosis are environmental rather than genetic. Business and tourist travel to areas of endemicity is increasing the chance of patients being diagnosed with coccidioidomycosis outside such areas. With as many as 150,000 new cases being estimated to occur each year, travel history should always be sought in the evaluation of patients (3, 5).
CLINICAL PRESENTATION AND COURSE
The spectrum of illness due to Coccidioides spp. is very broad and is predominantly driven by host defenses, inoculum size, and possibly specific organism virulence or resistance factors that are not well understood. About 60% of clinical infections occur with few or no respiratory symptoms. The 40% of patients that are symptomatic may present with an acute or subacute spectrum of illness, ranging from “flu-like” to progressive pneumonia. Most commonly, a self-limited “community-acquired pneumonia” occurs and is often misdiagnosed. Valdivia and colleagues reported that approximately 29% of 56 patients presenting with community-acquired pneumonia to a clinic in southern AZ were diagnosed with coccidioidomycosis (12).
Symptoms usually begin within 7 to 21 days of inhalation of arthroconidia. Symptomatic patients complain of fever, cough, chest discomfort, malaise, and fatigue. Twenty percent of primary coccidioidal pneumonia is associated with headache. Symptoms generally last less than 3 weeks, although protracted fatigue may become prominent. Transient skin manifestations, including rash and erythema nodosum, may be seen in 10% to 50% of patients. About 25% of cases of pulmonary disease appear similar to many community-acquired pneumonias, with pleuritic pain, cough (usually nonproductive), fever, arthralgias, and myalgias. Empirical therapy for presumed bacterial pneumonia is often given, and the natural course of symptom resolution deceives the clinician. Progressive pneumonia may involve the pleura and necrosis with cavitation. Problems occur if steroids are used or underlying host-compromising conditions exist. The majority of patients with extensive pulmonary involvement do not have extrapulmonary disease. The evaluation of the solitary pulmonary nodule is more complex when a past history of coccidioidomycosis is elicited. Such pulmonary nodules may mimic neoplasia and are usually serologically negative (1).
Disseminated coccidioidomycosis is estimated to occur in less than 5% of symptomatic patients and probably less than 1% of all infections. Patients of black or Asian (especially Filipino) ethnic backgrounds, pregnant women in the third trimester, and any immunocompromised patients appear to be at significant risk for disseminated disease. Dissemination may occur months to several years after the primary infection. The general role of the immune system in handling coccidioidal antigen is recognized, but it is unclear why some patients have few or no symptoms and others have disseminated disease. Host genes and in particular HLA class II and ABO blood group may play a role in dissemination and severity of infection.
Dissemination of spherules and endospores via the lymphatics and the bloodstream may occur to any organ system, but skin, lymph nodes, and the skeletal system are primarily involved. Meningeal disease is less common. The gastrointestinal tract is rarely involved. Infection after traumatic percutaneous inoculation with arthroconidia occurs rarely, mimicking cutaneous nocardiosis or sporotrichosis.
LABORATORY DIAGNOSIS
Hematology.
Elevated erythrocyte sedimentation rate and eosinophilia may be seen in coccidioidomycosis. Eosinophilia especially should heighten suspicion.
Direct detection. (i) Microscopy.
In immunocompetent patients, inflammatory changes normally occur in two phases, early and late. The early phase includes a mixed acute reaction consisting of an influx of polymorphonuclear neutrophils and, to a far lesser degree, granulomatous cells (13). Tissue eosinophilia may be present and may be seen surrounding the offending organism (Splendore-Höeppli phenomenon). As endospores mature into spherules, a granulomatous reaction predominates, with an influx of lymphocytes, plasma cells, and multinucleated giant cells. Mixed inflammatory reactions may occur as spherules release endospores, thereby precipitating the reoccurrence of a polymorphonuclear neutrophil response.
Coccidioidomycosis can be diagnosed microscopically by visualization of endospore-containing spherules in infected material. Potassium hydroxide (KOH) wet mounts are useful and readily available for microscopic evaluations but lack the sensitivity and specificity of several other staining methods. The calcofluor white (CFW) fluorescent stain affords the best ability to detect fungi by binding the chitin and cellulose found in their cell walls (13). In an observation of 374 culture-positive specimens in the Phoenix area, the overall sensitivity of the CFW stain was 22% (D. Sussland and M. A. Saubolle, unpublished data). CFW stains plant and fatty material nonspecifically. Reading the stain, therefore, requires well-trained personnel and strict attention to morphological detail.
The Grocott-methenamine silver stain is most sensitive in detecting fungi in histopathological preparations. However, it may overstain fungal material (potentially masking internal structures such as endospores within spherules) and tissue elements (e.g., mucus droplets and glycogen granules), as well as some bacteria. Other commonly used histological stains, such as the periodic acid-Schiff stain and the hematoxylin-eosin stain, are not considered to be as sensitive as the Grocott-methenamine silver stain. Occasionally, the Giemsa, Papanicolau, and mucicarmine stains may differentiate Coccidioides organisms in specimen preparations (13). The Gram stain does not normally stain Coccidioides spp. and should not be used as a primary screening method.
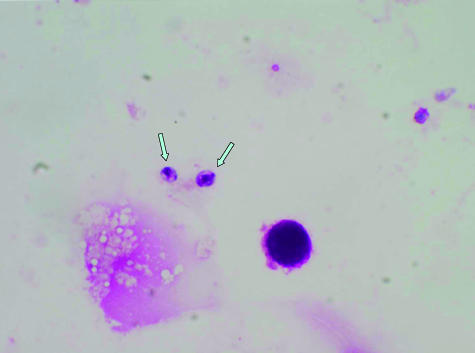
The presence of endospore-containing spherules is diagnostic of coccidioidomycosis. However, mycelial forms may also be observed in specimens collected from peripheries of cavitary or skin lesions; without the presence of spherules, these do not provide the identity of the fungus. At times, endospores may predominate as the morphological form in specimens and may be mistaken for yeast forms of Histoplasma, Cryptococcus, or Candida (Fig. 1). Small, immature spherules abutting each other may be confused with budding yeast forms of Blastomyces (11, 13).
FIG. 1.
Endospores seen in a Wright's stain of cerebrospinal fluid of a patient with coccidioidal meningitis. Magnification, ×1,000.
(ii) Molecular detection.
Although direct molecular probes are not available at this time, other nucleic acid amplification tests have recently been introduced for the direct detection of Coccidioides spp. in specimens. Nucleic acid amplification tests using PCR have been described by a number of noncommercial laboratories and found to be both sensitive and specific. The availability and full role of PCR in the diagnosis of coccidioidomycosis remain to be ascertained. Real-time PCR and repetitive-sequence-based PCR are likely to become important diagnostic methods.
Culture. (i) Safety.
Arthroconidia pose high risk in the clinical laboratory. Safety features for the mycology laboratory should at the minimum include negative air pressure in the work area, biosafety level 2 practices, presence of a class II biological safety cabinet, and procedures and practices delineating standard precautions. Some suggest that biosafety level 3 practices be maintained in the mycology laboratory. Care must be observed in the routine laboratory as well, since unsuspected Coccidioides sp. isolates grow well on routine bacterial media.
(ii) Select agent status.
Both species of Coccidioides are considered by the federal government to be select agents of bioterrorism, and laboratories isolating such isolates must follow strict mandates (7). Laboratories registered with the government to handle select agents must follow rules for defined work practices, documentation and inventory controls of isolates, background checks of all involved employees, strictly restricted access to work areas, and monthly reporting to the CDC and must pay registration fees. Laboratories that are not registered must appropriately destroy all isolates and advise the CDC of each isolate and its disposition in writing within 7 days of identification. Identified isolates cannot be transferred for any reason without prior written permission from the CDC.
(iii) Isolation.
Coccidioides spp. can be grown on most fungal media (Sabouraud medium, inhibitory mold agar, and medium containing cycloheximide) and bacterial media (sheep blood and chocolate agar media as well as selective yeast extract medium used for Legionella) (11). Specimens containing mixed flora should always be plated onto selective medium as well, since Coccidioides spp. do not compete well with other bacteria or molds. Growth is usually recognizable within 4 to 5 days on most media. Study of 1,513 cultures positive for Coccidioides spp. over a 6-year period in the Phoenix area found the average days to detection to be 4.4 days (range, 2 to 16 days) (D. Sussland and M. A. Saubolle, unpublished data). The average days to detection for smear-positive specimens was 2.6 days shorter than for those with negative smears (3.9 days versus 6.5 days, respectively). Yet the most common day of detection in both groups was the fourth day. Young colonies (2 to 3 days after visually detectable growth) do not have arthroconidia. Colonies are usually white to buff but may assume a variety of colors, especially upon aging. Production of characteristic arthroconidia occurs as the colony grows and may be used to presumptively identify the mold. If spherules with endospores are noted on direct microscopy, further confirmation of the identity is unnecessary.
Confirmation of an isolate as Coccidioides spp. is best achieved using a molecular genus-specific genetic probe (GenProbe, San Diego, CA). The probe recognizes both species but does not differentiate between them. Nonviable controls may be maintained by inactivating previously confirmed isolates. Autoclaving and formalin should not be used to kill an isolate, as these might cause false negative reactions to occur with the probes. With the introduction of the molecular probe method of identification, older methods, such as immunodiffusion of exoantigen for lines of identity, propagation of the spherule phase in Converse medium, or mouse inoculation studies, are no longer commonly used (11).
It is difficult to phenotypically differentiate between C. immitis and C. posadasii. It has been noted that C. posadasii grows significantly faster at 37°C in vitro than does C. immitis (B. Barker, S. Statt, J. Galgiani, and M. Orbach, Abstr. 6th Int. Symp. Coccidioidomycosis, abstr. 9, 2006). However, it is clinically unnecessary to routinely differentiate between the two species at present, as the two seem to have almost identical clinical presentations and antifungal susceptibility profiles. This may change as more is learned about the two species.
Predictably, the respiratory tract provides the highest recovery rate in culture of Coccidioides spp. The experience in a mycology section located in Phoenix (Table 1) (D. Sussland and M. A. Saubolle, unpublished data) showed an overall recovery rate of 3.2% from all specimens submitted for fungal culture (n = 55,788) over a 6-year period and a respiratory tract rate of 8.3% (n = 10,372). Recovery rates dropped to 0.4% for blood cultures (n = 5,026) and 0.6% for urine cultures (n = 649). The central nervous system (CNS) also had a poor recovery rate (0.9%). The standard method for diagnosis of CNS coccidioidomycosis is serological rather than by culture.
TABLE 1.
Recovery of Coccidioides spp. by source at Laboratory Sciences of Arizona in the Phoenix metropolitan area, 1998 to 2003a
| Source | Total no. of cultures submitted | No. of Coccidioides sp. isolates | Recovery rate (%) |
|---|---|---|---|
| Respiratory tract | 10,372 | 861 | 8.3 |
| Urinary tract | 649 | 4 | 0.6 |
| Nonsterile body sites (other than respiratory tract and urinary tract) | 25,628 | 648 | 2.5 |
| Blood | 5,026 | 20 | 0.4 |
| Bone marrow | 267 | 7 | 2.6 |
| CNS | 2,280 | 20 | 0.9 |
| Other normally sterile body sites | 11,566 | 246 | 2.1 |
| Total | 55,788 | 1,806 | 3.2 |
These are the unpublished data of D. Sussland and M. A. Saubolle.
There is no standard for in vitro susceptibility testing of Coccidioides spp. In some animal models, testing of molds does not correlate with therapeutic outcomes (8). Thus, routine susceptibility testing of Coccidioides spp. is not indicated.
Serologies.
Cell-mediated immunity is protective in the host. Skin testing with fungus-specific antigen preparations (spherulin or coccidioidin) was a mainstay in surveillance studies of coccidioidomycosis activity. Unfortunately, the preparations are no longer available in the United States.
Humoral antibodies are not protective against Coccidioides spp. but reflect the organism's level of activity in the infected host and are used for the diagnosis and prognosis of the disease. The timing and magnitude of the antibody response are directly related to the integrity of the patient's immune system and to the specific clinical presentation of infection. The serologic response can simplistically be broken down into the production of early (immunoglobulin M [IgM]) and late (IgG) antibodies (9, 10, 14). Early IgM (often referred to as tube precipitin or TP) antibody becomes measurable within 1 (50% of cases) to 3 (90% of cases) weeks of onset. IgG (often referred to as complement fixation or CF) antibody becomes measurable sometime between the second and third weeks of onset or later (up to several months). IgG antibody may remain for months, and its titer is usually related to the degree of infection. Serologic studies may be compromised in patients with decreased immune response (e.g., transplant patients or those with human immunodeficiency virus infection).
Enzyme immunoassays (EIA) are now available for detection of both IgM and IgG and are seemingly the most sensitive. A negative result by EIA does not have to be confirmed by any of the other methods. Immunodiffusion (IMDF) methods are available for detection of IgM and IgG as well, but they require longer incubation periods (up to 4 days) to rule out negatives. The IMDF tests are normally qualitative but can be altered to quantitate titers. This may be useful in testing sera which show anticomplementary activity in CF studies. Quantitative CF studies for IgG are used primarily for monitoring the activity and prognosis of coccidioidomycosis. Increasing CF titers or those above 1:32 suggest heightened activity and possible dissemination. Because of its lessened sensitivity, the CF test should not be used as the sole diagnostic tool for coccidioidomycosis. The CF test is useful for the diagnosis of meningeal disease. Pleural and synovial fluids may be tested by the CF test, but their diagnostic efficacy is controversial. Different CF values may be obtained by laboratories using differing test methods and reagents; thus, results must be interpreted cautiously and only in the context of the whole clinical picture. In measuring infection activity, it is best to concurrently test antibody levels in sera collected sequentially over a time period (i.e., the most recent against a previous). There may also be significant discrepancies between EIA, IMDF, and CF study results; this is especially true between results of EIA and IMDF IgM studies.
In one retrospective analysis of EIA, IMDF, and CF tests on culture-positive patients during the acute disease phase, data showed an overall test sensitivity of only 82%. Thus, negative serologies do not rule out coccidioidomycosis, especially early in the disease phase. Individually, the studies showed sensitivities of 83% for EIA, 71% for IMDF, and 56% for CF (C. R. Pollage, E. Billetdeaux, A. Phansalkar, M. Litwin, and C. A. Petti, Abstr. Ann. Meet. Am. Soc. Microbiol., abstr. F-005, 2006). Although EIA IgM and IgG were the most sensitive methods, they may show less specificity than the other methods, especially if the IgM-specific test alone is reactive.
Wheat et al. recently reported that the histoplasma urine antigen test was reactive in approximately 50% of patients with systemic coccidioidomycosis (L. Wheat, T. Kuberski, A. Myers, M. Durkin, and P. Connolly, Abstr. 6th Int. Symp. Coccidioidomycosis, abstr. 20, 2006). This seems to be due to cross-reaction between the two antigens in the urine test. A more sensitive and specific urinary antigen test for Coccidioides spp. is presently being sought.
THERAPEUTIC APPROACHES
Since the majority of patients with coccidioidomycosis are asymptomatic and do well, therapy may not be necessary in most cases. Before the introduction of azole antifungals in the early 1980s, therapy of coccidioidomycosis was limited to the more toxic intravenous amphotericin B. The oral azole antifungals simplify the therapeutic decision. Most documented cases of pneumonia are treated early even though there are no prospective clinical trials to justify early azole therapy in healthy hosts. Amphotericin B or the newer lipid formulations of amphotericin are still used for serious coccidioidal infection. Fluconazole is preferred for CNS involvement due to excellent CNS penetration (5). However, fluconazole, like all antifungals, is static in the CNS; thus, coccidioidal meningitis requires lifelong therapy. There is little clinical experience with the newer azoles voriconazole and posaconazole. The echinocandins (caspofungin, micafungin, and anidulafungin) have poor activity against Coccidioides spp.
Coccidioidomycosis is not a benign disease that afflicts only a few unfortunate or immunosuppressed hosts. Population growth in the southwestern United States will escalate the impact of this disease. Better therapies may include immunotherapy in the future. Much work is ongoing in the formulation of vaccines. However, a clinically efficacious vaccine is likely to be a long time in coming.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Ampel, N. 2000. Coccidioidomycosis, p. 59-77. In G. A. Sarosi and S. F. Davies (ed.), Fungal diseases of the lung. Lippincott Williams & Wilkins, Philadelphia, PA.
- 2.Bialek, R., J. Kern, T. Herrman, R. Tijerina, L. Ceceñas, U. Reischl, and G. M. González. 2004. PCR assays for identification of Coccidioides posadasii based on the nucleotide sequence of the antigen 2/proline-rich antigen. J. Clin. Microbiol. 42:778-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi, V., R. Ramani, S. Gromadzki, B. Rodeghier, H. Chang, and D. L. Morse. 2000. Coccidioidomycosis in New York State. Emerg. Infect. Dis. 6:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher, M. C., G. L. Koenig, T. J. White, and J. W. Taylor. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94:73-84. [PubMed] [Google Scholar]
- 5.Galgiani, J. N., N. M. Ampel, J. E. Blair, A. Catanzaro, R. H. Johnson, D. A. Stevens, and P. L. Williams. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217-1223. [DOI] [PubMed] [Google Scholar]
- 6.Kirkland, T. N., and J. Fierer. 1996. Coccidioidomycosis: a reemerging infectious disease. Emerg. Infect. Dis. 3:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller, J. M. 2006. The select agent rule and its impact on clinical laboratories. Clin. Microbiol. Newsl. 28:57-63. [Google Scholar]
- 8.Odds, F. C., F. V. Van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities and treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappagianis, D. 1996. Serology of coccidioidomycosis, p. 33-35. In H. E. Enstein and A. Catanzaro (ed.), Coccidioidomycosis: proceedings of the 5th international conference. National Foundation for Infectious Diseases, Washington, DC.
- 10.Pappagianis, D., and B. L. Zimmer. 1990. Serology of coccidioidomycosis. Clin. Microbiol. Rev. 3:247-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saubolle, M. A. 2000. Mycology and the clinical laboratory in the diagnosis of respiratory mycoses, p. 1-16. In G. A. Sarosi and S. F. Davies (ed.), Fungal diseases of the lung. Lippincott Williams & Wilkins, Philadelphia, PA.
- 12.Valdivia, L., D. Nix, M. Wright, E. Lindberg, T. Fagan, D. Lieberman, T. Stoffer, N. M. Ampel, and J. N. Galgiani. 2006. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg. Infect. Dis. 12:958-962. (First published June 2006; http://www.cdc.gov/ncidod/EID/vol12no06/06-0028.htm.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieden, M. A., and M. A. Saubolle. 1996. The histopathology of coccidioidomycosis, p. 12-17. In H. E. Einstein and A. Catanzaro (ed.), Coccidioidomycosis: proceedings of the 5th international conference. National Foundation for Infectious Diseases, Washington, DC.
- 14.Yeo, S. F., and B. Wong. 2002. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin. Microbiol. Rev. 15:465-484. [DOI] [PMC free article] [PubMed] [Google Scholar]