Abstract
Acinetobacter sp. isolates having multidrug resistance (MDR) patterns have become common in many medical centers worldwide, limiting therapeutic options. A five-center study tested 103 contemporary clinical Acinetobacter spp., including MDR strains, by reference broth microdilution and disk diffusion (15-μg disk content) methods against tigecycline. Applying U.S. Food and Drug Administration tigecycline breakpoint criteria for Enterobacteriaceae (susceptibility at ≤2 μg/ml [≤1 μg/ml by the European Committee on Antimicrobial Susceptibility Testing]; disk diffusion breakpoints at ≥19 mm and ≤14 mm) to Acinetobacter spp. led to an unacceptable error rate (23.3%). However, an adjustment of tigecycline disk diffusion breakpoints (susceptible/resistant) to ≥16/≤12 mm reduced intermethod errors to an acceptable level (only 9.7%, all minor).
Acinetobacter sp. isolates have emerged in recent years as among the most problematic pathogens to eradicate using available antimicrobial agents (1, 6, 7, 10, 25). The occurrences of Acinetobacter sp. infections have escalated in the National Nosocomial Infection Study to levels of 6.9, 2.4, 2.1, and 1.6% as causes of health care-associated pneumonia, bloodstream infections, surgical-site infections, and urinary tract infections, respectively (25). Similarly, the SENTRY Antimicrobial Surveillance Program lists Acinetobacter spp. as causing 2.3 to 3.0% of health care-associated pneumonia and as the eighth most common organism (4.0%) isolated from intensive-care unit (ICU) patients (12), a figure comparable to that in a French survey of all hospital infections (1.2%) (7). Complicating this increased prevalence, multidrug resistance (MDR) among Acinetobacter sp. isolates has risen markedly because of acquired or selected mechanisms of resistance, including antimicrobial inactivation enzymes, efflux pumps, target modifications, and altered porins (7).
Treatment of Acinetobacter sp. infections has largely been limited to a few broad-spectrum agents, including carbapenems, amikacin, some tetracyclines (doxycycline and minocycline), and the sulbactam component of ampicillin/sulbactam (1, 6, 15, 25, 26). As resistance to the carbapenems and other alternatives has emerged, the popularity of polymyxin class agents (colistin and polymyxin B) has increased, with documentation of clinical success (14-16, 26) but not without side effects, usually renal toxicity (27 to 58%) (15).
The search for therapeutic alternatives has recently focused on a new class of antimicrobial agents, glycylcyclines, represented by tigecycline (a 9-t-butylglycylamide derivative of minocycline) (2, 6, 17; Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). Tigecycline has a novel, often bactericidal mode of action characterized by binding to the 30S ribosomal subunit, thus blocking aminoacyl-tRNA entry into the acceptor site (e.g., inhibiting protein synthesis), an action that overcomes two types of tetracycline resistance (efflux and ribosomal protection) (2, 17, 20). However, some investigations (17, 19) have reported that tigecycline showed only bacteriostatic activity against bacteremic isolates of Acinetobacter spp. and other species. Nevertheless, tigecycline clearly displays inhibitory activity against Acinetobacter spp. (2, 8, 9, 13, 17, 19, 22, 23) and has been utilized for therapy against MDR strains despite the lack of a U.S. FDA-approved clinical indication and interpretive criteria for in vitro susceptibility testing (5, 5a, 11, 13; Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). This report summarizes the results from a multicenter investigation of tigecycline tested using the Clinical and Laboratory Standards Institute (CLSI, formerly the National Committee for Clinical Laboratory Standards [NCCLS]) methods (3, 4) to assess possible susceptibility breakpoints for Acinetobacter spp. and to address the perception that applying the U.S. FDA tigecycline breakpoints used for Enterobacteriaceae to Acinetobacter spp. results in unacceptable error rates, i.e., false resistance by the disk diffusion test (Wyeth Research, personal communication).
The study was designed utilizing multiple laboratories, with Acinetobacter sp. strains selected from contemporary clinical isolates at five geographically diverse locations. A total of 103 nonduplicated strains were identified locally (60 Acinetobacter baumannii, 2 A. lwoffii, and 41 other unspecified Acinetobacter species) (24) and tested for susceptibility by CLSI methods (3-5) using freshly prepared (<12-h-old) cation-adjusted Mueller-Hinton medium (2, 21) for frozen-form broth microdilution tests (TREK Diagnostics, Cleveland, Ohio). Tigecycline disks were used (15 μg; lot no. 5187044; BBL, Sparks, MD) for the disk diffusion procedure (11). Each institution utilized the current Mueller-Hinton agar lot in use at that facility. The quality control strains (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853) were concurrently tested on five or more occasions by each participant with tigecycline and control agents (gentamicin, tetracycline, and tobramycin); all results (100.0%) except those for gentamicin (96.2% by MIC tests only) were within the quality control ranges recommended by the CLSI (3-5). The inoculum colony counts for the broth microdilution method averaged 3.8 × 105 CFU/ml across all participant sites. This protocol design conformed to the NCCLS M23-A2 document recommendations (18).
Initial analyses examined tigecycline MIC and zone diameter results for serious discords (susceptible to resistant or vice versa), and tests with discordant results were repeated to assess reproducibility. The two occurrences of severe intermethod discord were resolved on repeat testing, and the entire collection (103 strains) was analyzed using the U.S. FDA tigecycline susceptible breakpoints listed for Enterobacteriaceae (≤2 μg/ml and ≥19 mm) applied to the Acinetobacter spp. (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). Resistance was defined as ≥8 μg/ml and ≤14 mm (Table 1) . A scattergram correlating tigecycline MICs and zone diameters around 15-μg disks was constructed (Fig. 1) and analyzed by the error rate-bounding method (18) to maximize intermethod agreement for the MIC breakpoint of ≤2 μg/ml (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). Generally, the goal of such calculations should be to minimize false-susceptible (very major) errors for the disk diffusion test at ≤1.5%, as well as intermethod minor and total errors at ≤10.0% (18). Also, the tigecycline MIC distribution and percentage of Acinetobacter sp. strains inhibited at ≤2 μg/ml for the collection were compared to those reported in four distinct surveillance reports (8, 9, 22, 23).
TABLE 1.
Proposed tigecycline interpretive criteria for Acinetobacter spp. and calculated intermethod error rates for the disk diffusion method
| MIC (μg/ml) interpretive criteria (correlate zone)a | Error rate (%)
|
||||
|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Very major | Major | Minor |
| ≤2 (≥19)b | 4 (15-18)b | ≥8 (≤14)b | 0.0 | 0.0 | 23.3 |
| ≤2 (≥17) | 4 (14-16) | ≥8 (≤13) | 0.0 | 0.0 | 11.7 |
| ≤2 (≥16)c | 4 (13-15)c | ≥8 (≤12)c | 0.0 | 0.0 | 9.7d |
Interpretive criteria proposed for CLSI method (3-5) results; error rates are derived from the scattergram presented in Fig. 1.
Indicates MIC and disk diffusion criteria for Enterobacteriaceae found in the tigecycline product package insert (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). The EUCAST MIC breakpoint is at ≤1 μg/ml.
Criteria that minimize intermethod error rates by modifying the disk diffusion test criteria only.
Acceptable level of intermethod discord or errors (18).
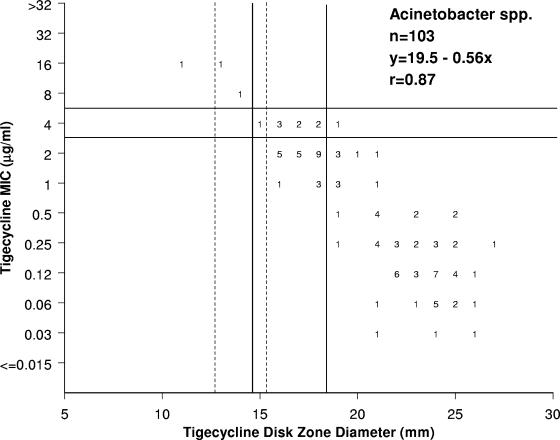
FIG. 1.
Scattergram comparing tigecycline MICs (μg/ml) and zones of inhibition around 15-μg tigecycline disks when tested against 103 Acinetobacter isolates. This was a multicenter (five-site) investigation with a diverse collection of recent clinical strains. The solid vertical and horizontal lines show the interpretive criteria for Enterobacteriaceae published in the U.S. FDA-approved product package insert (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA) for CLSI methods (3, 4). The dashed vertical lines illustrate the proposed breakpoints for Acinetobacter sp. testing for two options (Table 1).
Figure 1 shows the scattergram of tigecycline MIC results versus zone diameters around 15-μg disks. The 103 isolates of Acinetobacter spp. demonstrated a favorable linear correlation (r = 0.87) between the results, with three strains having tigecycline MICs at ≥8 μg/ml (possible resistance). Using the U.S. FDA Enterobacteriaceae susceptible breakpoint for tigecycline of ≤2 μg/ml for this Acinetobacter collection with the correlate zones, an unacceptably high minor-error rate (23.3%) was observed (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). These errors were dominantly (23/24 occurrences) false-intermediate results by the disk diffusion test for strains with tigecycline MICs of 1 or 2 μg/ml. By modifying the susceptible and resistant breakpoint zone diameters to ≥16 and ≤12 mm (Table 2), respectively, the minor- and total error rates were minimized at 9.7%. Our findings, when using the U.S. FDA tigecycline package insert Enterobacteriaceae breakpoints for the disk diffusion method (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA), were consistent with the anecdotal reports of high numbers of “tigecycline false-intermediate” Acinetobacter sp. isolates with the Kirby-Bauer method (Wyeth Research, personal communication). Another option, using disk diffusion test breakpoints at ≥17 (susceptible) and ≤13 mm (resistant), resulted in an overall error rate of 11.7% (unacceptable) (Table 1).
TABLE 2.
Summary of published tigecycline activity resultsa
| Organism source (no. tested/%)b | Tigecycline MIC (μg/ml)
|
% at MIC (μg/ml)
|
% by categoryc
|
Reference | |||
|---|---|---|---|---|---|---|---|
| 50% | 90% | ≤1 | ≤2 | Susceptible | Resistant | ||
| Bacteremia (326/1.2) | 0.5 | 2 | 74 | 95 | 94.5 | 0.9 | 23 |
| Bacteremia (49/NA) | 2 | 2 | - | 92 | 91.8 | 0.0 | 19 |
| ICU (223/2.4) | 1 | 2 | 65 | 93 | 93.3 | 0.9 | 22 |
| Respiratory tract (143/4.5) | 1 | 4 | 51 | 87 | 86.7 | 0.7 | 8 |
| SSTI (61/1.2) | 0.5 | 2 | 87 | 97 | 96.7 | 1.6 | 9 |
Tested against 753 Acinetobacter sp. isolates among 51,619 strains reported from an international surveillance program (8, 9, 22, 23) and 49 additional bacteremias (19).
Totals for 753 strains, with the percentage of Acinetobacter sp. isolates among the species reported for each specimen source (8, 9, 22, 23). Reference 19 studied only bloodstream isolates. NA, not applicable; SSTI, skin and soft tissue infection.
Categories were defined using the U.S. FDA tigecycline package insert criteria for Enterobacteriaceae (≤2 μg/ml, susceptible) (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA); resistance was defined as a tigecycline MIC at ≥8 μg/ml.
Table 2 shows tigecycline activities tested by reference methods against more than 800 strains of Acinetobacter spp. reported in other studies (8, 9, 19, 22, 23). The collection used in this study to determine the Acinetobacter sp. tigecycline disk diffusion breakpoints was slightly more tigecycline resistant (2.9%) than those of other published studies (0.0 to 1.6% resistant) (Table 2). The tigecycline MIC90 results ranged from 2 to 4 μg/ml (8, 9, 19, 22, 23), and the percentage of isolates with a tigecycline MIC at ≤2 μg/ml was 86.7 to 96.7%. Generally, Acinetobacter sp. isolates from the respiratory tract or in ICU patients had more elevated MIC results (8, 22). Compared to these listed surveillance program results, the five-center study collection was judged to be representative of current U.S. clinical material/strains. The tigecycline U.S. FDA-approved package insert lists Acinetobacter baumannii among species for which “at least 90% of these microorganisms exhibit in vitro MICs less than or equal to the susceptible breakpoint for tigecycline” (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). These data contrast with those for the strains reported by Kronvall et al. (13), which had a mean MIC of only 0.16 μg/ml compared to 1.4 μg/ml for our Acinetobacter sp. collection. When the statistical calculation of the “normalized resistance interpretation” (NRI) was used by these Swedish investigators (13), the NRI was 0.73, rounded to 1 μg/ml (European Committee on Antimicrobial Susceptibility Testing [EUCAST] Enterobacteriaceae breakpoint at ≤1 μg/ml). This NRI method used to define the so-called “wild-type” population appeared to be suboptimal for breakpoint determinations in our collection, since one mode of a bimodal distribution of tigecycline MICs occurred at 2 μg/ml (Fig. 1), and rounding of our mean MIC (1.4 μg/ml) favored the previously selected U.S. FDA breakpoint criteria for the Enterobacteriaceae (≤2 μg/ml) (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). The bimodality of tigecycline MICs among A. baumannii has been confirmed elsewhere (15a).
Like its parent compound (minocycline), tigecycline exhibits potent activity against Acinetobacter spp. comparable to that shown against the indicated species of Enterobacteriaceae (2, 8, 9, 13, 22, 23). Therefore, the suggested MIC susceptibility breakpoint (≤2 μg/ml) for these two gram-negative bacillary groups (Acinetobacter spp. and Enterobacteriaceae) should be consistent, as well as conforming, where possible, to the product package insert (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA). A modest change in the tigecycline 15-μg disk diffusion breakpoints (Table 1) of only 3 mm improved the tigecycline intermethod agreement and predictive accuracy to acceptable levels (18). Those laboratories asked to provide tigecycline in vitro susceptibility testing for Acinetobacter sp. isolates should attempt to comply by using a validated quantitative MIC method (broth microdilution or Etest [AB BIODISK, Solna, Sweden]), but when the disk diffusion test must be employed, these proposed interpretive modifications should be considered to maximize correlations and accuracy with the reference MIC method. We encourage the continued study of glycylcycline treatment alone or in combination (1) against MDR Acinetobacter sp. infections to assist in clarifying these breakpoint criteria in adequate numbers of patients and to further investigate the intermethod agreement of tigecycline in vitro tests for the Enterobacteriaceae, with ultimate action by the CLSI and other breakpoint organizations (harmonization with EUCAST). Those investigations, when available, should be presented to the U.S. FDA and CLSI for possible adjustments of breakpoint criteria (Tygacil package insert [June 2005], Wyeth Pharmaceuticals Inc., Philadelphia, PA).
Acknowledgments
We appreciate the excellent support provided at each center by numerous technologists and managers. Also, the following individuals contributed to the development of the protocol and the draft manuscript: N. O'Mara-Morrissey, J. Ross, M. Dowzicky, T. Fritsche, and P. Bradford.
This study and the associated work of the CLSI Acinetobacter Working Group were funded by an educational/research grant from Wyeth Research.
Footnotes
Published ahead of print on 8 November 2006.
REFERENCES
- 1.Aronson, N. E., J. W. Sanders, and K. A. Moran. 2006. In harm's way: infections in deployed American military forces. Clin. Infect. Dis. 43:1045-1051. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, P. A. 2004. Tigecycline: a first in class glycycycline. Clin. Microbiol. Newsl. 26:163-168. [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A9, 9th ed. CLSI, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A7, 7th ed. CLSI, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; M100-S16, 16th informational supplement. CLSI, Wayne, PA.
- 5a.Dukart, G., C. A. Cooper, N. Dartois, and E. J. Ellis-Grosse. 2006. Abstr. 44th Annu. Meet. Infect. Dis. Soc. Am., abstr 173.
- 6.Ferrara, A. M. 2006. Potentially multidrug-resistant non-fermentative Gram-negative pathogens causing nosocomial pneumonia. Int. J. Antimicrob. Agents. 27:183-195. [DOI] [PubMed] [Google Scholar]
- 7.Fournier, P. E., and H. Richet. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42:692-699. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche, T. R., H. S. Sader, M. G. Stilwell, M. J. Dowzicky, and R. N. Jones. 2005. Antimicrobial activity of tigecycline tested against organisms causing community-acquired respiratory tract infection and nosocomial pneumonia. Diagn. Microbiol. Infect. Dis. 52:187-193. [DOI] [PubMed] [Google Scholar]
- 9.Fritsche, T. R., H. S. Sader, M. G. Stilwell, M. J. Dowzicky, and R. N. Jones. 2005. Potency and spectrum of tigecycline tested against an international collection of bacterial pathogens associated with skin and soft tissue infections (2000-2004). Diagn. Microbiol. Infect. Dis. 52:195-201. [DOI] [PubMed] [Google Scholar]
- 10.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11:868-873. [DOI] [PubMed] [Google Scholar]
- 11.Jones, R. N. 1999. Disk diffusion susceptibility test development for the new glycylcycline, GAR-936. Diagn. Microbiol. Infect. Dis. 35:249-252. [DOI] [PubMed] [Google Scholar]
- 12.Jones, R. N. 2003. Global epidemiology of antimicrobial resistance among community-acquired and nosocomial pathogens: a five-year summary from the SENTRY Antimicrobial Surveillance Program (1997-2001). Semin. Respir. Crit. Care Med. 24:121-134. [DOI] [PubMed] [Google Scholar]
- 13.Kronvall, G., I. Karlsson, M. Walder, M. Sorberg, and L. E. Nilsson. 2006. Epidemiological MIC cut-off values for tigecycline calculated from Etest MIC values using normalized resistance interpretation. J. Antimicrob. Chemother. 57:498-505. [DOI] [PubMed] [Google Scholar]
- 14.Landman, D., J. M. Quale, D. Mayorga, A. Adedeji, K. Vangala, J. Ravishankar, C. Flores, and S. Brooks. 2002. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, N.Y.: the preantibiotic era has returned. Arch. Intern. Med. 162:1515-1520. [DOI] [PubMed] [Google Scholar]
- 15.Levin, A. S. 2003. Treatment of Acinetobacter spp. infections. Exp. Opin. Pharmacother. 4:1289-1296. [DOI] [PubMed] [Google Scholar]
- 15a.Lolans, K., T. W. Rice, L. Thompson. G. D. Wright, and J. P. Quinn. 2006. Abstr. 44th Annu. Meet. Infect. Dis. Soc. Am., abstr. 293.
- 16.Michalopoulos, A. S., S. Tsiodras, K. Rellos, S. Mentzelopoulos, and M. E. Falagas. 2005. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin. Microbiol. Infect. 11:115-121. [DOI] [PubMed] [Google Scholar]
- 17.Nathwani, D. 2005. Tigecycline: clinical evidence and formulary positioning. Int. J. Antimicrob. Agents 25:185-192. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2001. Approved guideline M23-A2: Development of in vitro susceptibility testing criteria and quality controls parameters, 2nd ed. NCCLS, Wayne, PA.
- 19.Pachon-Ibanez, M. E., M. E. Jimenez-Mejias, C. Pichardo, A. C. Llanos, and J. Pachon. 2004. Activity of tigecycline (GAR-936) against Acinetobacter baumannii strains, including those resistant to imipenem. Antimicrob Agents Chemother. 48:4479-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen, P. J., P. A. Bradford, W. J. Weiss, T. M. Murphy, P. E. Sum, and S. J. Projan. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen, P. J., and P. A. Bradford. 2005. Effect of medium age and supplementation with the biocatalytic oxygen-reducing reagent oxyrase on in vitro activities of tigecycline against recent clinical isolates. Antimicrob. Agents Chemother. 49:3910-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sader, H. S., R. N. Jones, M. J. Dowzicky, and T. R. Fritsche. 2005. Antimicrobial activity of tigecycline tested against nosocomial bacterial pathogens from patients hospitalized in the intensive care unit. Diagn. Microbiol. Infect. Dis. 52:203-208. [DOI] [PubMed] [Google Scholar]
- 23.Sader, H. S., R. N. Jones, M. G. Stilwell, M. J. Dowzicky, and T. R. Fritsche. 2005. Tigecycline activity tested against 26,474 bloodstream infection isolates: a collection from 6 continents. Diagn. Microbiol. Infect. Dis. 52:181-186. [DOI] [PubMed] [Google Scholar]
- 24.Schreckenberger, P. C., M. I. Daneshvar, R. S. Weyant, and D. G. Hollis. 2003. Acinetobacter, Achromobacter, Chryseobacterium, Moraxella, and other non-fermentative gram-negative rods, p. 749-779. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 25.Talbot, G. H., J. Bradley, J. E. Edwards, Jr., D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 26.Van Looveren, M., and H. Goossens. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10:684-704. [DOI] [PubMed] [Google Scholar]