Abstract
We developed a real-time PCR which allowed the highly sensitive detection of Naegleria fowleri in histological brain tissue sections from experimentally infected mice. This genus-specific small-subunit (18S) rRNA gene-based PCR can complement conventional (immuno-) histology for the diagnosis of primary amoebic meningoencephalitis in paraffin-embedded brain necropsy specimens that had been fixed in formalin buffered with phosphate-buffered saline.
The free-living amoeba Naegleria fowleri is an etiological agent of primary amoebic meningoencephalitis (PAM), a rare but fulminating and mostly fatal disease of the central nervous system (CNS) (1, 5, 9, 10, 15, 24, 25). The diagnosis of PAM is mostly performed by (immuno-) histological examination of formalin-fixed, paraffin-embedded brain necropsy specimens (1, 4, 8, 18), but the highly sensitive PCR technique will gain increasing importance in the diagnostic detection of this amoebic pathogen. Normally, diagnostic PCRs require fresh native test samples (ex vivo or postmortem samples) or trophozoites that have been obtained by in vitro cultivation from the appropriate patient specimen.
In most cases, archival samples are stored as formalin-fixed and paraffin-embedded tissue specimens. Although this method is suitable for long-term storage and subsequent retesting by histology, at least partial DNA destruction results from formalin fixation, which may negatively affect the efficiency of PCR (13, 17, 23). Therefore, PCR methods that are compatible with the use of fixed tissue specimens as the substrate are required. Several Naegleria sp.-specific PCRs essentially focused on the identification or genotyping of in vitro-cultivated Naegleria isolates (e.g., primary cultures of environmental samples) have been reported (2, 12, 14, 16, 19, 21). However, none of these PCRs has proven to be suitable for the detection of Naegleria in histological samples. For this reason, we have developed a new PCR to be used for the detection of N. fowleri DNA in formalin-fixed and paraffin-embedded tissue specimens. For the evaluation of the PCR protocol presented here, formalin-fixed, paraffin-embedded brain tissue sections from experimentally N. fowleri-infected mice were investigated.
For the infection experiment, trophozoites from pathogenic N. fowleri strain 30863 (ATCC, Manassas, VA) (Table 1) were axenically cultivated as described by Gianinazzi et al. (6). In order to increase the virulence of this strain for infection in the mouse model, the trophozoites were exposed to the murine fibroblast cell line L-929 (ATCC, Rockville, MD) by coculture on a monocellular layer of L-929 cells until the majority of the murine cells were lysed (11, 28). Upon cocultivation, 5 × 105 trophozoites were intranasally inoculated into 6-week-old C57BL/6 mice (purchased from Charles River GmbH, Germany; the mice were kept at biosafety level 3, according to the Swiss regulations for animal experiments), as described previously (6).
TABLE 1.
Determination of the specifity of the Naegleria sp.-specific PCR
| Species | Isolate | PCR result |
|---|---|---|
| Acanthamoeba astronyxis | 26 | − |
| A. castellanii | 26 | − |
| A. castellanii | 26 | − |
| A. castellanii | 26 | − |
| A. culbertsoni | ATCC 30171 | − |
| A. hatchetti | 26 | − |
| A. lenticulata | 26 | − |
| A. polyphaga | ATCC 30461 | − |
| A. polyphaga | 26 | − |
| A. rhysodes | 26 | − |
| Balamuthia mandrillaris | ATCC 50209 | − |
| Naegleria fowleri | ATCC 30174 | + |
| N. fowleri | ATCC 30214 | + |
| N. fowleri | ATCC 30863 | + |
| N. fowleri | ATCC 30893 | + |
| N. fowleri | ATCC 30894 | + |
| N. lovaniensis | ATCC 30569 | + |
| N. gruberi | ATCC 30133 | + |
| Entamoeba histolytica | ATCC 30015 | − |
| Echinococcus granulosus | 7 | − |
| Echinococcus multilocularis | 7 | − |
| Taenia solium | 7 | − |
| Streptococcus pneumoniae | 3 | − |
| Listeria monocytogenes | 20 | − |
| DNA sample of noninfected human brain tissue (negative control) | NSa | − |
NS, not specified.
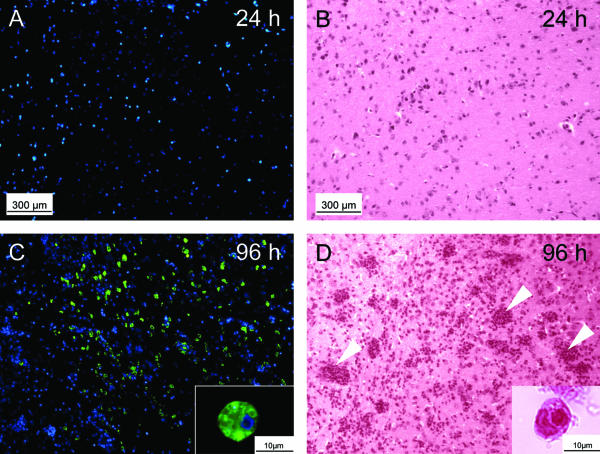
Following necropsy of the mice at 24, 48, 72, and 96 h postinfection (p.i.), the brains were carefully removed, fixed in 4% formalin (buffered with phosphate-buffered saline [PBS]) for 4 days, embedded in paraffin, and finally cut into 6-μm-thick serial sagittal sections. In these sections, the N. fowleri infection was demonstrated by immunohistochemistry (IHC) and hematoxylin-eosin (HE) staining, as described previously (6). IHC revealed no trophozoites at 24 h p.i. (Fig. 1A), but a few trophozoites were detectable at 48 h p.i. (<10 trophozoites per microscopic field) and 72 h p.i.(<100 trophozoites per microscopic field) (data not shown). The number of trophozoites markedly increased between 72 and 96 h p.i. (>1,000 trophozoites per microscopic field; Fig. 1C). The inflammatory response, as documented in corresponding adjacent HE-stained brain sections by the detection of neutrophilic cells that had infiltrated into the brain parenchyma, was not seen at 24 h p.i. (Fig. 1B) or 48 h p.i. (data not shown). However, inflammation substantially intensified between 72 h p.i. (data not shown) and 96 h p.i. (Fig. 1D), matching the increase in the numbers of trophozoites found within the brain parenchyma. Higher-magnification views of the tissue sections evaluated by IHC (Fig. 1C) and HE staining (Fig. 1D) demonstrated the presence of N. fowleri trophozoites. Conversely, no morphological structures comparable to the encysted form of N. fowleri were detected. Brain sections of the mock-infected control animal were negative for trophozoites and neutrophilic cells (data not shown).
FIG. 1.
Analyses of trophozoite proliferation and inflammatory response during Naegleria fowleri infections in mice. The results of immunofluorescence analyses that included specific staining for trophozoites (green, trophozoites; blue, 4′,6′diamidino-2-phenylindole counterstain for cell nuclei) in the mouse brain at 24 h p.i. (A) and 96 h p.i. (C) are shown. Corresponding adjacent histological sections taken at 24 h p.i. (B) and 96 h p.i. (D) were stained with HE and demonstrate the degree of the neutrophilic inflammatory response. No trophozoites were visible in the brain at 24 h p. i. (A), but large numbers of trophozoites (green) were detectable at 96 h p. i. (C). Intense inflammation (infiltration of neutrophilic inflammatory cells into the brain parenchyma, as indicated by arrowheads) was detectable at 96 h p.i. (D). Between 72 h p.i. (data not shown) and 96 h p.i. (C) the number of neutrophils was greatly increased, simultaneously including an increase in trophozoite number between 72 h p.i. (data not shown) and 96 h p.i. (C). The insets show typical examples of immunohistologically stained (C) and HE-stained (D) trophic amoebae at higher magnifications. No morphological structures corresponding to encysted amoebae were found.
For quantitative real-time PCR-based detection of N. fowleri, genomic DNA was extracted from histological tissue sections as described by Müller et al. (17). PCR was carried out on a LightCycler instrument (Roche Diagnostics, Rotkreuz, Switzerland) by using SYBR green I as a double-stranded DNA-specific fluorescent dye and continuous fluorescence monitoring, as described previously (27). Amplification of the ribosomal small-subunit (SSU) (18S) rRNA gene was performed by using the Naegleria sp.-specific primer pair Nae3-For (5′-CAAACACCGTTATGACAGGG-3′) and Nae3-Rev (5′-CTGGTTTCCCTCACCTTACG-3′). Selection of the primers was based on the alignment of sequences of SSU (18S) rRNA gene sequences from N. fowleri (GenBank accession number AY376150), Naegleria lovaniensis (GenBank accession number U80062.1), and Naegleria gruberi (GenBank accession number M18732.1) (data not shown). The primers generated a 183-bp amplification product (representing nucleotide positions 141 to 323 of the N. fowleri SSU [18S] rRNA gene sequence; GenBank accession number AY376150) and were positioned within stretches that were identical among the SSU (18S) rRNA gene sequences of the different Naegleria species listed above. Conversely, the primer alignment did not exhibit striking sequence matches with SSU (18S) rRNA genes from free-living amoeba (FLA) species (Acanthamoeba astronyxis, GenBank accession number AF479546.1; Acanthamoeba castellanii, GenBank accession number AF526424.1; Acanthamoeba culbertsoni, GenBank accession number AF479542.1; Acanthamoeba hatchetti, GenBank accession number AF260722.1; Acanthamoeba lenticulata, GenBank accession number U94732.1; Acanthamoeba polyphaga, GenBank accession number AF479557.1; Acanthamoeba rhysodes, GenBank accession number AF479553.1; Balamuthia mandrillaris, GenBank accession number AF263351.1) other than Naegleria spp. Alignment and comparison of the nucleotide sequences were done by using the MultAlin and the ESPript1.9 computer software, available at the ExPASy Molecular Biology Server. The nucleotide sequence authenticity of the 183-bp N. fowleri SSU (18S) rRNA gene amplification product was confirmed by automated sequencing through a commercial sequencing service (Microsynth, Balgach, Switzerland).
The real-time PCR was done with 4 μl of a 1:4-diluted genomic DNA preparation (see above) and the LightCycler-FastStart DNA Master SYBR green I kit in a 10-μl standard reaction mixture supplemented with MgCl2 to a final concentration of 3.5 mM and containing a 0.5 μM concentration (each) of the forward and the reverse primers (Invitrogen, Basle, Switzerland). PCR was started by initiating the “Hot-Start” Taq DNA polymerase reaction at 95°C (15 min). Subsequent DNA amplification was done in 50 cycles (denaturation [95°C, 15 s], annealing [58°C, 30 s], and extension [72°C, 45 s]; the temperature transition rate in all cycle steps was 20°C/s). The fluorescence signals from the amplification products were quantitatively assessed in triplicate by applying the standard software (version 3.5.3) of the LightCycler instrument. The external standards were obtained as follows: approximately 200 μl of uninfected mouse brain tissue was spiked with 2 × 106 to 2 × 102 N. fowleri trophozoites, and the DNA was prepared from the spiked samples as described above. From these DNA preparations, 1 μl (with 3 μl H2O) containing genomic DNA equivalents from 104 N. fowleri trophozoites to a single N. fowleri trophozoite was used for the standard amplification reactions. Control experiments for identification of the PCR products included a DNA melting point analysis, as described by Ririe et al. (22) (data not shown). In all PCR tests performed, the identical melting temperatures of the amplicons (melting-point peak, 82.8°C ± 0.5°C) from the samples and the respective standards indicated specific amplification reactions without unwanted primer-dimer formation (data not shown). This overall specificity of the reactions was confirmed by subsequent agarose gel electrophoresis (2% gels), which monitored the PCR products as single DNA bands of the expected sizes (data not shown). The presence of possible PCR-inhibition effects were excluded by demonstrating amplification products in samples simultaneously amplified by PCR in the presence of genomic N. fowleri DNA at an amount equivalent to that from approximately 10 trophozoites (data not shown).
An initial evaluation of the methodological sensitivity of the real-time Naegleria PCR was performed by testing the amplification reaction with genomic DNA from different numbers of N. fowleri trophozoites (data not shown). That test revealed an extremely high methodological sensitivity of the PCR, in that genomic DNA from a single N. fowleri trophozoite was detected. Furthermore, the PCR was tested with genomic DNAs prepared from different N. fowleri strains as well as N. lovaniensis and N. gruberi as additional positive controls (Table 1). The PCR was assessed for the absence of nonspecific amplification reactions by testing genomic DNAs from a histological sample of noninfected human brain tissue (negative control DNA) as well as from the non-Naegleria FLA species (different Acanthamoeba species and B. mandrillaris) listed in Table 1. Nonspecific amplification was also revealed to be absent in PCRs with genomic DNA preparations representing a selection of parasitic and bacterial pathogens causing CNS disease in humans (Table 1). In this analysis, PCR amplification products were exclusively observed for the genomic DNA isolated from the Naegleria species (Table 1).
To experimentally evaluate the applicability of the real-time Naegleria PCR in routine histology, formalin-fixed and paraffin-embedded brain tissue samples originating from immunohistochemically confirmed N. fowleri-positive and -negative mice (Fig. 1) were examined for the presence of genomic N. fowleri DNA (Fig. 2). PCR inhibition was excluded by demonstrating amplification products in samples simultaneously amplified by PCR in the presence of an amount of genomic N. fowleri DNA equivalent to approximately 10 trophozoites (data not shown).
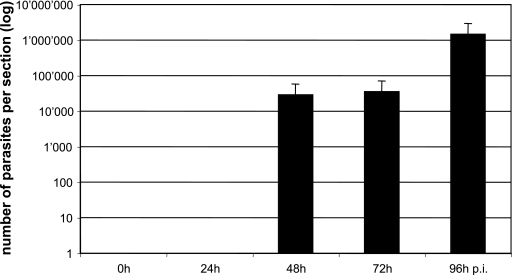
FIG. 2.
Analysis of diagnostic applicability of the real-time Naegleria spp. PCR for histological specimens. The figure shows the mean logarithmic values (plus standard deviations) from triplicate determinations representing amplification reactions from formalin-fixed, paraffin-embedded histological mouse brain tissue sampled at 0 (experimental time point prior to infection), 24, 48, 72, and 96 h p.i. with Naegleria fowleri. Note that all samples that scored immunohistologically N. fowleri-positive also provided a specific PCR amplification signal.
All samples that initially yielded positive findings by IHC (samples taken between 48 and 96 h p.i.; see above) also provided positive results by PCR (Fig. 2). The high methodological sensitivity of the PCR was particularly revealed by the fact that amplification products were also detected in fixed brain tissue, sampled at day 48 h p.i., that only contained extremely low numbers of parasites (<10 parasites per microscopic field; see above).
In the daily routine, tissue samples are often stored temporarily or even over the long term in 4% formalin solution in the absence of PBS. It is feasible that nonbuffered formalin fixation promotes the hydrolytic destruction of the sample DNA and thus complicates or even excludes the possibility of PCR-based diagnosis of Naegleria infections in the respective histological specimens. Our investigation with histological brain samples from N. fowleri-infected mice revealed that exclusive fixation with formalin in the presence of PBS allowed the subsequent detection of N. fowleri in histological specimens. Under these conditions, the PCR even scored positive when the samples had been stored for more than 1 year in fixation solution (data not shown).
In conclusion, the present report describes for the first time a highly sensitive PCR test for the detection of Naegleria sp. DNA in formalin-fixed and paraffin-embedded tissue, thus basically allowing retrospective diagnostic assessment of archival specimens. Moreover, the PCR is supposed to represent a new supplementary tool for the diagnosis of complicated PAM cases, in that it enables detection of the amoeba under circumstances in which microscopic demonstration of the parasite is impossible.
Acknowledgments
The work has been supported by the Swiss National Science Foundation (SCOPES no. 7IP062584 and 632-66057.01) and by the Federal Office for Civil Protection (project no. 350001627).
We are indebted to Nadia Schürch and Martin Schütz from the SPIEZ Laboratory for their valuable support and logistic contribution to the work.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Barnett, N. D., A. M. Kaplan, R. J. Hopkin, M. A. Saubolle, and M. F. Rudinsky. 1996. Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr. Neurol. 15:230-234. [DOI] [PubMed] [Google Scholar]
- 2.Behets, J., F. Seghi, P. Declerck, L. Verelst, L. Duvivier, A. Van Damme, and A. Ollevier. 2003. Detection of Naegleria spp. and Naegleria fowleri: a comparison of flagellation tests, ELISA, and PCR. Water Sci. Technol. 47(3):117-122. [PubMed] [Google Scholar]
- 3.Bifrare, Y. D., C. Gianinazzi, H. Imboden, S. L. Leib, and M. G. Täuber. 2003. Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus 13:481-488. [DOI] [PubMed] [Google Scholar]
- 4.Cogo, P. E., M. Scagli, S. Gatti, F. Rossetti, R. Alaggio, A. M. Laverda, L. Zhou, L. Xiao, and G. S. Visvesvara. 2004. Fatal Naegleria fowleri meningoencephalitis, Italy. Emerg. Infect. Dis. 10:1835-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cursons, R. T., J. Sleigh, D. Hood, and D. Pullon. 2003. A case of primary amoebic meningoencephalitis: North Island, New Zealand. N. Z. Med. J. 116:U712. [PubMed] [Google Scholar]
- 6.Gianinazzi, C., M. Schild, N. Müller, S. L. Leib, F. Simon, S. Nuñez, P. Joss, and B. Gottstein. 2005. Organotypic slice cultures from rat brain tissue: a new approach to study Naegleria fowleri CNS infection in vitro. Parasitology 131:797-804. [DOI] [PubMed] [Google Scholar]
- 7.Gottstein, B., and M. R. Mowatt. 1991. Sequencing and characterization of an Echinococcus multilocularis DNA probe and its use in the polymerase chain reaction (PCR). Mol. Biochem. Parasitol. 44:183-194. [DOI] [PubMed] [Google Scholar]
- 8.Gyori, E. 2003. December 2002: 19-year old male with febrile illness after jet ski accident. Brain Pathol. 13:237-239. [PubMed] [Google Scholar]
- 9.Jaffar-Bandjee, M. C., J. L. Alessandri, B. Molet, J. Clouzeau, L. Jacquemot, S. Samperiz, and J. C. Saly. 2005. Primary amebic meningoencephalitis: 1st case observed in Madagascar. Bull. Soc. Pathol. Exot. 98:11-13. [PubMed] [Google Scholar]
- 10.Jain, R., S. Prabhakar, M. Modi, R. Bhatia, and R. Sehgal. 2002. Naegleria meningitis: a rare survival. Neurol. India 50:470-472. [PubMed] [Google Scholar]
- 11.John, D. T., and R. A. John. 1994. Enhancement of virulence of Naegleria fowleri by growth in Vero-cell cultures. J. Parasitol. 80:149-151. [PubMed] [Google Scholar]
- 12.Kilvington, S., and J. Bleeching. 1995. Development of a PCR for identification of Naegleria fowleri from the environment. Appl. Environ. Microbiol. 61:3764-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann, U., and H. Kreipe. 2001. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods 25:409-418. [DOI] [PubMed] [Google Scholar]
- 14.Marciano-Cabral, F., R. MacLean, A. Menesah, and L. LaPat-Podasko. 2003. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl. Environ. Microbiol. 69:5864-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin, G. L., M. H. Vodkin, and H. W. Huizinga. 1991. Amplification of repetitive DNA for the specific detection of Naegleria fowleri. J. Clin. Microbiol. 29:227-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller, N., V. Zimmermann, U. Forster, M. Bienz, B. Gottstein, and M. Welle. 2003. PCR-based detection of canine Leishmania infections in formalin-fixed and paraffin-embedded skin biopsies: elaboration of a protocol for quality assessment of the diagnostic amplification reaction. Vet. Parasitol. 114:223-229. [DOI] [PubMed] [Google Scholar]
- 18.Okuda, D. T., and S. Coons. 2003. Naegleria fowleri meningoencephalitis. Neurology 61:E1. [DOI] [PubMed] [Google Scholar]
- 19.Pélandakis, M., and P. Pernin. 2002. Use of multiplex PCR and PCR restriction enzyme analysis for detection and exploitation of the variability in the free-living amoeba Naegleria in the environment. Appl. Environ. Microbiol. 68:2060-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remer, K. A., T. W. Jungi, R. Fatzer, M. G. Täuber, and S. L. Leib. 2001. Nitric oxide is protective in listeric meningoencephalitis of rats. Infect. Immun. 69:4086-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Réveiller, F. L., P. A. Cabanes, and F. Marciano-Cabral. 2002. Development of a nested PCR assay to detect the pathogenic free-living amoeba Naegleria fowleri. Parasitol. Res. 88:443-450. [DOI] [PubMed] [Google Scholar]
- 22.Ririe, K. M., R. P. Rasmussen, and C. T. Wittwer. 1997. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal. Biochem. 245:154-160. [DOI] [PubMed] [Google Scholar]
- 23.Sato, Y., R. Sugie, B. Tsuchiya, T. Kameya, M. Natori, and K. Mukai. 2001. Comparison of the DNA extraction methods for polymerase chain reaction amplification from formalin-fixed and paraffin-embedded tissues. Diagn. Mol. Pathol. 10:265-271. [DOI] [PubMed] [Google Scholar]
- 24.Shenoy, S., G. Wilson, H. V. Prashanth, K. Vidyalakshmi, B. Dhanashree, and R. Bharath. 2002. Primary meningoencephalitis by Naegleria fowleri: first reported case from Mangalore, South India. J. Clin. Microbiol. 40:309-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephany, J. D., G. S. Pearl, and O. R. Gonzalez. 2004. Pathologic quiz case: headache in an 8-year-old child. Primary amebic meningoencephalitis due to Naegleria fowleri. Arch. Pathol. Lab. Med. 128:e33-e34. [DOI] [PubMed] [Google Scholar]
- 26.Tsvetkova, N., M. Schild, S. Panaiotov, R. Kurdova-Mintcheva, B. Gottstein, J. Walochnik, H. Aspöck, M. Siles Lucas, and N. Müller. 2004. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol. Res. 92:405-413. [DOI] [PubMed] [Google Scholar]
- 27.Wittwer, C. T., K. M. Ririe, R. V. Andrew, D. A. David, R. A. Gundry, and U. J. Balis. 1997. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques 22:176-181. [DOI] [PubMed] [Google Scholar]
- 28.Wong, M. M., S. L. Karr, Jr., and C. K. Chow. 1977. Changes in the virulence of Neagleria fowleri maintained in vitro. J. Parasitol. 63:872-878. [PubMed] [Google Scholar]