Abstract
Clinical laboratories frequently face the problem of delayed availability of commercially prepared approved reagents for performing susceptibility testing of new antimicrobials. Although this problem is encountered more often with antibacterial agents, it is also an issue with antifungal agents. A current example is voriconazole, a new triazole antifungal with an expanded spectrum and potency against Candida spp., Aspergillus spp., and other opportunistic fungal pathogens. The present study addresses the use of fluconazole as a surrogate marker to predict the susceptibility of Candida spp. to voriconazole. Reference broth microdilution MIC results for 13,338 strains of Candida spp. isolated from more than 200 medical centers worldwide were used. Voriconazole MICs and interpretive categories (susceptible, ≤1 μg/ml; susceptible dose dependent, 2 μg/ml; resistant, ≥4 μg/ml) were compared with those of fluconazole by regression statistics and error rate bounding analyses. For all 13,338 isolates, the absolute categorical agreement was 91.6% (false susceptible or very major error [VME], 0.0%). Since voriconazole is 16- to 32-fold more potent than fluconazole, the performance of fluconazole as a surrogate marker for voriconazole susceptibility was improved by designating those isolates with fluconazole MICs of ≤32 μg/ml as being susceptible to voriconazole, resulting in a categorical agreement of 97% with 0.1% VME. Clinical laboratories performing antifungal susceptibility testing of fluconazole against Candida spp. can reliably use these results as surrogate markers until commercial FDA-approved voriconazole susceptibility tests become available.
Voriconazole is a new triazole antifungal agent with broad-spectrum activity against Candida spp., Cryptococcus neoformans, Aspergillus spp., and other opportunistic fungal pathogens (2, 9, 12, 21, 22, 25, 27). The in vitro activity of voriconazole against Candida spp. has been documented by broth dilution, agar disk diffusion, and Etest methods (24, 25, 27, 28, 30), and most recently clinically relevant interpretive breakpoints for MIC and disk diffusion testing of Candida spp. have been established by the Clinical and Laboratory Standards Institute (CLSI) (formerly NCCLS) (30).
The U.S. Food and Drug Administration (FDA) has approved voriconazole for treatment of invasive candidiasis (including candidemia) in nonneutropenic patients (6, 12). Voriconazole has also been FDA approved for treatment of esophageal candidiasis, invasive aspergillosis, and serious infections caused by Fusarium spp. and by Scedosporium apiospermum in patients who are intolerant or refractory to other antifungal agents (9, 19, 21, 22). Similar indications for the use of voriconazole have been approved in Europe, including the use of this agent for the treatment of fluconazole-resistant serious invasive Candida infections (6, 12).
Voriconazole has been shown to be efficacious in both primary (12) and salvage (19, 22) therapy of invasive candidiasis. However, several investigators have recently urged caution regarding its use in heavily azole-exposed patients due to the potential for cross-resistance, especially with fluconazole-resistant strains of Candida glabrata (1, 6, 8, 13, 20).
Previously, we have shown that in vitro cross-resistance between fluconazole and voriconazole does exist among clinical isolates of Candida spp. (25, 27, 30). The clinical relevance of this cross-resistance has been documented in case series from three different institutions (1, 13, 20), where clinically significant microbial resistance to voriconazole has been reported among immunocompromised patients (e.g., intensive care and stem cell transplant patients) with a high level of azole (i.e., fluconazole before voriconazole) exposure. Although most of the resistant isolates in these series were C. glabrata, a number of C. albicans, C. tropicalis, and C. parapsilosis isolates were also found to have reduced susceptibility or resistance to both fluconazole and voriconazole (1, 13, 20).
Thus, decreased susceptibility to fluconazole may precede, or even predict, decreased susceptibility to voriconazole, and diligent monitoring of candidal infections in highly compromised patients must be continued despite voriconazole coverage, particularly when voriconazole use is preceded by prolonged fluconazole exposure (1, 13, 20). These concerns have led Magill et al. (13) to institute reflexive fluconazole susceptibility testing of patients' initial blood isolates as a means of identifying those that may not respond optimally to either fluconazole or voriconazole therapy.
Despite the fact that interpretive breakpoints for voriconazole and Candida have been established by CLSI (30), the manufacture of commercially available antifungal susceptibility test systems that incorporate voriconazole is still under development, and none are FDA approved (3, 24). Currently, the Sensititre YeastOne colorimetric antifungal plate (TREK Diagnostic Systems, Cleveland, OH) is FDA approved for testing Candida spp. against fluconazole, itraconazole, and flucytosine (3). Previously, we have shown that fluconazole may serve as a surrogate marker for evaluating the susceptibility of Candida spp. to ravuconazole, an investigative triazole (29). This same approach should be applicable to voriconazole, allowing laboratories to use an FDA-approved commercial MIC panel containing fluconazole to predict the susceptibility of Candida isolates to voriconazole as a temporary measure until FDA approval for voriconazole testing has been granted.
In the present study we utilized a large database of susceptibility test results for fluconazole and voriconazole, all determined by CLSI broth microdilution (BMD) methods, to first provide a very robust analysis of cross-resistance between the two agents and additionally to examine the usefulness of fluconazole as a surrogate marker for evaluating voriconazole susceptibility and resistance among Candida spp.
MATERIALS AND METHODS
Organisms.
A total of 13,338 clinical isolates of Candida spp. obtained from more than 200 medical centers worldwide were tested (23). The collection included the following species and numbers of isolates: 7,725 isolates of C. albicans, 1,966 isolates of C. glabrata, 1,623 isolates of C. parapsilosis, 1,253 isolates of C. tropicalis, 312 isolates of C. krusei, 134 isolates of C. lusitaniae, 103 isolates of C. dubliniensis, 92 isolates of C. guilliermondii, 34 isolates of C. pelliculosa, 33 isolates of C. kefyr, 19 isolates of C. famata, 19 isolates of C. rugosa, 7 isolates of C. lipolytica, 5 isolates of C. zeylanoides, 3 isolates of C. inconspicua, 2 isolates of C. lambica, 2 isolates of C. sake, 1 isolate of C. norvegensis, and 5 isolates of Candida spp. not otherwise identified. All were incident isolates from individual patients and were obtained from blood or other normally sterile body fluids (23, 25-27). Isolates were identified by using Vitek and API yeast identification systems (bioMerieux, Inc, Hazelwood, MO) supplemented by conventional methods as needed (7). The C. dubliniensis isolates were from mucosal infections and were identified by specific probe hybridization (10). Isolates were stored as water suspensions until used. Prior to testing, each isolate was passaged at least twice on potato dextrose agar (Remel, Lenexa, KS) and CHROMagar (Hardy Laboratories, Santa Maria, CA) to ensure purity and viability.
Susceptibility testing.
Reference antifungal susceptibility testing of all isolates was performed by BMD as described by the CLSI (15). Reference powders of fluconazole and voriconazole were obtained from Pfizer Inc. (Groton, CT).
MIC interpretive criteria for fluconazole were those published by Rex et al. (32, 33) and recently confirmed by Pfaller et al. (31). Breakpoints were as follows: susceptible (S), ≤8 μg/ml; susceptible dose dependent (SDD), 16 to 32 μg/ml; and resistant (R), ≥64 μg/ml. The interpretive breakpoints for voriconazole were those proposed by the CLSI and published by Pfaller et al. (30): S, ≤1 μg/ml; SDD, 2 μg/ml; and R, ≥4 μg/ml.
Analysis of results.
All MIC results (in μg/ml) for fluconazole were directly compared with those for voriconazole by regression statistics and by scattergram (Fig. 1). The error rate bounding method to minimize intermethod interpretive error was also applied using the interpretive breakpoints described above. Acceptable error limits used in this comparison were those cited by the CLSI (8) and by other authors (4, 11).
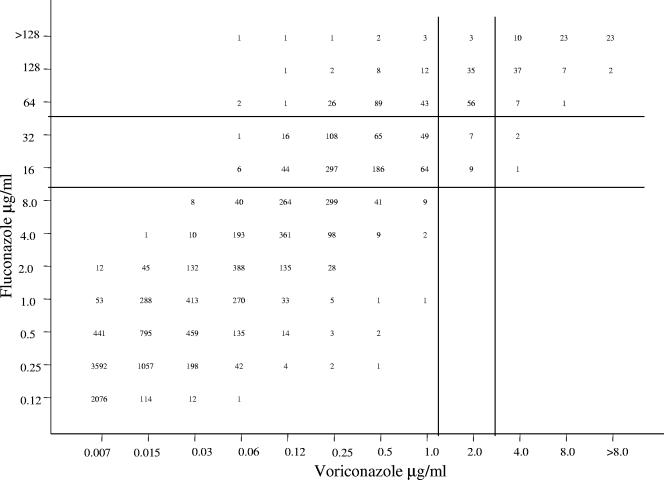
FIG. 1.
Scattergram comparing fluconazole and voriconazole MICs for 13,338 strains of Candida spp. An excellent correlation was observed (r = 0.93; y = 4.5 + 1.1x). The horizontal and vertical lines designate the respective breakpoints for fluconazole and voriconazole. (Adapted from reference 30.)
The definitions of errors used in this analysis were as follows: a very major error (VME), or a false-susceptible error, was an S result for the surrogate marker fluconazole and an R result for voriconazole; a major error (ME), or a false-resistant error, was an R result for fluconazole and an S result for voriconazole; and minor errors occurred when the result for one of the agents was S or R and that for the other agent was SDD. In general, for an agent to be considered a reliable surrogate, the VME rate should be ≤1.5% of all results, and the absolute categorical agreement between methods should be ≥90% (4, 11, 14).
RESULTS AND DISCUSSION
Table 1 depicts the MIC distribution profiles for fluconazole and voriconazole determined for 13,338 strains of Candida spp. by using CLSI (15)-validated BMD methods. Overall, 12,087 isolates (90.6%) were S, 855 (6.4%) were SDD, and 396 (3.0%) were categorized as R to fluconazole. Conversely, 13,115 (98.3%) were S, 110 (0.8%) were SDD, and 113 (0.9%) were R to voriconazole at MIC breakpoints of ≤1 μg/ml, 2 μg/ml, and ≥4 μg/ml, respectively. The modal MIC for voriconazole was 0.007 μg/ml, compared to 0.25 μg/ml for fluconazole. Decreased potencies of both fluconazole and voriconazole were observed among C. glabrata (modal MICs of 8 μg/ml and 0.12 to 0.25 μg/ml, respectively) and C. krusei (modal MICs of 32 μg/ml and 0.25 μg/ml, respectively), although >99% of C. krusei isolates were susceptible to voriconazole at the ≤1-μg/ml breakpoint.
TABLE 1.
Fluconazole and voriconazole MIC distribution profiles for 13,338 clinical isolates of Candida spp. tested by Clinical and Laboratory Standards Institute-recommended broth microdilution methodsa
| Species (no. of isolates tested) | Antifungal agent | No. of isolates at MIC (μg/ml) of:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.007 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | ||
| C. albicans (7,725) | Fluconazole | 2,121 | 4,387 | 751 | 210 | 104 | 33 | 32 | 31 | 7 | 49 | ||||
| Voriconazole | 5,624 | 1,417 | 372 | 181 | 39 | 38 | 17 | 12 | 10 | 1 | 14 | ||||
| C. glabrata (1,966) | Fluconazole | 1 | 4 | 6 | 18 | 164 | 477 | 547 | 491 | 77 | 181 | ||||
| Voriconazole | 3 | 5 | 29 | 237 | 584 | 584 | 223 | 139 | 89 | 50 | 21 | 2 | |||
| C. parapsilosis (1,623) | Fluconazole | 4 | 163 | 636 | 479 | 213 | 55 | 27 | 30 | 8 | 8 | ||||
| Voriconazole | 265 | 589 | 409 | 219 | 70 | 47 | 12 | 7 | 4 | 1 | |||||
| C. tropicalis (1,253) | Fluconazole | 21 | 240 | 390 | 322 | 202 | 36 | 14 | 7 | 2 | 19 | ||||
| Voriconazole | 120 | 203 | 382 | 386 | 100 | 27 | 8 | 6 | 3 | 4 | 9 | 5 | |||
| C. krusei (312) | Fluconazole | 2 | 7 | 31 | 146 | 126 | |||||||||
| Voriconazole | 1 | 28 | 137 | 126 | 18 | 1 | 1 | ||||||||
| C. lusitaniae (134) | Fluconazole | 5 | 44 | 43 | 22 | 11 | 3 | 2 | 1 | 2 | 1 | ||||
| Voriconazole | 77 | 36 | 8 | 7 | 2 | 2 | 1 | 1 | |||||||
| C. dubliniensis (103) | Fluconazole | 46 | 39 | 4 | 1 | 4 | 2 | 4 | 3 | ||||||
| Voriconazole | 60 | 24 | 8 | 1 | 2 | 4 | 2 | 2 | |||||||
| C. guilliermondii (92) | Fluconazole | 1 | 1 | 3 | 32 | 31 | 15 | 5 | 1 | 3 | |||||
| Voriconazole | 2 | 3 | 14 | 36 | 18 | 9 | 7 | 2 | 1 | ||||||
| C. pelliculosa (34) | Fluconazole | 6 | 21 | 7 | |||||||||||
| Voriconazole | 1 | 20 | 11 | 2 | |||||||||||
| C. kefyr (33) | Fluconazole | 4 | 16 | 11 | 2 | ||||||||||
| Voriconazole | 18 | 12 | 3 | ||||||||||||
| C. famata (19) | Fluconazole | 1 | 3 | 3 | 1 | 4 | 2 | 5 | |||||||
| Voriconazole | 2 | 3 | 2 | 2 | 2 | 5 | 3 | ||||||||
| C. rugosa (19) | Fluconazole | 13 | 2 | 4 | |||||||||||
| Voriconazole | 10 | 1 | 5 | 1 | 2 | ||||||||||
| Candida spp.b (25) | Fluconazole | 1 | 1 | 3 | 2 | 2 | 7 | 2 | 1 | 6 | |||||
| Voriconazole | 3 | 2 | 4 | 2 | 7 | 1 | 3 | 1 | 1 | 1 | |||||
| Total (13,338) | Fluconazole | 2,203 | 4,896 | 1,849 | 1,064 | 740 | 674 | 661 | 607 | 248 | 396 | ||||
| Voriconazole | 6,174 | 2,300 | 1,232 | 1,079 | 874 | 869 | 404 | 183 | 110 | 57 | 31 | 25 | |||
Broth microdilution MICs determined in accordance with CLSI M27-A2.
Includes C. lipolytica (7 isolates), C. zeylanoides (5 isolates), C. inconspicua (3 isolates), C. lambica (2 isolates), C. sake (2 isolates), C. norvegensis (1 isolate), and Candida spp. not otherwise identified (5 isolates).
The extent of cross-resistance between fluconazole and voriconazole may be seen more clearly in Table 2 and Fig. 1. Although the MICs for voriconazole were at least 16- to 32-fold lower than the corresponding fluconazole MICs for each isolate tested, there was a strong positive correlation (r = 0.93) between voriconazole and fluconazole MICs (Fig. 1). All of the fluconazole-susceptible isolates were susceptible to voriconazole, as were 97.8% of the fluconazole-SDD isolates (Table 2). Among 396 fluconazole-resistant isolates, 192 (48%) were susceptible, 94 (24%) were SDD, and 110 (28%) were resistant to voriconazole.
TABLE 2.
Use of fluconazole to predict susceptibility patterns of voriconazole, using 13,338 clinical isolates of Candida spp. from the Global Antifungal Surveillance Program
| Species (no. of isolates tested) | Fluconazole susceptibility categorya | No. (%) in voriconazole categoryb:
|
||
|---|---|---|---|---|
| S (≤1 μg/ml) | SDD (2 μg/ml) | R (≥4 μg/ml) | ||
| All Candida (13,338) | S | 12,087 (90.6) | 0 (0.0) | 0 (0.0) |
| SDD | 836 (6.3) | 16 (0.1) | 3 (0.1) | |
| R | 192 (1.4) | 94 (0.7) | 110 (0.8) | |
| All Candida minus C. krusei (13,026) | S | 12,078 (92.7) | 0 (0.0) | 0 (0.0) |
| SDD | 659 (5.1) | 16 (0.1) | 3 (0.1) | |
| R | 68 (0.5) | 93 (0.7) | 109 (0.8) | |
| C. albicans (7,725) | S | 7,638 (98.9) | 0. (0.0) | 0 (0.0) |
| SDD | 37 (0.5) | 1 (0.01) | 0 (0.0) | |
| R | 25 (0.3) | 9 (0.1) | 15 (0.19) | |
| C. glabrata (1,966) | S | 1,217 (61.9) | 0 (0.0) | 0 (0.0) |
| SDD | 557 (28.3) | 10 (0.5) | 1 (0.1) | |
| R | 30 (1.5) | 79 (4.0) | 72 (3.7) | |
| C. parapsilosis (1,623) | S | 1,577 (97.2) | 0. (0.0) | 0 (0.0) |
| SDD | 36 (2.2) | 2 (0.1) | 0 (0.0) | |
| R | 5 (0.3) | 2 (0.1) | 1 (0.1) | |
| C. tropicalis (1,253) | S | 1,223 (97.6) | 0 (0.0) | 0 (0.0) |
| SDD | 5 (0.4) | 2 (0.2) | 2 (0.2) | |
| R | 2 (0.2) | 1 (0.1) | 16 (1.3) | |
| C. krusei (312) | S | 9 (2.8) | 0 (0.0) | 0 (0.0) |
| SDD | 177 (56.7) | 0 (0.0) | 0 (0.0) | |
| R | 124 (39.7) | 1 (0.4) | 1 (0.4) | |
| C. lusitaniae (134) | S | 130 (97.0) | 0 (0.0) | 0 (0.0) |
| SDD | 2 (1.4) | 1 (0.8) | 0 (0.0) | |
| R | 1 (0.8) | 0 (0.0) | 0 (0.0) | |
| C. dubliniensis (103) | S | 94 (91.3) | 0 (0.0) | 0 (0.0) |
| SDD | 6 (5.8) | 0 (0.0) | 0 (0.0) | |
| R | 1 (1.0) | 0 (0.0) | 2 (1.9) | |
| C. guilliermondii (92) | S | 83 (90.2) | 0 (0.0) | 0 (0.0) |
| SDD | 6 (6.5) | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 2 (2.2) | 1 (1.1) | |
| C. pellliculosa (34) | S | 34 (100) | 0 (0.0) | 0 (0.0) |
| SDD | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| C. kefyr (33) | S | 33 (100) | 0 (0.0) | 0 (0.0) |
| SDD | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| C. famata (19) | S | 14 (73.7) | 0 (0.0) | 0 (0.0) |
| SDD | 5 (26.3) | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| C. rugosa (19) | S | 19 (100) | 0 (0.0) | 0 (0.0) |
| SDD | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
S, MIC of ≤8 μg/ml; SDD, MIC of 16 to 32 μg/ml; R, MIC of ≥64 μg/ml.
Voriconazole susceptibility categories are as described by Pfaller et al. (30).
The CLSI does not recommend that laboratories test C. krusei against fluconazole, given its poor clinical response to this agent and the fact that the “intrinsic” resistance manifested by this species may be underrepresented by the in vitro results (5, 15, 26, 29, 31, 32). In contrast, voriconazole appears quite active against C. krusei (310 of 312 isolates [99%] were susceptible at an MIC of ≤1 μg/ml [Table 2]), and the clinical activity looks promising as well (5, 12, 19, 22). When the C. krusei results are eliminated from the total, we again find that 100% of fluconazole-susceptible isolates and 97% of fluconazole-SDD isolates are susceptible to voriconazole but that only 25% of fluconazole-resistant isolates are susceptible to voriconazole (Table 2). Alternatively, if one considers all isolates identified as C. krusei to be resistant to fluconazole, then 378 of 582 fluconazole-resistant isolates (65%) would be susceptible to voriconazole (data not shown).
When the fluconazole test result category (S, SDD, or R) was used to predict the voriconazole category, the absolute categorical agreement between the test results was 91.6%, with no VME (false-susceptible error), 1.4% ME (false-resistant error), and a 7.0% minor error rate (Table 3). Given the fact that the fluconazole results clearly do not predict the susceptibility of C. krusei to voriconazole (Tables 1 and 2), we have also factored the C. krusei results out of the analysis, with a resulting improvement in categorical agreement (93.6%) and a decrease in both ME (0.5%) and minor errors (5.9%) (Table 3).
TABLE 3.
Absolute categorical agreement and error rates when the azole surrogate fluconazole result was used to predict voriconazole susceptibility of Candida spp.
| Organism(s) tested | No. of isolates | %a:
|
|||
|---|---|---|---|---|---|
| Agreement | VME | ME | Minor errors | ||
| All Candida | 13,338 | 91.6 | 0.0 | 1.4 | 7.0 |
| All Candida minus C. krusei | 13,026 | 93.6 (97.7) | 0.0 (0.1) | 0.5 (1.4) | 5.9 (0.8) |
| C. albicans | 7,725 | 99.1 | 0.0 | 0.3 | 0.6 |
| C. glabrata | 1,966 | 66.1 (93.9) | 0.0 (0.1) | 1.5 (1.5) | 32.4 (4.5) |
| C. parapsilosis | 1,623 | 97.4 | 0.0 | 0.3 | 2.3 |
| C. tropicalis | 1,253 | 99.1 | 0.0 | 0.2 | 0.7 |
| C. krusei | 312 | 3.2 | 0.0 | 39.7 | 57.1 |
| C. lusitaniae | 134 | 97.8 | 0.0 | 0.8 | 1.4 |
| C. dubliniensis | 103 | 93.2 | 0.0 | 1.0 | 5.8 |
| C. guilliermondii | 92 | 91.3 | 0.0 | 0.0 | 8.7 |
| C. pelliculosa | 34 | 100 | 0.0 | 0.0 | 0.0 |
| C. kefyr | 33 | 100 | 0.0 | 0.0 | 0.0 |
| C. famata | 19 | 73.7 (100) | 0.0 | 0.0 | 26.3 (0.0) |
| C. rugosa | 19 | 100 | 0.0 | 0.0 | 0.0 |
Values in parentheses are categorical agreement and error rates obtained using the following categories for fluconazole: susceptible, MIC of ≤32 μg/ml (S and SDD combined); resistant, MIC of ≥64 μg/ml.
The absolute categorical agreements for 12 individual species of Candida are shown in Table 3. A categorical agreement of 91% or better (range, 91.3 to 100%) was observed for all species except C. glabrata, C. famata, and C. krusei.
As seen with C. krusei, the fluconazole results also underestimated the activity of voriconazole against C. glabrata and C. famata (Tables 2 and 3). All fluconazole-susceptible isolates of these two species were also susceptible to voriconazole (Table 2). Likewise, 98% of C. glabrata isolates and all five C. famata isolates that were SDD to fluconazole were susceptible to voriconazole. In contrast, only 16.5% of fluconazole-resistant isolates of C. glabrata were susceptible to voriconazole, and 40% were resistant. Importantly, 72 of 73 voriconazole-resistant isolates (98.6%) were also resistant to fluconazole.
Clearly, it is most important to detect those isolates of C. glabrata that may be resistant to voriconazole, and in that regard fluconazole performs quite well as a surrogate marker; there were no false-susceptible results (VME) and only 1.5% false-resistant results (ME). Given that 98% of C. glabrata isolates that were SDD to fluconazole were susceptible to voriconazole, one may improve the ability of the fluconazole MIC test to predict susceptibility of C. glabrata to voriconazole by combining the fluconazole S and SDD categories and using fluconazole MICs of ≤32 μg/ml to identify voriconazole-susceptible isolates and MICs of ≥64 μg/ml to identify voriconazole resistance. Using this criterion, the categorical agreement for C. glabrata improves to 93.9%, with 0.1% VME, 1.5% ME, and 4.5% minor errors (Table 3). Similarly, the categorical agreement for C. famata improves from 73.7% to 100%. Applying this modified criterion to the entire collection of isolates (minus C. krusei) results in an overall categorical agreement of 97.7%, with 0.1% VME and 1.4% ME (Table 3).
Previously, we conducted a similar analysis using fluconazole results to predict the susceptibility of Candida spp. to ravuconazole as a proof of concept regarding the use of surrogate markers or class representatives in antifungal susceptibility testing (29). This strategy has been used for decades in antibacterial susceptibility testing to develop practical alternatives for the microbiology laboratory when specific diagnostic susceptibility testing reagents are limited or unavailable (11, 14, 16-18). Given the lack of FDA-approved testing systems for voriconazole, the approach described in the present study provides a useful strategy for laboratories in the effort to optimize antifungal therapy of candidal infections.
In addition to providing a strategy to predict voriconazole susceptibility and resistance among Candida spp., these results provide strong support for the concerns of several investigators regarding the issue of cross-resistance between fluconazole and voriconazole (1, 8, 13, 20). By using an extensive global collection of clinically important isolates encompassing more than 12 species, we validate concerns originating from single-center experiences and rightly focus attention on C. glabrata as the species most likely to demonstrate cross-resistance among the azoles.
Fluconazole functioned well as a surrogate marker for voriconazole susceptibility when applied to this collection of clinically significant isolates of Candida spp. The absolute categorical agreement of 91.6% (93.6% without C. krusei), with no VME among more than 13,000 isolates tested, easily meets the recognized criteria for a reliable surrogate marker as applied to antibacterial susceptibility testing (4, 11, 14, 16-18). Given the greater potency of voriconazole over fluconazole, the use of fluconazole as a surrogate marker for voriconazole susceptibility was clearly improved by designating those isolates with fluconazole MICs of ≤32 μg/ml (S and SDD categories combined) as being susceptible to voriconazole, with the resistant category staying the same at ≥64 μg/ml. The resulting 97.7% categorical agreement and 0.1% VME rate is excellent for a surrogate marker test.
In conclusion, we have demonstrated the existence of cross-resistance between fluconazole and voriconazole, with the greatest emphasis on C. glabrata. Furthermore, we have shown that the availability of voriconazole susceptibility testing results for Candida spp., in any medical center currently performing antifungal susceptibility testing of fluconazole, can be accomplished by using the fluconazole result as a surrogate marker for voriconazole susceptibility and resistance. Specifically, fluconazole MICs of ≤32 μg/ml predict susceptibility and MICs of ≥64 μg/ml predict resistance to voriconazole. The occurrence of false-susceptible and false-resistant errors with this expanded application of the “class representative” concept to selected triazoles was very low and was acceptable for surrogate marker testing. By using a predictor agent with a generally narrower spectrum of activity or reduced potency, such as fluconazole, a conservative and safe categorical estimation of activity can be made until specific voriconazole-containing FDA-approved products are available. As commercial voriconazole susceptibility products become available, they should rapidly replace the interim use of this surrogate marker for clinical testing.
Acknowledgments
This study was supported in part by unrestricted research grants from Pfizer Inc.
Linda Elliott provided excellent support in the preparation of the manuscript. We appreciate contributions of all participants in the Global Antifungal Surveillance Program. For a complete listing of participants, please go to http://www.medicine.uiowa.edu/pathology/path_folder/research/acknowledgments/artemis_participants.pdf.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Alexander, B. D., W. A. Schell, J. L. Miller, G. D. Long, and J. R. Perfect. 2005. Candida glabrata fungemia in transplant patients receiving voriconazole after fluconazole. Transplantion 80:868-871. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, S. Killian, H. A. Norris, and M. A. Ghannoum. 1999. Multicenter comparison of the Sensititre Yeast One Colorimetric Antifungal Panel with the National Committee for Clinical Laboratory Standards M27-A reference method for testing clinical isolates of common and emerging Candida spp., Cryptococcus spp., and other yeasts and yeast-like organisms. J. Clin. Microbiol. 37:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferraro, M. J., and J. H. Jorgensen. 1995. Instrument-based antibacterial susceptibility testing, p. 1379-1384. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, DC.
- 5.Fukuoka, T., D. A. Johnston, C. A. Winslow, M. J. de Groot, C. Burt, C. A. Hitchocok, and S. G. Filler. 2003. Genetic basis for differential activities of fluconazole and voriconazole against Candida krusei. Antimicrob. Agents Chemother. 47:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graybill, J. R. 2005. Voriconazole for candidosis: an important addition? Lancet 366:1413-1414. [DOI] [PubMed] [Google Scholar]
- 7.Hazen, K. C., and S. A. Howell. 2003. Candida, Cryptococcus, and other yeasts of medical importance, p. 1693-1711. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, DC.
- 8.Imhof, A., A. Balajee, D. N. Fredricks, J. A. Englund, and K. A. Marr. 2004. Breakthrough fungal infections in stem cell transplant patients receiving voriconazole. Clin. Infect. Dis. 39:743-746. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 10.Joly, S., C. Pujol, M. Rysz, K. Vargas, and D. R. Soll. 1999. Development and characterization of complex DNA fingerprinting probes for the infectious yeast Candida dubliniensis. J. Clin. Microbiol. 37:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, R. N., and M. A. Pfaller, and the SENTRY Antimicrobial Surveillance Program Participants Group (USA). 2001. Can. antimicrobial susceptibility testing results for ciprofloxacin or levofloxacin predict susceptibility to a newer fluoroquinolone, gatifloxacin? Report from the SENTRY Antimicrobial Surveillance Program (1997-99). Diagn. Microbiol. Infect. Dis. 39:237-243. [DOI] [PubMed] [Google Scholar]
- 12.Kullberg, B. J., J. D. Sobel, M. Ruhnke, P. G. Pappas, C. Viscoli, J. H. Rex, J. D. Cleary, E. Rubinstein, L. W. R. Church, J. M. Brown, H. T. Schlammn, I. T. Oberska, F. Hilton, and M. R. Hodges. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomized non-inferiority trial. Lancet 366:1435-1442. [DOI] [PubMed] [Google Scholar]
- 13.Magill, S. S., C. Shields, C. L. Sears, M. Choti, and W. G. Merz. 2006. Triazole cross-resistance among Candida spp.: case report, occurrence among bloodstream isolates, and implications for antifungal therapy. J. Clin. Microbiol. 44:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 2nd ed., M23-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed., M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests; approved standard, 8th ed., M2-A8. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 17.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 6th ed., M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 18.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing; 13th informational supplement, M100-S13. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Ostrosky-Zeichner, L., A. M. L. Oude Lashof, B. J. Kullberg, and J. H. Rex. 2003. Voriconazole salvage treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22:651-655. [DOI] [PubMed] [Google Scholar]
- 20.Panackal, A. A., J. L. Gribskov, J. F. Staab, K. A. Kirby, M. Rinaldi, and K. A. Marr. 2006. Clinical significance of azole antifungal drug cross-resistance in Candida glabrata. J. Clin. Microbiol. 44:1740-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson, M. M., P. D. Rogers, J. D. Cleary, and S. W. Chapman. 2003. Voriconazole: a new triazole antifungal agent. Ann. Pharmacother. 37:420-432. [DOI] [PubMed] [Google Scholar]
- 22.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lustar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., and D. J. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, H. Huynh, and R. J. Hollis. 2002. Clinical evaluation of a frozen commercially prepared microdilution panel for antifungal susceptibility testing of seven antifungal agents, including the new triazoles posaconazole, ravuconazole, and voriconazole. J. Clin. Microbiol. 40:1694-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis. 2003. Activities of fluconazole and voriconazole against 1,526 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 41:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., D. J. Diekema, L. Boyken, S. A. Messer, S. Tendolkar, and R. J. Hollis. 2003. Evaluation of Etest and disk diffusion methods for determining susceptibilities of 235 bloodstream isolates of Candida glabrata to fluconazole and voriconazole. J. Clin. Microbiol. 41:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Cross-resistance between fluconazole and ravuconazole and the use of fluconazole as a surrogate marker to predict susceptibility and resistance to ravuconazole among 12,796 clinical isolates of Candida spp. J. Clin. Microbiol. 42:3137-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infection. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 33.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]