Abstract
Coupled cellular respiration requires that ATP and ADP be efficiently exchanged between the cytosol and the mitochondrial matrix. When growth factors are withdrawn from dependent cells, metabolism is disrupted by a defect in ATP/ADP exchange across the mitochondrial membranes. Unexpectedly, we find that this defect results from loss of outer mitochondrial membrane permeability to metabolic anions. This decrease in anion permeability correlates with the changes in conductance properties that accompany closure of the voltage-dependent anion channel (also known as mitochondrial porin). Loss of outer membrane permeability (i) results in the accumulation of stored metabolic energy within the intermembrane space in the form of creatine phosphate, (ii) is prevented by the outer mitochondrial membrane proteins Bcl-xL and Bcl-2, and (iii) can be reversed by growth factor readdition. If outer membrane impermeability persists, the disruption of mitochondrial homeostasis culminates in loss of outer mitochondrial membrane integrity, cytochrome c redistribution, and apoptosis. The recognition that outer membrane permeability is regulated under physiological conditions has important implications for the understanding of bioenergetics and cell survival.
In multicellular organisms, cell survival depends on the presence of exogenous factors that regulate cellular metabolism. When cells are deprived of growth factors, they undergo a perturbation in mitochondrial physiology that results in cytochrome c redistribution to the cytosol and cell death (1, 2). An alteration in mitochondrial physiology that may be responsible for cytochrome c redistribution is a disruption in ATP/ADP exchange between the mitochondria and the cytosol (3). Cytosolic ADP must be transported across both mitochondrial membranes for the F1F0-ATPase to generate ATP in the matrix. The principal mediators of adenine nucleotide exchange between the matrix and the cytosol are the adenine nucleotide translocator (ANT) on the inner membrane and the voltage dependent anion channel (VDAC) on the outer membrane (4, 5). It has been proposed that Bcl-2 proteins can regulate cell death through an effect on mitochondrial ion exchange (1–3). However, Bcl-2 proteins have been localized to the outer mitochondrial membrane (6–9), and the only known mechanisms for regulating mitochondrial homeostasis involve proteins at the inner membrane.
Historically, the outer membrane has not been considered a barrier to transport because VDAC is a large diameter channel (≈3 nm) that is permeable to uncharged molecules up to ≈5,000 Da in the open configuration (10). However, it has also been shown in vitro that a conserved property of eukaryotic VDAC channels is the ability to adopt multiple conductance states (10, 11). In vitro, VDAC assumes closed configurations in the presence of either positive or negative transmembrane potentials. However, the ability of VDAC to change conductance states has not been demonstrated in intact cells, nor is it clear whether these channel properties are important for the regulation of mitochondrial physiology.
Methods
Cell Lines, Mitochondrial Isolation, Oxygen Consumption, ADP Uptake, and Subcellular Fractionation.
The murine FL5.12 and Baf3 pro-B cell lines were cultured as described previously (12, 13). Multiple independently derived Bcl-xL- and control-transfected FL5.12 lines were used for these experiments.
Mitochondrial isolation, cellular oxygen consumption rates, and ANT-dependent ADP import were also measured as described previously (2, 3). Where indicated, mitoplasts were prepared from isolated mitochondria by using digitonin (Wako Pure Chemicals, Osaka) (0.2 μg/μg mitochondrial protein) according the method of Schnaitman and Greenawalt (14).
Mitochondrial and cytosolic (S-100) fractions were prepared and analyzed by Western blot as described previously (2).
Measurement of ATP, ADP, and Phosphocreatine.
ATP and ADP were measured by the luciferin/luciferase method as described previously (3). Phosphocreatine was measured by using HPLC as described previously (15). HPLC was performed by using a Zorbax RxC8 reverse phase column, eluted with an aqueous solution containing 15 mM KH2PO4, 2.3 mM tetrabutylammonium sulfate (pH 3.0–3.7) at a flow rate of 2 ml/min. For each experiment, the phosphocreatine peak was determined by coinjection of purified phosphocreatine (Sigma). A longer HPLC run was required for the separation of phosphocreatine in Baf3 cells than was required in FL5.12 cells. Creatine levels were determined similarly. Where indicated, antimycin A (Sigma) was added at a concentration of 10 μg/ml to the culture media for 30 min before extraction.
Measurement of Creatine Kinase Activity.
Cells were lysed by a 5-min incubation on ice in 10 mM Hepes/10 mM KCl/1 mM DTT/0.1 mM EDTA/0.1 mM EGTA/0.05% saponin. Creatine kinase activity in the lysates was determined by measuring the amount of creatine generated in 30 min after the addition of phosphocreatine and ADP by using a standard commercial colorimetric assay for creatine (Sigma).
VDAC Channel Studies.
Solvent-free planar phospholipid membranes were generated by the monolayer method (16, 17) using the neutral phospholipid diphytanoylphosphatidylcholine and cholesterol (5:1 by weight). The recordings were made under voltage-clamp conditions (17) by using aqueous solutions that were 200 mM vs. 100 mM Na2Creatine-PO3. VDAC channels dissolved in 1% Triton X-100 were isolated and purified as described (5). On the insertion of a single channel, current recordings in response to triangular voltage waves (5 MHz, ±60 mV) were used to determine the conductance and reversal potential for the channel in the different conductance states. The ion flux of Na+, creatine-PO32−, HPO42−, and succinate2− through the channel at zero voltage was calculated as described previously (18). There are a variety of low-conducting “closed” states, and what is reported is the mean. The values for ATP were obtained by direct measurement of ATP flux by using a luciferin/luciferase assay (5).
Results
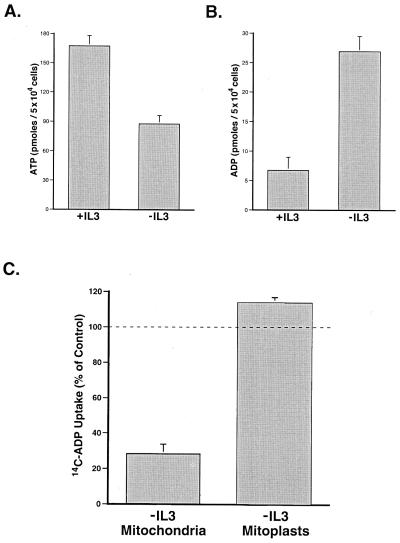
The exchange of metabolites between the matrix and the cytosol is a potential regulatory event for mitochondrial function that involves transport across both mitochondrial membranes. For instance, oxidative phosphorylation can be controlled by the rate of adenine nucleotide exchange between the matrix and the cytosol (19, 20). When IL-3-dependent FL5.12 cells are withdrawn from IL-3, the cells undergo a metabolic arrest that results in death between 18 and 24 h. In the presence of growth factors, mitochondria maintain cellular ATP levels by coupling the rate of oxidative phosphorylation to the cytosolic ATP/ADP ratio (21). However, after 12 h of growth factor deprivation, a significant decrease in cellular ATP is observed (Fig. 1A). The decrease in ATP is accompanied by an increase in cellular ADP as well as a decreased rate of electron transport, as measured by oxygen consumption (Fig. 1B and data not shown). These changes in cellular adenine nucleotide levels suggest that oxidative phosphorylation is no longer coupled to cytosolic ADP.
Figure 1.
Growth factor withdrawal results in a mitochondrial ATP/ADP exchange defect that is corrected by removal of the outer mitochondrial membrane. (A) IL-3-dependent FL5.12 cells were cultured in the presence (+IL3) or absence (−IL3) of IL-3 for 12 h before the determination of cellular ATP. The data shown are the mean (+ one SEM) of four independent experiments. The difference in ATP was statistically significant by Student's t test (P < 0.01). (B) Cells were cultured in the presence (+IL3) or absence (−IL3) of IL-3 for 12 h, and the amount of cellular ADP present was determined. The data shown are the mean (+SEM) of four independent measurements. The difference in ADP was statistically significant by Student's t test (P < 0.01). (C) Mitochondria were isolated from cells cultured in the presence or absence of IL-3 for 12 h. Where indicated, the outer mitochondrial membrane was removed by using digitonin (mitoplasts). The resultant mitoplasts contained less than 1% of the intermembrane space molecule phosphocreatine found in mitochondria yet retained the ability to carry out the ANT-specific import of ADP. ANT-dependent ADP uptake of mitochondria and mitoplasts was assessed by comparing the uptake of 14C-ADP in the presence or absence of atractyloside, a specific inhibitor of the ANT. The mean ANT-dependent uptake for mitochondria and mitoplasts from cells deprived of IL-3 (−IL3) is shown as a percentage of that observed in mitochondria and mitoplasts from cells growing in the presence of IL-3 (+SEM). The difference in ADP uptake between mitochondria and mitoplasts was statistically significant by Student's t test (P < 0.01).
Loss of ADP-coupled respiration can occur as a result of defective mitochondrial ATP/ADP exchange (19). Consistent with this hypothesis, mitochondria isolated from cells deprived of growth factor demonstrate a substantially reduced ability to take up ADP when compared with mitochondria prepared from cells growing in the presence of IL-3 (Fig. 1C). The inability of mitochondria to exchange adenine nucleotides with the cytosol could be caused by a defect in ANT function at the inner membrane or in the passage through VDAC in the outer membrane. To test whether decreased transport across the outer membrane could be responsible for the decreased uptake of ADP, the outer mitochondrial membrane was removed by using digitonin and the resulting mitoplasts tested for the uptake of ADP. In contrast to mitochondria, mitoplasts prepared from growth factor-deprived cells have a restored ability to take up ADP (Fig. 1C). In fact, mitoplasts prepared from IL-3-withdrawn cells reproducibly import more ADP than mitoplasts from cells cultured continuously in the presence of IL-3. This is consistent with the reported ADP-depleted state of mitochondria after growth factor withdrawal (3). These data suggest that a primary defect in the ANT is not responsible for the decreased rate of ATP/ADP exchange present in growth factor-deprived cells.
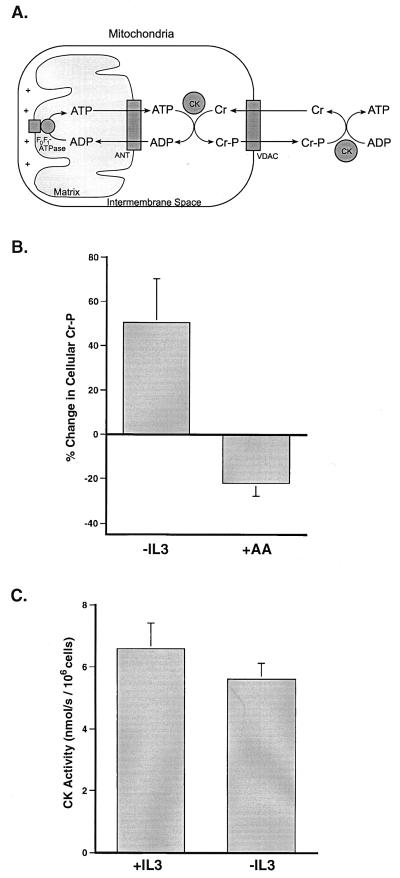
To evaluate further the outer mitochondrial membrane's role in the adenine nucleotide exchange defect, we examined changes in cellular creatine phosphate (phosphocreatine) after growth factor withdrawal. The enzyme creatine kinase transfers a phosphate moiety between ATP/ADP and creatine/phosphocreatine and allows phosphocreatine to act as an ATP buffer (Fig. 2A). Creatine kinase exists in several isoforms that are localized either to the mitochondrial intermembrane space or to the cytosol (22, 23). It has been postulated that phosphocreatine is generated in the intermembrane space because it is the only subcellular compartment that contains a sufficiently high ATP/ADP ratio to thermodynamically favor phosphocreatine synthesis (24). Phosphocreatine generated in the intermembrane space is believed to diffuse through outer membrane channels into the cytosol where its higher phosphorylation potential can be used to regenerate ATP and buffer changes in the cytosolic ATP/ADP ratio (Fig. 2A) (25). Because mitochondrial creatine kinase activity is present in the intermembrane space and absent from the matrix, the production of phosphocreatine in mitochondria depends on the bioenergetic state of the intermembrane space (23, 26–29).
Figure 2.
Growth factor-deprived cells accumulate creatine phosphate. (A) A schematic representation of the shuttle mechanism through which creatine/creatine phosphate (Cr/Cr-P) can buffer cytosolic ATP levels. (B) Cells were withdrawn from IL-3 for 12 h or treated for 30 min in the presence of IL-3 with the electron transport inhibitor, antimycin A, before the preparation of perchloric acid extracts. The amount of phosphocreatine present in the extracts was determined by using HPLC. The percent change in cellular phosphocreatine measured after IL-3 withdrawal (−IL3) and the addition of antimycin A (+AA) is shown. The data presented are the mean (+SEM) of at least four independent experiments. The changes in phosphocreatine observed in both cases were statistically significant by Student's t test (P < 0.05). (C) Creatine kinase (CK) catalyzes the reaction: ADP + Cr-P ↔ ATP + Cr, hence creatine kinase activity was assessed by measuring the ability of saponin-lysed cells to convert creatine phosphate to creatine in the presence of ADP. At the low concentrations used, saponin preferentially disrupts the plasma membrane of cells while leaving intracellular membranes intact. The CK activity present in lysates prepared from equal numbers of cells cultured in the presence (+IL3) or absence (−IL3) of IL-3 for 12 h is depicted. Data shown are the mean (+ one SD) of three independent measurements.
Despite decreased cellular ATP levels, total phosphocreatine levels are increased in cells deprived of growth factor for 12 h (Fig. 2B). To confirm that phosphocreatine pools in these cells are capable of responding to decreased cellular ATP levels, cells growing in the presence of IL-3 were treated acutely with the electron transport inhibitor antimycin A. In contrast to cells withdrawn from growth factor, cells treated with antimycin A demonstrated a decrease in cellular phosphocreatine levels (Fig. 2B). The amount of creatine present in these cells was large relative to phosphocreatine and varied little regardless of treatment (data not shown). Thus, changes in the amount of phosphocreatine will be responsible for any significant changes in the phosphocreatine/creatine ratio. In addition, cellular inorganic phosphate decreased slightly after growth factor withdrawal, suggesting that the increases observed in phosphocreatine will underestimate increases in the phosphocreatine/phosphate ratio.
The increase in phosphocreatine after growth factor withdrawal did not appear to result from inactivation of cytosolic creatine kinase. Cells growing in the presence of IL-3 or withdrawn from IL-3 for 12 h were disrupted with saponin, and their ability to convert ADP + phosphocreatine to ATP + creatine in the cytosol was assessed by using exogenous substrates (Fig. 2C). The creatine kinase activity observed after 12 h of growth factor withdrawal was only slightly reduced. The activity observed should not allow the accumulation of phosphocreatine in the presence of decreased cellular ATP levels.
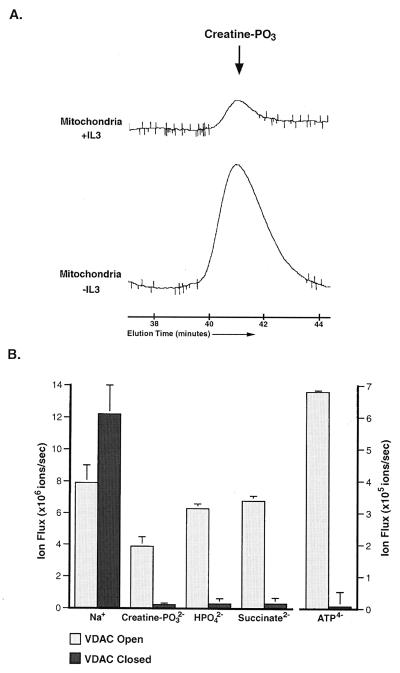
Another possible explanation for the accumulation of phosphocreatine after growth factor withdrawal is that the phosphocreatine is compartmentalized in a location where it is not available to buffer the falling ATP levels. Because one isoform of creatine kinase is located in the mitochondrial intermembrane space, phosphocreatine could be accumulating in mitochondria. To test this possibility, phosphocreatine was measured in mitochondria isolated from cells grown in the presence of IL-3 or deprived of growth factor for 12 h. Over 100-fold more phosphocreatine was present in mitochondria isolated from growth factor-deprived cells than in mitochondria from cells growing in the presence of IL-3 (Fig. 3A). Removal of the outer mitochondrial membrane with digitonin resulted in the loss of 99.5 ± 0.2% of the mitochondrial phosphocreatine, suggesting that the accumulated phosphocreatine is in the intermembrane space.
Figure 3.
Anion flux through the outer mitochondrial membrane and through isolated VDAC channels can be regulated. (A) Mitochondria were isolated from cells cultured in the presence (+IL3) or absence (−IL3) of IL-3 for 12 h, and perchloric acid extracts normalized to mitochondrial protein were prepared. The amount of creatine phosphate (creatine–PO3) present in the mitochondrial extracts was determined by HPLC. The peak corresponding to the amount of creatine–PO3 present in the mitochondria under each condition is shown. The data presented are representative of results from four independent experiments, and the increase observed in all cases was statistically significant by Student's t test (P < 0.02). The creatine-PO3 peak of both samples was confirmed by coinjection of purified creatine–PO3 (not shown). (B) The net ion flux through single VDAC channels in the high-conducting (open) state and in the low-conducting (closed) state. All fluxes shown are the mean (+SEM) and reflect ion flow in the absence of an electrical potential and in the presence of a concentration difference of 100 mM salt. The difference in phosphocreatine flux through VDAC in the open and closed states was statistically significant by Student's t test (P < 0.01).
For mitochondrial phosphocreatine levels to increase after growth factor withdrawal, adenine nucleotide exchange must be intact across the inner mitochondrial membrane. In addition, the transport of both adenine nucleotides and phosphocreatine must be limited across the outer mitochondrial membrane, otherwise both would be used to support cytosolic ATP levels. The primary transporter of metabolites across the outer mitochondrial membrane is VDAC. In the closed states, the ability of VDAC to pass small negatively charged metabolites is compromised (18). At physiological pH, ADP, ATP, and phosphocreatine all carry a net negative charge. To determine whether VDAC can adopt a conformation that would limit the flux of adenine nucleotides and phosphocreatine, we measured the permeability of VDAC to various ions in a planar phospholipid membrane. When VDAC is in the open conformation, the channel is freely permeable to both sodium and phosphocreatine (Fig. 3B). The higher flux of sodium as compared with phosphocreatine through the open channel reflects the more rapid diffusion of the smaller and less-charged sodium ion. In the closed configuration, VDAC retained the ability to pass the positively charged sodium ion, but exhibited a 15-fold decrease in its permeability to creatine-PO32−. In addition, the permeability of VDAC to ATP4−, HPO42−, and succinate2− was markedly decreased when VDAC adopted a closed configuration (Fig. 3B). This demonstrates that a general property of VDAC closure is a limitation of anion flux with little effect on cation permeability. Because the decrease in permeability for these small molecules depends on charge rather than size, the permeability of VDAC to uncharged molecules such as creatine should be unaffected. Furthermore, mitochondrial creatine levels were in excess and essentially unchanged after growth factor withdrawal (data not shown), suggesting that creatine transport across the outer membrane does not limit the production of phosphocreatine. Thus, it appears that after growth factor withdrawal, channels in the outer membrane, including VDAC, exist in a low conductance state that limits the passage of anionic metabolites. Restriction of adenine nucleotide and phosphocreatine flux through VDAC provides an explanation for the accumulation of phosphocreatine in the intermembrane space of mitochondria.
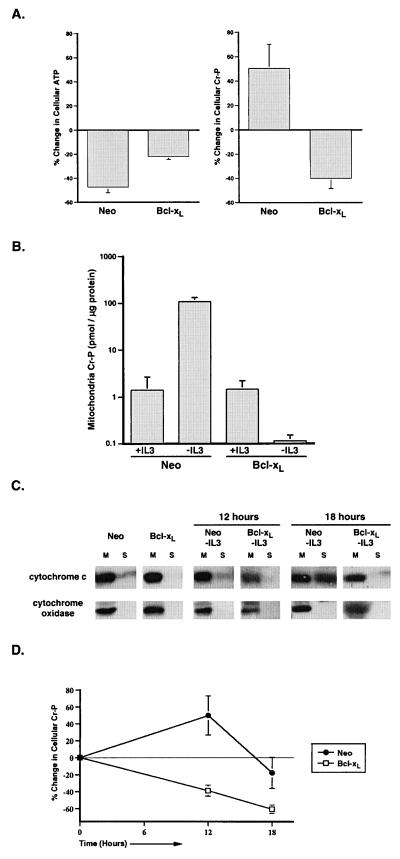
The Bcl-2 related protein, Bcl-xL, has been shown to maintain adenine nucleotide exchange across mitochondrial membranes after growth factor withdrawal (3). In addition, Bcl-xL is located on the outer mitochondrial membrane, and in vitro studies have demonstrated that the induction of transmembrane potential, which would induce closure of VDAC, can also promote the insertion of Bcl-xL into membranes forming a potential-dissipating channel (30). To test whether Bcl-xL can regulate the permeability of the outer mitochondrial membrane, FL5.12 cells stably transfected with Bcl-xL or a control vector (Neo) were examined after IL-3 withdrawal. Cellular ATP was depleted in both Bcl-xL- and control-transfected cells after 12 h of IL-3 withdrawal, although the decrease was greater in the control transfectants. However, in contrast to control-transfected cells, phosphocreatine decreased in Bcl-xL-transfected cells deprived of growth factor (Fig. 4A). To determine whether the mitochondrial phosphocreatine pools reflected the changes in total phosphocreatine levels, phosphocreatine was measured in mitochondria isolated from control- and Bcl-xL-transfected cells cultured for 12 h in the presence or absence of IL-3. Although mitochondrial phosphocreatine increased approximately 100-fold in control-transfected cells upon growth factor withdrawal, phosphocreatine levels decreased in mitochondria from Bcl-xL-transfected cells (Fig. 4B). These data suggest that after growth factor withdrawal, Bcl-xL expression enables phosphocreatine pools to remain coupled to cellular ATP levels by maintaining the exchange of phosphocreatine and adenine nucleotides across the outer mitochondrial membrane.
Figure 4.
Loss of outer mitochondrial membrane permeability after growth factor withdrawal is prevented by Bcl-xL expression. (A) Bcl-xL- and control- (Neo) transfected FL5.12 cells were cultured in the presence or absence of IL-3 for 12 h before the determination of ATP and phosphocreatine. The amount of ATP was determined by the luciferin/luciferase method, and the amount of phosphocreatine was determined by HPLC. The percent change in cellular ATP and creatine phosphate (Cr-P) measured on IL-3 withdrawal for each population is shown. The data presented are the mean (+SEM) of at least four independent determinations. The changes in ATP and phosphocreatine observed in both cases were statistically significant by Student's t test (P < 0.01). (B) Bcl-xL- and control- (Neo) transfected cells were cultured in the presence or absence of IL-3 for 12 h, and perchloric acid extracts normalized to mitochondrial protein were prepared. The mean (+SEM) amount of creatine phosphate present in the mitochondrial extracts from four independent experiments is shown. (C) Bcl-xL- and control- (Neo) transfected cells that were cultured in the presence of IL-3 or deprived of IL-3 (-IL3) for the time indicated were mechanically lysed and separated into mitochondrial (M) and S-100 (S) fractions. The cytosol is represented in the S-100 fraction. The amount of cytochrome c and cytochrome oxidase subunit IV in each fraction was determined by Western blot analysis. (D) Perchloric acid extracts were prepared from equal numbers of Bcl-xL- and control- (Neo) transfected cells that were withdrawn from IL- 3 for the period indicated. Cellular phosphocreatine was measured by using HPLC, and the percent change in phosphocreatine over time is graphed. The data presented are the mean (+SEM) of five independent experiments.
The above data demonstrated that the maintenance of outer membrane permeability is a feature of surviving cells. It remained possible that outer membrane impermeability is present only in dead or dying cells. To explore this possibility, Bcl-xL- and control-transfected cells were cultured in the presence or absence of IL-3 for 12 h and then cloned by limiting dilution in IL-3-containing medium. All four populations exhibited comparable cloning efficiencies that were each greater than 70%. Despite the impairment of outer membrane permeability observed in control transfectants deprived of growth factor for 12 h, these cells retained a cloning efficiency similar to that observed in cells cultured continuously in the presence of IL-3. Therefore, the disruption of metabolite exchange across the outer membrane is a reversible event regulated by growth factor signal transduction and prevented by Bcl-xL expression.
If phosphocreatine were trapped in the intermembrane space, a disruption of the outer membrane should release phosphocreatine into the cytosol and allow it to buffer the decreased cellular ATP levels. After 12 h of IL-3 deprivation, cytochrome c copurifies only with the mitochondrial fraction in both Bcl-xL- and control-transfected cells, demonstrating that the outer mitochondrial membrane remains intact (Fig. 4C).
However, after 18 h of growth factor withdrawal, a significant amount of cytochrome c can be copurified with the cytosolic fraction in control-transfected cells but not in Bcl-xL-expressing cells. Coincident with the disruption of the outer mitochondrial membrane, cellular phosphocreatine levels begin to decline in control-transfected cells. In contrast, Bcl-xL transfectants exhibit decreased phosphocreatine levels at all time points without loss of outer mitochondrial membrane integrity (Fig. 4D).
To determine whether decreased outer mitochondrial membrane permeability to anions is a general feature of growth factor-deprived cells, an independently derived cell line, Baf3, and a Bcl-2-transfected derivative were obtained and examined (12). When Baf3 cells are deprived of growth factor, the majority of cells die within 24 h, and this death is prevented by expression of the Bcl-xL-related protein, Bcl-2. Although greater than 90% of the cells remain viable after 12 h of growth factor withdrawal, a significant decrease in cellular ATP was observed (Fig. 5A). This decrease in ATP was accompanied by an increase in cellular ADP (Fig. 5B). As in FL5.12 cells, total phosphocreatine levels increased in Baf3 cells after 12 h of growth factor withdrawal despite the fall in ATP (Fig. 5C). Phosphocreatine levels were also increased in mitochondria isolated from growth factor-deprived cells, suggesting that phosphocreatine is accumulating in the intermembrane space (Fig. 5D). These data suggest that the ability of metabolic anions to equilibrate across the outer mitochondrial membrane is disrupted in growth factor-withdrawn cells.
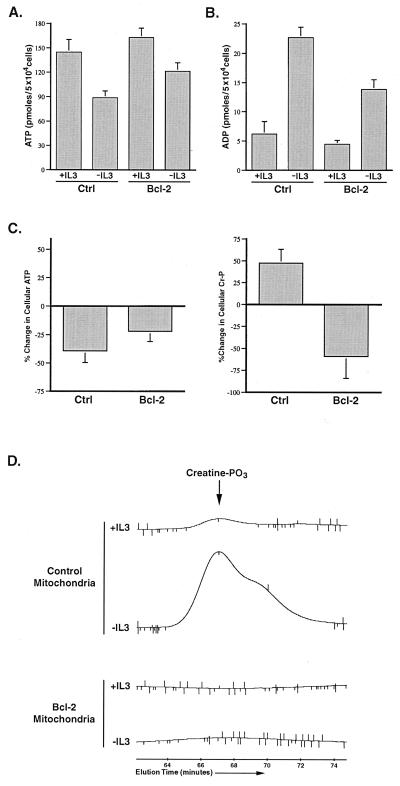
Figure 5.
Growth factor withdrawal in Baf3 cells results in mitochondrial creatine phosphate accumulation that is prevented by Bcl-2 expression. (A) Control (Ctrl) and Bcl-2-transfected Baf3 cells were cultured in the presence (+IL3) or absence (−IL3) of IL-3 for 12 h before the determination of cellular ATP. The data shown are the mean (+SEM) of four independent experiments. The fall in ATP observed on IL-3 withdrawal was statistically significant by Student's t test (P < 0.05). (B) Control (Ctrl) and Bcl-2-transfected cells were cultured in the presence (+IL3) or absence (−IL3) of IL-3 for 12 h, and the amount of cellular ADP present was determined. The data shown are the mean (+SEM) of four independent measurements. The increase in ADP on IL-3 withdrawal was statistically significant by Student's t test (P < 0.05). (C) Control and Bcl-2-transfected cells were cultured in the presence or absence of IL-3 for 12 h before the determination of ATP and phosphocreatine. The percent change in cellular ATP and creatine phosphate (Cr-P) measured on IL-3 withdrawal for each population is shown. The data presented are the mean (+SEM) of four independent determinations. The changes in ATP and phosphocreatine observed in both cases were statistically significant by Student's t test (P < 0.05). (D) Control and Bcl-2-transfected cells were cultured in the presence or absence of IL-3 for 12 h, and perchloric acid extracts normalized to mitochondrial protein were prepared. The amount of creatine phosphate (creatine-PO3) present in the mitochondrial extracts was determined by HPLC. The peak corresponding to the amount of creatine-PO3 present in the mitochondria under each condition is shown. The creatine-PO3 peak in the samples was confirmed by coinjection of purified creatine-PO3 (not shown).
Because Bcl-2 expression protects Baf3 cells from death after growth factor withdrawal, a Baf3 line expressing Bcl-2 was used to test whether outer membrane permeability could be regulated by Bcl-2. After 12 h of IL-3 withdrawal, the cellular ATP/ADP ratio was decreased, although not to the same extent as that observed in control cells deprived of growth factor (Fig. 5). However, in contrast to the results obtained with control cells, phosphocreatine levels decreased in Bcl-2 expressing cells, and phosphocreatine did not accumulate in mitochondria. These data suggest that Bcl-2 expression enables phosphocreatine levels to remain coupled to the cellular ATP/ADP ratio. In addition, these data suggest that, like Bcl-xL, Bcl-2 can function to maintain outer mitochondrial membrane permeability to metabolic anions after growth factor withdrawal.
Discussion
It has been believed that the outer mitochondrial membrane is constitutively permeable to metabolites involved in oxidative phosphorylation. Here we demonstrate that mitochondrial outer membrane permeability is subject to regulation by growth factor withdrawal and the activity of the antiapoptotic proteins Bcl-xL and Bcl-2. The change in permeability correlates with properties of VDAC permeability observed in planar phospholipid membranes. For example, in the open state, VDAC is permeable to phosphocreatine, whereas in a closed configuration, the permeability of VDAC to phosphocreatine is greatly reduced. Thus, it appears that the ability of VDAC to adopt closed configurations may have important implications for the control of cellular bioenergetics and the regulation of apoptosis.
The disruption of mitochondrial ATP/ADP exchange with the cytosol is among the earliest identified defects in cells withdrawn from growth factor that may contribute to the initiation of apoptosis (3). Here we demonstrate that the defect is in the transport of complex anions, including adenine nucleotides, across the outer mitochondrial membrane. These data also suggest that the ANT retains the ability to exchange adenine nucleotides between the matrix and the intermembrane space, but that this function is limited by transport across the outer membrane. The outer mitochondrial membrane proteins Bcl-xL and Bcl-2 appear to maintain mitochondrial ATP/ADP exchange across the inner membrane by facilitating outer membrane permeability to adenine nucleotides. Because both Bcl-xL and Bcl-2 ion channels show some selectivity for monovalent cations in vitro, it is unlikely that these proteins function as anion channels to directly maintain outer membrane permeability to anionic metabolites (30, 31). However, Bcl-xL and Bcl-2 may act to influence the properties of other outer membrane proteins to maintain their ability to pass complex anions. Recently it has been reported that Bcl-xL can interact with VDAC and regulate its gating properties in vitro (32). After growth factor withdrawal, VDAC must assume a closed configuration to render it impermeable to phosphocreatine and ADP. Thus, it is possible that in vivo, Bcl-xL and Bcl-2 function to maintain VDAC and/or other outer membrane channels in an open configuration. The ability to maintain metabolic coupling between the mitochondria and the cytosol is a mechanism by which Bcl-2 proteins could function to promote cell survival. In contrast, Shimizu and colleagues (32) have suggested that the proapoptotic Bcl-2 family members Bax and Bak disrupt the integrity of VDAC, allowing it to adopt a nonphysiologic open state that permits the apoptogenic release of cytochrome c. The ability of Bcl-2 proteins to regulate the open state and integrity of VDAC could thus account for their ability to be either anti- or proapoptotic.
Under normal physiological conditions, VDAC exists in an open configuration that permits the exchange of anionic metabolites across the outer mitochondrial membrane (18). However, in this physiological open state, VDAC is not permeable to cytochrome c. The ability of Bcl-xL to prevent cytochrome c permeability of VDAC does not suggest that Bcl-xL induces VDAC to adopt a closed conformation that is impermeable to metabolic anions such as ADP and phosphocreatine. In fact, the present data suggest that Bcl-xL promotes the maintenance of VDAC in a physiological open state after growth factor withdrawal.
Although the loss of outer membrane anion permeability appears to contribute to the initiation of apoptosis after growth factor withdrawal, it is not an essential step in all apoptosis paradigms. For instance, we have found that the mitochondrial exchange defects demonstrated here do not play an obligatory role in Fas- or TNF-induced cell death. Nevertheless, the shared ability of Bcl-xL and Bcl-2 to prevent cell death after growth factor withdrawal correlates with the ability of these proteins to prevent the accumulation of metabolic anion gradients across the outer mitochondrial membrane.
One of the earliest known responses to growth factor withdrawal is a metabolic arrest characterized by a decrease in the mitochondrial membrane potential as well as a decrease in the rate of electron transport (3, 33). How this metabolic arrest relates to outer mitochondrial membrane impermeability remains unclear. Because growth factors are known to regulate glycolysis (34, 35), it is tempting to speculate that changes in the regulation of glycolysis after growth factor withdrawal result in a perturbation of cellular metabolism that leads to VDAC closure. For example, NADH can induce VDAC closure in planar phospholipid membranes (36). Regardless of the mechanism by which it is induced, VDAC closure would be predicted to have profound effects on cellular bioenergetics. In addition to the effects on adenine nucleotide and phosphocreatine exchange between the cytosol and the mitochondria, the ability of VDAC to switch between an open and closed configuration is likely to affect a number of other exchange reactions, including NADH exchange through the malate–aspartate and glycerol phosphate shuttles.
The ability of VDAC to switch between open and closed configurations in vivo may regulate mitochondrial adaptation to additional physiological stresses. For instance, the acute mitochondrial response to cellular anoxia has been characterized as a simultaneous disruption in the exchange of multiple anions between the matrix and the cytosol (37). The complex disruption in ion exchange that accompanies anoxia could be explained by a single defect in anion transport across the outer membrane, analogous to that observed in this study. Thus, VDAC closure could serve to isolate mitochondria from the cytosol during periods of cellular stress. Consistent with this hypothesis, Gram-negative bacteria use VDAC-like porins in their outer membranes as part of a stress response to regulate the periplasmic space and enable the inner membrane to maintain homeostasis (38–40). The ability of VDAC to regulate the environment of the intermembrane space might be a similar adaptive response for mitochondria, reflecting their origin as endosymbiotic organisms.
Acknowledgments
We thank members of the Thompson laboratory for thoughtful discussions and comments on the manuscript and J. Ashman for technical advice. We also thank T. Look for the gift of Baf3 cells and S. Talapatra and D. Plas for helpful advice. M.G.V. is supported in part by the University of Chicago Cancer Research Foundation Women's Board, and N.S.C. is a fellow supported by the American Lung Association. In addition, this work was supported in part by grants from the National Cancer Institute and the National Institutes of Health.
Abbreviations
- ANT
adenine nucleotide translocator
- VDAC
voltage dependent anion channel
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090082297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090082297
References
- 1.Reed J C. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden M G, Chandel N S, Schumacker P T, Thompson C B. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- 4.Wallace D C. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 5.Rostovtseva T, Colombini M. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise L H, Thompson C B, Nunez G. Development (Cambridge, UK) 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 7.de Jong D, Prins F A, Mason D Y, Reed J C, van Ommen G B, Kluin P M. Cancer Res. 1994;54:256–260. [PubMed] [Google Scholar]
- 8.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 9.Nguyen M, Millar D G, Yong V W, Korsmeyer S J, Shore G C. J Biol Chem. 1993;268:25265–25268. [PubMed] [Google Scholar]
- 10.Mannella C A. Trends Biochem Sci. 1992;17:315–320. doi: 10.1016/0968-0004(92)90444-e. [DOI] [PubMed] [Google Scholar]
- 11.Colombini M. J Membr Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- 12.Inukai T, Inoue A, Kurosawa H, Kumiko G, Shinjyo T, Ozawa K, Mao M, Inaba T, Look A T. Mol Cell. 1999;4:343–352. doi: 10.1016/s1097-2765(00)80336-6. [DOI] [PubMed] [Google Scholar]
- 13.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 14.Schnaitman C, Greenawalt J W. J Cell Biol. 1968;38:158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budinger G R, Chandel N, Shao Z H, Li C Q, Melmed A, Becker L B, Schumacker P T. Am J Physiol. 1996;270:L44–L53. doi: 10.1152/ajplung.1996.270.1.L44. [DOI] [PubMed] [Google Scholar]
- 16.Montal M, Mueller P. Proc Natl Acad Sci USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombini M. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- 18.Hodge T, Colombini M. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 19.Brown G C. Biochem J. 1992;284:1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erecinska M, Wilson D F. J Membr Biol. 1982;70:1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- 21.Saraste M. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 22.Wallimann T. Curr Biol. 1994;4:42–46. doi: 10.1016/s0960-9822(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 23.Wyss M, Smeitink J, Wevers R A, Wallimann T. Biochim Biophys Acta. 1992;1102:119–166. doi: 10.1016/0005-2728(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 24.Saks V A, Chernousova G B, Gukovsky D E, Smirnov V N, Chazov E I. Eur J Biochem. 1975;57:273–290. doi: 10.1111/j.1432-1033.1975.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 25.Bessman S P, Geiger P J. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- 26.Jacobus W E, Lehninger A L. J Biol Chem. 1973;248:4803–4810. [PubMed] [Google Scholar]
- 27.Bessman S P, Fonyo A. Biochem Biophys Res Commun. 1966;22:597–602. doi: 10.1016/0006-291x(66)90317-2. [DOI] [PubMed] [Google Scholar]
- 28.Klingenberg M. FEBS Lett. 1970;6:145–154. doi: 10.1016/0014-5793(70)80044-8. [DOI] [PubMed] [Google Scholar]
- 29.Scholte H R, Weijers P J, Wit-Peeters E M. Biochim Biophys Acta. 1973;291:764–773. doi: 10.1016/0005-2736(73)90479-3. [DOI] [PubMed] [Google Scholar]
- 30.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 31.Lam M, Bhat M B, Nunez G, Ma J, Distelhorst C W. J Biol Chem. 1998;273:17307–17310. doi: 10.1074/jbc.273.28.17307. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 33.Garland J M, Halestrap A. J Biol Chem. 1997;272:4680–4688. doi: 10.1074/jbc.272.8.4680. [DOI] [PubMed] [Google Scholar]
- 34.Hue L, Rousseau G G. Adv Enzyme Regul. 1993;33:97–110. doi: 10.1016/0065-2571(93)90011-2. [DOI] [PubMed] [Google Scholar]
- 35.Rozengurt E. Curr Top Cell Regul. 1980;17:59–88. doi: 10.1016/b978-0-12-152817-1.50007-9. [DOI] [PubMed] [Google Scholar]
- 36.Zizi M, Forte M, Blachly-Dyson E, Colombini M. J Biol Chem. 1994;269:1614–1616. [PubMed] [Google Scholar]
- 37.Aw T Y, Andersson B S, Jones D P. Am J Physiol. 1987;252:C356–C361. doi: 10.1152/ajpcell.1987.252.4.C356. [DOI] [PubMed] [Google Scholar]
- 38.Zambrano M M, Kolter R. Cell. 1996;86:181–184. doi: 10.1016/s0092-8674(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 39.Rudel T, Schmid A, Benz R, Kolb H A, Lang F, Meyer T F. Cell. 1996;85:391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 40.Schindler H, Rosenbusch J P. Proc Natl Acad Sci USA. 1978;75:3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]