Abstract
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Subcommittee on Antifungal Susceptibility Testing recently published a standard for determining the susceptibility of fermentative yeasts to antifungals. From the beginning, the EUCAST and its North American counterpart, the CLSI, decided to work together in order to establish common standards. As part of this exercise, the susceptibility of a set of 475 yeast isolates was tested by both standards. The intraclass correlation coefficient and the equations defining the linear regression between both methods were estimated. Both methods produced very similar results, with an intraclass correlation coefficient of 0.954 (0.945 to 0.962), although linear regression analysis shows that the EUCAST standard resulted in slightly lower MICs. There were only eight isolates showing at least four twofold dilution MIC differences between both standards. After 24 h of incubation, the MICs obtained by the CLSI method were equivalent to those obtained by the EUCAST standard. In summary, both methods produce very similar MICs, indicating that methodology does not pose any obstacle to obtaining uniform standards for antifungal susceptibility testing of yeasts.
The Subcommittee on Antifungal Susceptibility Testing (AFST) of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has published a standard for the determination of antifungal susceptibility testing of fermentative yeasts (7). Hitherto, only the M27-A2 reference method for broth dilution antifungal susceptibility testing of yeasts issued by the CLSI (formerly NCCLS) was available (4). Besides being established to devise a standard European methodology for antifungal susceptibility testing, determine breakpoints for antifungal drugs, and establish expert rules for interpreting these antimicrobial susceptibility tests, the AFST also set out to produce results concordant with those obtained using the CLSI method. Moreover, the EUCAST and CLSI opted to work together with the aim of establishing common standards.
The standards of the EUCAST AFST and CLSI for determining the MICs of yeasts to antifungal drugs differ in several important respects (Table 1) and might be expected to result in major differences between the MICs generated using each method for the same strains. To investigate this, a series of Candida species were tested by both methods to determine the relationship between the MICs obtained and to identify any discrepancies that might have occurred.
TABLE 1.
Differences between the EUCAST AFST and CLSI M27-A2 yeast standards
| Method | Glucose supplementation (%) | Shape of well | Inoculum (CFU/ml) | Incubation time (h) | Reading | Endpoint for azole drugs |
|---|---|---|---|---|---|---|
| EUCAST AFST | 2 | Flat bottom | 0.5 × 105-2.5 × 105 | 24 | Spectrophotometric | Lowest concn of drug that inhibits growth by 50% of that of the control |
| CLSI M27-A2 | 0.2 | Round bottom | 0.5 × 103-2.5 × 103 | 48 | Visual | Lowest concn of drug that inhibits growth substantially compared with that of the control |
MATERIALS AND METHODS
Microorganisms.
A set of 475 distinct clinical isolates containing 149 isolates of Candida albicans, 86 of Candida glabrata, 73 of Candida krusei, 87 of Candida parapsilosis, and 80 of Candida tropicalis was selected.
Antifungal susceptibility testing.
The methods described in CLSI M27-A2 (4) and EUCAST E.Dis. 7.1 (7) were followed strictly for testing the susceptibility to fluconazole. Strains were tested once by both methods at the same time. Candida krusei ATCC 6258 and C. parapsilosis ATCC 22019 were included for quality control. MICs were read at 24 h for the EUCAST AFST method and at 48 h for the CLSI M27-A2 method.
Statistical analysis.
Statistical analysis was done using SPSS, version 13.0 (SPSS, S.L. Madrid, Spain).
MIC values were transformed to log2. Linear regression analysis for both methods was done to test the linearity of the relationship between the CLSI M27-A2 and EUCAST AFST MICs. A two-way random effect model was utilized to calculate the intraclass correlation coefficient (ICC) with a confidence interval of 95% using the following equation: ICC = (group mean square − error mean square)/(group mean square + error mean square) (3). The ICC has a maximum value of 1 if there is a perfect correlation and a minimum value of −1 if there is a complete absence of correlation.
A difference of at least four twofold dilutions was considered a major discrepancy between the EUCAST AFST and CLSI M27-A2 methods.
RESULTS
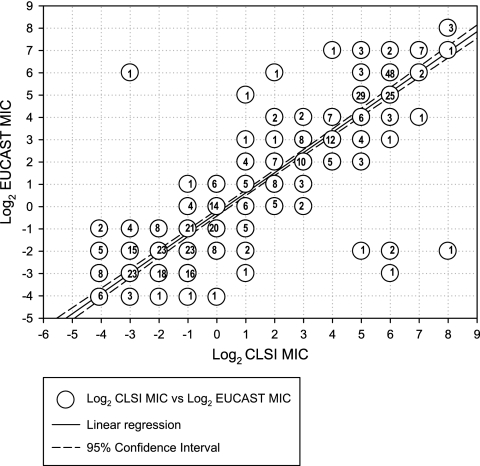
The set of 475 strains was used for defining the mathematical equivalencies between the EUCAST and CLSI methods. The regression line between MICs obtained by each method showed that the MICs were generally equivalent (Fig. 1). Mean MICs generated by both methods were equivalent up to a mean MIC of 2 mg/liter (Table 2), but thereafter, MICs generated by the CLSI M27-A2 method were twofold higher than those generated by the EUCAST AFST method.
FIG. 1.
Regression line of log2 MICs obtained by the EUCAST E.Dis 7.1 and CLSI M27-A2 methods.
TABLE 2.
Equivalencies between MICs obtained by the CLSI M27-A2 and EUCAST E.Dis 7.1 AFST methods
| MIC (mg/liter) by CLSI M27-A2 | MIC (mg/liter) after using equivalence equationa | Conversion to closest EUCAST AFST MIC (mg/liter) |
|---|---|---|
| 0.015 | 0.019 | 0.015 |
| 0.03 | 0.035 | 0.03 |
| 0.06 | 0.065 | 0.06 |
| 0.12 | 0.126 | 0.12 |
| 0.25 | 0.235 | 0.25 |
| 0.5 | 0.39 | 0.5 |
| 1 | 0.821 | 1 |
| 2 | 1.535 | 2 |
| 4 | 2.870 | 2 |
| 8 | 5.367 | 4 |
| 16 | 10.035 | 8 |
| 32 | 18.765 | 16 |
| 64 | 35.090 | 32 |
| 128 | 65.617 | 64 |
| 256 | 122.701 | 128 |
log2 EUCAST MIC = 0.903 × log2 CLSI AFST MIC − 0.285.
There were eight isolates that showed a major discrepancy (Table 3). Four isolates of C. tropicalis and one isolate of C. albicans exhibited high MICs by the CLSI M27-A2 method and low MICs by the EUCAST AFST method. However, the MICs obtained by the CLSI M27-A2 method at 24 h were within the range of those obtained by the EUCAST M27-A2 method. The remaining three C. albicans isolates with discrepant MICs exhibited high MICs by the EUCAST AFST method and low MICs by the CLSI M27-A2 method.
TABLE 3.
Major MIC discrepancies between the EUCAST E.Dis 7.1 and CLSI M27-A2 methods
| Species | EUCAST MIC (mg/liter) | CLSI MIC (mg/liter) at:
|
|
|---|---|---|---|
| 24 h | 48 h | ||
| C. albicans | 32 | 1 | 2 |
| C. albicans | 64 | NAa | 0.125 |
| C. albicans | 64 | NA | 4 |
| C. albicans | 0.25 | 1 | 256 |
| C. tropicalis | 0.25 | 1 | 32 |
| C. tropicalis | 0.25 | 0.25 | 64 |
| C. tropicalis | 0.25 | 0.5 | 64 |
| C. tropicalis | 0.125 | 1 | 64 |
NA, not available.
ICCs were above 0.83 in every case (Table 4). The overall ICC was 0.954 (95% confidence interval, 0.945 to 0.962). The slope and intercept values indicate that the CLSI M27-A2 method results in slightly higher MICs than the EUCAST AFST method.
TABLE 4.
ICCs and 95% confidence intervals for fluconazole MICs obtained by the EUCAST E.Dis 7.1 and CLSI M27-A2 methods
| Species | No. of isolates | ICC | 95% CI ICCa | CLSI vs EUCASTb
|
EUCAST vs CLSIc
|
||
|---|---|---|---|---|---|---|---|
| Slope A | Intercept A | Slope B | Intercept B | ||||
| C. albicans | 149 | 0.948 | 0.928-0.962 | 0.915 | −0.079 | 0.886 | −0.026 |
| C. glabrata | 86 | 0.895 | 0.839-0.932 | 0.761 | 1.285 | 0.866 | −0.109 |
| C. krusei | 73 | 0.833 | 0.734-0.895 | 0.612 | 2.447 | 0.855 | 0.394 |
| C. parapsilosis | 87 | 0.876 | 0.810-0.919 | 0.837 | 0.385 | 0.739 | −0.540 |
| C. tropicalis | 80 | 0.891 | 0.830-0.930 | 0.764 | 1.066 | 0.871 | −0.923 |
| All | 475 | 0.954 | 0.945-0.962 | 0.922 | 0.511 | 0.903 | −0.285 |
95% confidence interval for the intraclass correlation coefficient.
Log2 MIC obtained by CLSI method = slope A × log2 MIC obtained by EUCAST method + intercept A.
Log2 MIC obtained by EUCAST method = slope B × log2 MIC obtained by CLSI method + intercept B.
DISCUSSION
The results of this study showed there were few major differences between the MICs resulting from the CLSI M27-A2 and EUCAST AFST methods. Not only did the regression analysis indicate close proximity of the MICs generated by each method but the ICCs confirmed this. Moreover, the relationship held true for values of up to 2 mg/liter. Thereafter, mean MICs generated by the CLSI M27-A2 method were twice those resulting from the EUCAST AFST method. Major discrepancies were found only for 8 isolates (1.6%) of the 475 tested.
These results were not expected given the differences between the two methods in terms of inoculum, incubation time, and concentration of glucose in the medium. The simplest explanation may well be that the extra glucose and higher inoculum allow sufficient growth for reading the MICs after 24 h rather than having to wait an extra 24 h (8). Besides assuming that a final density of 108 CFU/ml is needed for visible growth, that a lag period of 4 h occurs in the medium, and that a generation time of 120 min is achieved under these conditions, the EUCAST AFST method with the 100-fold-higher inoculum would patently reach this threshold well ahead of the CLSI M27-A2 method. It has previously been documented that a higher inoculum size significantly shortened the length of the lag phase and that the supplementation of glucose increased the growth of Candida spp. Thus, this medium-inoculum combination shortened the lag phase and yielded elevated optical densities after 24 h (2). Whatever the explanation, these results are encouraging as they suggest that there is no major difference between the two methods except that the EUCAST AFST method is quicker. Moreover, it would appear that the MICs generated by either method are equivalent up to 2 mg/liter, that is, within the susceptibility range for clinical success. That MICs of 4 mg/liter and higher generated by the CLSI M27-A2 method tend to be twofold higher than those obtained with the EUCAST AFST method may not pose any real difficulties since this difference is within the acceptable range.
Of the eight isolates showing significantly discrepant MICs, five showed high MICs with the CLSI M27-A2 method and low MICs with the EUCAST AFST method. Fortunately, the results of MICs obtained after 24 h with the CLSI M27-A2 method were concordant with those generated with the EUCAST AFST method. Two investigations of a murine model of invasive candidosis of several isolates with low MICs at 24 h and high MICs at 48 h (1, 6) concluded that the MICs read at 24 h for a 50% reduction in growth correlated with the in vivo response, i.e., under conditions adopted by the EUCAST AFST method (7). In addition, Revankar et al. showed that oropharyngeal candidosis caused by strains susceptible at 24 h and resistant at 48 h (significant trailing growth) responded to doses of fluconazole as low as 100 mg/day (5).
In summary, the CLSI M27-A2 and EUCAST AFST standards for yeasts result in essentially equivalent MICs. This would suggest that the method for determining the MICs will not account for any differences that might be found between the breakpoints set by the CLSI and those eventually adopted by the EUCAST AFST.
Footnotes
Published ahead of print on 8 November 2006.
REFERENCES
- 1.Arthington-Skaggs, B. A., D. W. Warnock, and C. J. Morrison. 2000. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 44:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, and J. L. Rodriguez-Tudela. 2001. Influence of glucose supplementation and inoculum size on growth kinetics and antifungal susceptibility testing of Candida spp. J. Clin. Microbiol. 39:525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGraw, K. O., and S. P. Wong. 1996. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1:30-46. [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 5.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Tudela, J. L., F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, D. Denning, J. P. Donnelly, B. Dupont, W. Fegeler, C. Moore, M. Richardson, P. E. Verweij, and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2003. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 9:I-VIII. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Tudela, J. L., and J. V. Martinez-Suarez. 1994. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob. Agents Chemother. 38:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]