Abstract
We describe the isolation of Laribacter hongkongensis in Hangzhou City, People's Republic of China. One strain of bacterium, named LHHZ242, had many of the same phenotypic and genotypic characteristics as Laribacter hongkongensis described in previous publications. This discovery proves that Laribacter hongkongensis is also associated with community-acquired gastroenteritis outside Hong Kong.
Laribacter hongkongensis was first isolated from the blood and empyemic pus of a 57-year-old Chinese man with alcoholic cirrhosis and bacterial thoracic empyema in Hong Kong in 2001. Thereafter, this newly discovered bacterium was isolated from the stools of three Asian patients and three European patients with community-acquired diarrhea in Hong Kong (5). This bacillus is a facultative anaerobic, motile, non-spore forming, nonfermentative, urease positive, gram negative, and seagull shaped and belongs to the family Neisseriaceae of the β subclass of the class Proteobacteria (7). Patrick Woo and colleagues reported on a case-control study that showed the presence of an association between L. hongkongensis and community-acquired diarrhea, the consumption of fish, and travel (6). No reports of the isolation of L. hongkongensis outside Hong Kong in the past 5 years have yet been published. In order to determine whether Laribacter hongkongensis is related to some community-acquired cases of gastroenteritis outside of Hong Kong, a study was carried out with patients with community-acquired gastroenteritis in Hangzhou First People's Hospital in the city of Hangzhou, People's Republic of China, by using modified cefoperazone MacConkey (M+) agar.
All fecal swabs in our study were freshly collected from outpatients with community-acquired diarrhea in an outpatient service at Hangzhou First People's Hospital from August to November 2005. The specimens were directly inoculated in six culture agars: xylosine-lysine-deoxycholate (XLD) agar, thiosulfate-citrate-bile salts-sucrose (TCBS) agar, salmonella-shigella (SS) agar, campylobacter blood-free selective agar (CCDA), MacConkey agar, and M+ agar. Modified cefoperazone MacConkey agar was cefoperazone MacConkey agar (1) in which the original concentration was reduced by 50% (final cefoperazone concentration, 16 mg/liter). The cultures were incubated under aerobic conditions at 37°C for 48 h on XLD, TCBS, SS, and M+ agars. The CCDA cultures were incubated in 5 to 10% CO2 at 37°C for 48 h. Small and colorless colonies that grew on MacConkey and M+ agars but that did not grow on XLD agar, TCBS agar, SS agar, or CCDA or that grew very poorly on those agars were picked out. Among these bacterial colonies, all oxidase-, arginine-, and urease-positive bacteria were screened out.
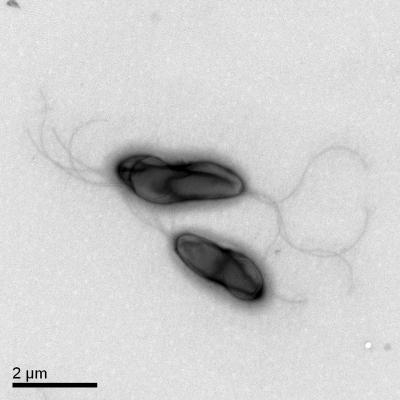
Among 275 stool samples tested, one strain of bacterium, named LHHZ242, that was positive by the oxidase, arginine, and Christensen urea agar tests was isolated from the stool of one patient. It was a seagull-shaped, gram-negative, and non-spore-forming bacterium that grew on M+ agar as a colorless colony 1 mm in diameter. The cells of LHHZ242 varied in length from 2.5 to 3.5 μm and were spiral, slender rods, as determined by transmission electron microscopy (Fig. 1). The bacterial cells had bipolar tufted flagella. Up to five flagella were detected at one end; and the length of each flagellum was about 9 μm, which was about three times the length of the cell body. All these phenotypic characteristics were most like those of Laribacter hongkongensis gen. nov., sp. nov., described by Yuen et al. (7).
FIG. 1.
Transmission electron micrograph of strain LHHZ242.
We also identified strain LHHZ242 by 16S rRNA gene sequencing. Comparison of the gene sequences of different bacterial species has shown that the 16S rRNA gene is highly conserved within a species and among species of the same genus and can be used as the new “gold standard” for the identification of bacteria to the species level (2, 3). The bacterial DNA of LHHZ242 was extracted by use of a genomic DNA extraction kit (Shanghai Sangon Biological Engineering Technology and Service Co., Ltd.). The primers used for 16S rRNA gene amplification were LPW264 (5′-GAGTTTGATCMTGGCTCAG-3′) and LPW265 (5′-GNTACCTTGTTACGACTT-3′) (Gibco BRL, Rockville, MD).
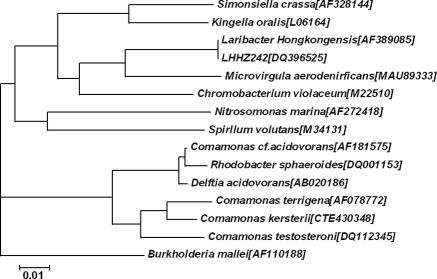
The 50-μl PCR mixture contained 2 μl of 100 μmol/liter of each deoxynucleoside triphosphate, 1 μl of 50 pmol of each primer, 5 μl of 10× buffer (which contained 25 mmol/liter MgCl2), 2 U Taq polymerase, and 4 μl of template DNA. The PCR temperature program was controlled at 94°C for 3 min, followed by 94°C for 1 min and then 55°C for 1 min and 72°C for 2 min (for 35 cycles), with a final extension at 72°C for 5 min. DNase I-treated double-distilled water was used as the control agent in the study. Ten microliters of each amplified product was electrophoresed in a 1.0% (wt/vol) agarose gel with a molecular size marker (DNA marker DL2000; Katara Biotechnology Co., Ltd.). Electrophoresis was performed in Tris-borate-EDTA buffer at 100 V for 1.5 h. The gel was stained with ethidium bromide (0.5 μg/ml) for 15 min, rinsed, and photographed under UV light illumination. The PCR product was sequenced directly by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd. The 16S rRNA gene sequence of the bacterium was about 1,413 bp, and a search for sequences homologous to the sequence was performed by use of the BLAST program. The result indicated that LHHZ242 had 100% homology with Laribacter hongkongensis (Fig. 2).
FIG. 2.
Phylogenetic tree showing the relationship of strain LHHZ242 to L. hongkongensis and other members of the β subclass of the class Proteobacteria. The designations in brackets are GenBank accession numbers.
The patient from whom LHHZ242 was recovered was a 58-year-old male who was a native of Hangzhou City. On 13 November 2005 he had watery stools at a rate of more than 10 stools per day. He took a traditional Chinese medicine for 2 days, with no effect. He went to see his doctor in an outpatient service in Hangzhou First People's Hospital on 15 November 2005. The clinical diagnosis was dyspeptic enteritis. Fleroxacin at 0.4 g was injected intravenously. An oral bifidobacterium preparation and an allicin capsule were ordered for the patient. His diarrhea discontinued on the second day.
The patient complained of watery stools but had no abdominal pain, vomiting, fever, or tenesmus; the stool sample had no special odor. He had not traveled outside of Hangzhou City in the 2 weeks before he became ill, and he had not eaten any aquatic product and had not eaten outside. He had consumed one chicken at home. All members of his family were healthy during the time that the patient became ill. He had a history of peptic ulcer but no other basic diseases, and he rarely took medications. He had not taken any antibiotics within the 2 weeks before he became ill. Except for strain LHHZ242, no nosogenic bacteria were found in his feces. From this evidence, we believed that strain LHHZ242 must be L. hongkongensis. This evidence suggests that LHHZ242 is the first L. hongkongensis strain associated with a case of community-acquired gastroenteritis in the city of Hangzhou.
As most patients with community-acquired gastroenteritis have a history of antibiotic use before they seek treatment, it has been very difficult to isolate L. hongkongensis by using the selective medium cefoperazone MacConkey agar, as reported by Lau et al. (1). Thus, we modified cefoperazone MacConkey agar by reducing the concentration from 32 μg/ml to 16 μg/ml, and the result was better than that previously obtained with the cefoperazone MacConkey agar used in our study in the city of Hangzhou.
L. hongkongensis is believed to be associated with community-acquired gastroenteritis and traveler's diarrhea (5). The isolation of L. hongkongensis from patients who reside in Hong Kong or who have recently traveled to Asia, Europe, North and South America, and Africa implies that the bacterium is likely to be part of a global epidemic. However, its etiology is not clearly understood. Freshwater fish are one of the sources of transmission of this bacterium (4). Some extensive epidemiologic studies should be carried out to ascertain the etiologic association between L. hongkongensis and diarrhea and to identify the host and the routes of transmission of L. hongkongensis.
Nucleotide sequence accession number.
The sequence of strain LHHZ242 has been submitted to the GenBank sequence database and can be found under accession no. DQ396525.
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Lau, S. K. P., P. C. Y. Woo, W. T. Hui, et al. 2003. Cefoperazone MacConkey agar for selective isolation of Laribacter hongkongensis. J. Clin. Microbiol. 41:4839-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen, G. J., R. Overbeek, N. Larsen, T. L. Marsh, M. J. McCaughey, M. A. Maciukenas, W. M. Kuan, T. J. Macke, Y. Xing, and C. R. Woese. 1992. The ribosomal database project. Nucleic Acids Res. Suppl. 20:2199-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen, G. J., and C. R. Woese. 1993. Ribosomal RNA: a key to phylogeny. FASEB J. 7:113-123. [DOI] [PubMed] [Google Scholar]
- 4.Teng, J. L. L., P. C. Y. Woo, S. S. Ma, T. H. Sit, L. T. Ng, W. T. Hui, S. K. P. Lau, and K. Y. Yuen. 2005. Eco-epidemiology of Laribacter hongkongensis, a novel bacterium associated with gastroenteritis. J. Clin. Microbiol. 43:919-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo, P. C. Y., P. Kuhnert, A. P. Burnens, J. L. L. Teng, S. K. P. Lau, T. L. Que, H. H. Yau, and K. Y. Yuen. 2003. Laribacter hongkongensis: a potential cause of infectious diarrhea. Diagn. Microbiol. Infect. Dis. 47:551-556. [DOI] [PubMed] [Google Scholar]
- 6.Woo, P. C. Y., S. K. P. Lau, J. L. L. Teng, T. L. Que, R. W. H. Yung, W. K. Luk, R. W. M. Lai, W. T. Hui, S. S. Y. Wong, H. H. Yau, and K. Y. Yuen. 2004. Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case-control study. Lancet 363:1941-1947. [DOI] [PubMed] [Google Scholar]
- 7.Yuen, K. Y., P. C. Y. Woo, J. L. L. Teng, K. W. Leung, M. K. Wong, and S. K. P. Lau. 2001. Laribacter hongkongensis gen. nov., sp. nov., a novel gram-negative bacterium isolated from a cirrhotic patient with bacteremia and empyema. J. Clin. Microbiol. 39:4227-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]