Abstract
A multilaboratory collaborative study was performed in order to propose quality control limits for posaconazole disk diffusion susceptibility tests on Mueller-Hinton agar supplemented with 2% glucose and 0.5 μg of methylene blue per ml. Replicate tests were performed on three lots of prepared media using 5-μg posaconazole disks in each of eight laboratories to generate data to propose quality control zone diameter ranges for tests of Candida albicans ATCC 90028 (24 to 34 mm), C. parapsilosis ATCC 22019 (25 to 36 mm), C. tropicalis ATCC 750 (23 to 33 mm), and C. krusei ATCC 6258 (23 to 31 mm).
Patients suffering from a variety of immune deficiencies are frequently colonized by or infected with a variety of fungi, especially Candida spp. These individuals often receive a variety of antifungal agents during the course of their infection. One class of antifungal agents that is frequently used is the group of compounds known as azoles. These agents have been shown to be highly effective in the treatment of yeast infections; however, prolonged exposure to azole compounds, such as fluconazole, can lead to the selection of strains that have diminished susceptibilities to these compounds (4, 5). As a result, clinical laboratories are frequently asked to perform susceptibility tests on isolates from such patients in order to determine when strains with diminished susceptibility have been selected (3). For many laboratories, the disk diffusion procedure is a very simple and practical method for performing susceptibility tests on yeasts.
Beginning in 1996, Barry et al. developed a disk diffusion procedure using RPMI 1640 broth supplemented with 2% glucose and 1.5% agar (1). Subsequent studies modified the procedure to use Mueller-Hinton (MH) agar supplemented with 2% glucose because this medium was considered to be more readily available in the average hospital laboratory. Further studies were able to show that the addition of a low concentration of methylene blue (0.5 μg/ml) made the zones of inhibition clearer, easier to read, and more precise (2, 9). Ultimately, this technique was approved by the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards) as the disk diffusion method of choice for performing antifungal susceptibility testing on yeasts (4, 5). Other disk diffusion techniques have been described in the literature (7, 8, 9), but well-documented quality control parameters have not yet been defined for these methods.
An eight-laboratory collaborative study was performed in order to determine the disk diffusion ranges of posaconazole against four CLSI-approved quality control strains. (Participating facilities included the Clinical Microbiology Institute, Wilsonville, OR; the New York State Department of Health, Albany, NY; Case Western Reserve University, Cleveland, OH; the University of Rochester Medical Center, Rochester, NY; the University of Iowa, Iowa City, IA; the University of Texas Houston Medical School, Houston, TX; the University of Alberta Hospital, Edmonton, Alberta, Canada; and the University of Texas Health Science Center, San Antonio, TX.) Three lots of Mueller-Hinton agar (Acumedia lot 0101-135, Difco lot 5145501, and BDMS lot 5080554) were prepared as MH plus 2% glucose and 0.5 μg of methylene blue (MH-GMB) agar plates. Each test plate received two 5-μg posaconazole disks (BDMS lot 5053171 and Oxoid lot 357352) and one 25-μg fluconazole disk as the control.
On each of 10 different test days, a standardized inoculum suspension was prepared for each of the control strains that were to be tested. The strains were grown on Sabouraud dextrose or potato dextrose agar for 24 h prior to the performance of the test. Each inoculum was adjusted to match the turbidity of a 0.5 McFarland standard using a spectrophotometer set at a 530-nm wavelength. This procedure should yield a yeast stock suspension of approximately 1 × 106 to 5 × 106 cells per ml. Each strain was then streaked onto each of the three lots of MH-GMB agar plates. A sterile applicator swab was moistened in this cell suspension and then used to inoculate the surface of each 150-mm agar plate. After inoculation of the plates, two posaconazole disks and one fluconazole disk were applied approximately 35 mm apart to the surface of the medium. The inverted plates were then incubated at 35°C for 20 to 24 h in room air. Calipers were used to measure the diameter of each zone of inhibition at the point where there was a sharp decline in the density of growth. Pinpoint microcolonies and large colonies within the zones were ignored. The measurement of inhibitory zone diameters is somewhat subjective and is likely to improve with experience.
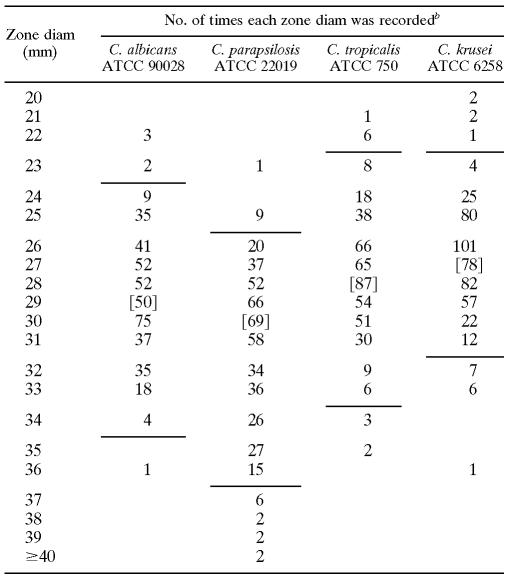
The resulting zone diameters were analyzed to establish tentative quality control statistics for the proposed disk test procedure on each agar medium. Quality control ranges were proposed according to the method of Gavan et al. (6), which is based upon the all laboratory median ± one-half the range of the medians for each lab. The quality control ranges proposed by this method were then adjusted as needed to include ≥95% of the data observed. Whenever the zone diameters for the control drug were outside of the approved quality control ranges, all of the posaconazole results associated with the out-of-range values for that day's testing were eliminated from the calculation. The two lots of posaconazole disks gave essentially identical results (data not shown). Reasonable control limits could be defined to include a 9- to 12-mm range of zone diameters for each of the control strains (Table 1) . That is only slightly broader than the 8- to 10-mm range that is commonly applied to tests of bacteria. Because of the nature of the endpoints that are being observed, tests of antifungal agents cannot be expected to be as precise as those of antibacterial agents. By adding methylene blue to the agar medium, zones of inhibition could be measured with a reasonable degree of precision when the standard control strains were being tested.
TABLE 1.
Results of replicate tests in eight laboratories using 5-μg posaconazole disks on MH-GMBa
MH agar was supplemented by adding GMB before autoclaving.
Based on ten replicates of two disks on each of three lots of media in eight laboratories per strain. The quality control limits (with the percent included) for tests with each control strain were as follows: C. albicans ATCC 90028, 24 to 34 mm (96.5%); C. parapsilosis ATCC 22019, 25 to 36 mm (97.2%); C. tropicalis, 23 to 33 mm (97.3%); and C. krusei, 23 to 31 mm (96.0%). The percent included refers to the percentage of zones that fell within the proposed control limits, as calculated for the data from all eight laboratories. Brackets designate the all-laboratory median zone diameters for each data set.
These quality control limits have now been approved by the CLSI subcommittee for antifungal susceptibility testing and will be presented in an upcoming document (CLSI M44-A2).
Acknowledgments
This study was supported by a grant from the Schering-Plough Research Institute.
Footnotes
Published ahead of print on 8 November 2006.
REFERENCES
- 1.Barry, A. L., and S. D. Brown. 1996. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J. Clin. Microbiol. 34:2154-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., M. A. Pfaller, R. P. Rennie, P. C. Fuchs, and S. D. Brown. 2002. Precision and accuracy of fluconazole susceptibility testing by broth microdilution, E test, and disk diffusion methods. Antimicrob. Agents Chemother. 46:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bille, J. 1997. When should Candida isolates be tested for susceptibility to azole antifungal agents? Eur. J. Clin. Microbiol. Infect. Dis. 16:281-282. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 1997. Methods for antifungal disk diffusion susceptibility testing of yeasts; approved standard M44-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Fan-Harvard, P., D. Capano, S. M. Smith, A. Mangia, and R. H. K. Eng. 1991. Development of resistance in Candida isolates from patients receiving prolonged antifungal therapy. Antimicrob. Agents Chemother. 35:2302-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavan, T. L., R. N. Jones, A. L. Barry, P. C. Fuchs, E. H. Gerlach, J. M. Matsen, L. B. Reller, C. Thornsberry, and L. D. Thrupp. 1981. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J. Clin. Microbiol. 14:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick, W. R., T. M. Turner, A. W. Fothergill, D. J. McCarthy, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Fluconazole disk diffusion susceptibility testing of Candida species. J. Clin. Microbiol. 36:3429-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May, J. L., A. King, and C. A. Warren. 1997. Fluconazole disc diffusion testing for the routine laboratory. J. Antimicrob. Chemother. 40:511-516. [DOI] [PubMed] [Google Scholar]
- 9.Sandven, P. 1999. Detection of fluconazole-resistant Candida strains by a disc screening test. J. Clin. Microbiol. 27:3856-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]