Abstract
An 8-month-old boy developed a necrotic lung mass from which Burkholderia glumae was recovered, leading to the diagnosis of chronic granulomatous disease (CGD). While other Burkholderia species have been identified as important pathogens in persons with CGD, B. glumae has not been previously reported to cause human infection.
CASE REPORT
An 8-month-old boy presented to his pediatrician with a 3-day history of fevers in the absence of other symptoms. History and physical examination provided no obvious source for the fevers. Laboratory evaluation revealed a white blood cell count of 29,000 per μl (49% neutrophils, 16% bands, and 29% lymphocytes), a hematocrit of 29% and a platelet count of 451,000 per μl. Blood cultures obtained at this visit were negative. He continued to have fevers during the next 4 days, after which time a chest radiograph revealed bilateral perihilar infiltrates. Treatment with oral amoxicillin-clavulanate was initiated, but treatment was switched to intramuscular ceftriaxone after 4 days. After 2 days of ceftriaxone, therapy was changed to oral cefdinir, which the patient received for 8 days. Throughout this time he continued to have daily fevers as high as 105°F. A repeat chest radiograph again demonstrated bilateral infiltrates. Therapy with azithromycin was initiated, and the patient was referred to a pediatric pulmonology clinic for further evaluation.
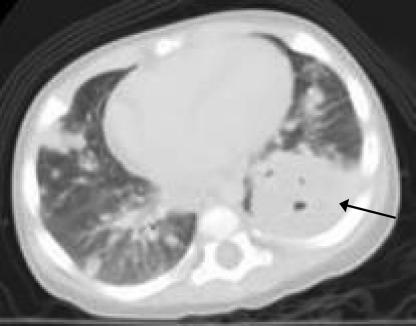
In the clinic, the patient's physical examination was significant for decreased breath sounds in the middle left and upper right lung fields. A chest radiograph showed heterogeneous, multifocal, and somewhat nodular opacities that were most confluent in the right upper and left lower lobes. Computerized tomography (CT) of the chest, abdomen, and pelvis revealed a large (3.9 by 4.4 cm) mass within the left lower lobe and numerous nodules, the largest of which measured 2.2 by 2.1 cm, distributed diffusely throughout the lungs (Fig. 1). Enlarged cervical lymph nodes and diffuse mediastinal lymphadenopathy were present. The patient was admitted to the hospital, and an open biopsy of the left lung revealed a necrotic, exophytic tumor involving the majority of the lower lobe. Several specimens were obtained from the main mass, and a wedge biopsy specimen containing two small nodules was obtained from the apical segment of the left upper lobe. A peripheral nodule in the left lower lobe was also resected. There was no evidence of pleura-based disease. Initial gram stains of the surgical specimens revealed no organisms and rare white blood cells. Histopathology revealed a diffuse pattern of acute pneumonia, with focal areas of necrosis and areas of organization and coalescence into poorly formed granulomata. The cellular composition of the inflammation was polymorphous; no malignant cells were identified. These findings were deemed most consistent with an acute infectious etiology; however, no bacteria, fungi, or mycobacteria were identified on special histochemical stains (periodic acid-Schiff, Gomori methenamine silver, and Brown Brenn) performed on multiple tissue samples from different sites.
FIG. 1.
CT of the chest demonstrating a 3.9- by 4.4-cm mass within the lower lobe of the left lung (arrow) and numerous smaller nodules in both lungs.
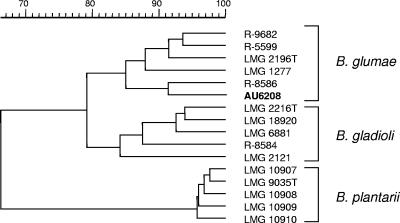
Blood cultures taken on separate days, two of which were taken prior to initiation of antibiotic therapy, remained negative. However, a surgical specimen culture was positive for a nonfermenting gram-negative rod after 2 days of incubation. This organism was oxidase negative and catalase positive, did not grow on cetrimide, and did grow on Trypticase soy agar at 42°C. The organism was presumptively identified as Burkholderia cepacia (similarity index of 0.572) by analysis of cell wall fatty acid composition (Microbial Identification System, version 4; MIDI, Inc. Newark, DE). Since the biochemical profile was not consistent with B. cepacia complex, the analysis of cell wall fatty acid composition was repeated the following day and again gave a presumptive identification of Burkholderia cepacia (similarity index, 0.669). A 16S rRNA-based PCR-based assay specific for Burkholderia spp. was positive; however,16S rRNA- and recA-based PCR assays specific for Burkholderia gladioli and species within the Burkholderia cepacia complex were negative (22, 23, 37). This strain, designated AU6208, was further analyzed by comparing its whole-cell protein profile with those of several reference strains. Whole-cell protein electrophoresis profiles correlate very well with levels of whole-genome DNA-DNA hybridizations; the latter being the decisive criterion for species delineation (7, 35). Preparation of a protein extract and sodium dodecyl sulfate-polyacrylamide gel electrophoresis were performed as described previously (24, 34). A numerical analysis of protein profiles of AU6208 and phylogenetically related reference strains, including Burkholderia glumae, B. gladioli, and Burkholderia plantarii, was performed using the GelCompar 4.0 software package (Applied Maths, Sint Martens Latem, Belgium) as described previously (6, 7, 34). Figure 2 demonstrates that strain AU6208 clusters among the B. glumae reference strains. Finally, DNA sequence analysis of the complete 16S rRNA gene of AU6208 demonstrated a similarity level of 99.9% with sequences of B. glumae strains, including the species type strain ATCC 33617 (36). This nucleotide sequence has been deposited in the GenBank database (see “Nucleotide sequence accession number” below). Antimicrobial susceptibility testing of AU6208 indicated susceptibility to piperacillin, ceftazidime, gentamicin, trimethoprim-sulfamethoxazole, and ciprofloxacin.
FIG. 2.
Dendrogram derived from the unweighted-pair group average linkage of correlation coefficients between the protein patterns of strain AU6208 and reference strains of B. glumae, B. gladioli, and B. plantarii. The rule at the top shows the percent similarity.
After surgery, therapy with cefotaxime and gentamicin was initiated, but therapy was changed to ceftazidime and gentamicin once susceptibility results were available. The patient defervesced within 1 week of surgery, and a repeat chest CT demonstrated an improvement in the bilateral pulmonary nodules. The presence of poorly formed granulomata in the setting of extensive pneumonia in the patient's lung biopsy specimens and the recovery of an unusual Burkholderia species suggested the possibility of an underlying granulocyte disorder. Further evaluation revealed a normal absolute lymphocyte count, slightly elevated immunoglobulin G (IgG) and IgA with normal IgM and IgE, normal total hemolytic complement (CH50), and a normal sweat chloride test. The neutrophil oxidative burst assay was clearly abnormal on two occasions, consistent with a diagnosis of chronic granulomatous disease (CGD). Commercially available sequencing of the CYBB gene was normal, ruling out the common X-linked form of CGD. The 2-base deletion in the gene NCF1 most commonly associated with autosomal recessive CGD was not present, and the sequence of CYBA, responsible for a small percentage of autosomal cases, was also normal. The patient's mutation is presumed to be in NCF2, the other of the less common forms of autosomal recessive CGD. After completion of a 4-week course of intravenous antibiotics, prophylactic treatment with trimethoprim-sulfamethoxazole was begun. There has been no evidence of recurrent pneumonia during the subsequent 31 months of follow-up during which the patient successfully underwent an allogeneic hematopoietic stem cell transplant from an HLA-identical sibling.
The genus Burkholderia comprises in excess of 40 species, most of which are typically nonpathogenic for humans. Important exceptions are Burkholderia mallei and Burkholderia pseudomallei, which cause glanders and melioidosis, respectively, and are considered potential agents of bioterrorism. The closely related species within the B. cepacia complex are well-known opportunistic pathogens affecting persons with cystic fibrosis or CGD (8). To our knowledge, there have been no previous descriptions of human infection caused by B. glumae, an important pathogen of plants. In the case described here, the recovery and identification of this unusual species from an invasive pulmonary infection in a young child led to the diagnosis of CGD.
CGD was first described in 1967 as a primary immunodeficiency characterized by defects in superoxide-generating NADPH oxidase (16, 25). These defects result in the inability of neutrophils to generate superoxide derivatives, such as hydrogen peroxide, hydrophilous acids, and hydroxyl radicals, which in turn impairs killing of intracellular microbes (15). Any one of the four subunits of NADPH oxidase may be involved in CGD. The most common form (approximately 70% of cases) is an X-linked mutation in the gene for phagocyte oxidase cytochrome glycoprotein of 91 kDa (gp91phox), while other forms involve autosomal recessive mutations in genes for other NADPH oxidase components, such as p22phox, p47phox, and p67phox (15, 38). Patients with CGD are at increased risk for invasive infections caused by various catalase-positive bacteria including Staphylococcus aureus, Aspergillus spp., Nocardia spp., and Serratia marcescens (12, 20). Certain Burkholderia species, particularly those within the B. cepacia complex, are well-recognized pathogens in CGD patients (1, 2, 19, 30). In fact, these species are especially virulent in CGD, causing sepsis and being responsible for a significant proportion of fatalities in these patients (38). More recently, B. pseudomallei and B. gladioli also have been reported to cause infection in CGD (3, 11, 26). Although the precise reasons for the enhanced susceptibility of CGD patients to infection with Burkholderia species are not clear, these species, in addition to producing catalase, are resistant to nonoxidative killing by neutrophil cationic antibacterial peptides and proteolytic enzymes (31). Bylund and colleagues (5) also recently demonstrated that ingested Burkholderia induced significant levels of necrosis in neutrophils from patients with CGD, suggesting yet another mechanism to explain the severe pathology seen with Burkholderia infection in this patient population.
B. glumae, first described in Japan in 1976 (33), has been isolated in association with various plants including tomato, hot pepper, eggplant, potato, perilla, sesame, and sunflower (17). It is found epiphytically on rice leaves and in the rice rhizosphere (32) and is an important pathogen of rice, being implicated as a major cause of rice grain discoloration and seedling rot (9, 10, 14, 33). It has been associated with epidemics of rice panicle blight in the southeastern United States (29), producing yield losses as high as 40% (27). There is some evidence to suggest that this disease is seed borne, transmitted from plants infected the previous year (30). Two toxins, fervenulin and toxoflavin, appear to be involved in the phytopathology of B. glumae (28).
The identification of B. glumae presents a challenge to the clinical microbiology laboratory. Burkholderia species, in general, can be difficult to distinguish from other closely related genera such as Ralstonia, Cupriavidus, and Pandoraea (21). Further, commercial bacterial identification systems do not reliably differentiate individual Burkholderia species, most of which are not human pathogens (4, 18). In this case, cellular fatty acid analysis indicated that the recovered organism was a likely Burkholderia species. Definitive identification was provided by a polyphasic analysis that included sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteins and DNA sequence analysis of the 16S rRNA gene. This case underscores recommendations that encourage the use of reference laboratories capable of performing genetics-based assays for the identification of Burkholderia and related species (13). Such laboratories play an important role in contributing to the clinical care of infected patients and to studies of the epidemiology of unusual organisms in human disease.
In summary, the isolation of B. glumae from surgical specimens from lung lesions in the patient described demonstrates this species' human pathogenic potential and expands the spectrum of Burkholderia species involved in infection of patients with CGD. The identification of this typically nonpathogenic Burkholderia species resulted in a high index of suspicion of an underlying immunodeficiency, which ultimately led to the diagnosis of CGD.
Nucleotide sequence accession number.
The nucleotide sequence of strain AU6208 has been deposited in the GenBank database under accession number DQ97837.
Acknowledgments
This work was supported by the Cystic Fibrosis Foundation.
Footnotes
Published ahead of print on 29 November 2006.
REFERENCES
- 1.Ahlin, A., M. De Boer, D. Roos, J. Leusen, C. I. Smith, U. Sundin, H. Rabbani, J. Palmblad, and G. Elinder. 1995. Prevalence, genetics and clinical presentation of chronic granulomatous disease in Sweden. Acta Paediatr. 84:1386-1394. [DOI] [PubMed] [Google Scholar]
- 2.Bottone, E. J., S. D. Douglas, A. R. Rausen, and G. T. Keusch. 1975. Association of Pseudomonas cepacia with chronic granulomatous disease. J. Clin. Microbiol. 1:425-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyanton, B. L., Jr., L. M. Noroski, H. Reddy, M. K. Dishop, M. J. Hicks, J. Versalovic, and E. H. Moylett. 2005. Burkholderia gladioli osteomyelitis in association with chronic granulomatous disease: case report and review. Pediatr. Infect. Dis. J. 24:837-839. [DOI] [PubMed] [Google Scholar]
- 4.Brisse, S., S. Stefani, J. Verhoef, A. Van Belkum, P. Vandamme, and W. Goessens. 2002. Comparative evaluation of the BD Phoenix and VITEK 2 automated instruments for identification of isolates of the Burkholderia cepacia complex. J. Clin. Microbiol. 40:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bylund, J., P. A. Campsall, R. C. Ma, B. A. Conway, and D. P. Speert. 2005. Burkholderia cenocepacia induces neutrophil necrosis in chronic granulomatous disease. J. Immunol. 174:3562-3569. [DOI] [PubMed] [Google Scholar]
- 6.Coenye, T., M. Gillis, and P. Vandamme. 2000. Pseudomonas antimicrobica Attafuah and Bradbury 1990 is a junior synonym of Burkholderia gladioli (Severini 1913) Yabuuchi et al. 1993. Int. J. Syst. Evol. Microbiol. 50(Pt. 6):2135-2139. [DOI] [PubMed] [Google Scholar]
- 7.Coenye, T., B. Holmes, K. Kersters, J. R. W. Govan, and P. Vandamme. 1999. Burkholderia cocovenenans (van Damme et al. 1960) Gillis et al. 1995 and Burkholderia vandii Urakami et al. 1994 are junior synonyms of Burkholderia gladioli (Severini 1913) Yabuuchi et al. 1993 and Burkholderia plantarii (Azegami et al. 1987) Urakami et al. 1994, respectively. Int. J. Syst. Bacteriol. 49:37-42. [DOI] [PubMed] [Google Scholar]
- 8.Coenye, T., P. Vandamme, J. R. W. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottyn, B., M. T. Cerez, M. F. Van Outryve, J. Barroga, J. Swings, and T. W. Mew. 1996. Bacterial diseases of rice. I. Pathogeneic bacteria associated with sheath rot complex and grain discoloration of rice in the Philippines. Plant Dis. 80:429-437. [Google Scholar]
- 10.Cottyn, B., M. F. Van Outryve, M. T. Cerez, M. De Cleene, J. Swings, and T. W. Mew. 1996. Bacterial diseases of rice. II. Characterization of pathogenic bacteria associated with sheath rot complex and discoloration of rice in the Philippines. Plant Dis. 80:438-445. [Google Scholar]
- 11.Dorman, S. E., V. J. Gill, J. I. Gallin, and S. M. Holland. 1998. Burkholderia pseudomallei infection in a Puerto Rican patient with chronic granulomatous disease: case report and review of occurrences in the Americas. Clin. Infect. Dis. 26:889-894. [DOI] [PubMed] [Google Scholar]
- 12.Gallin, J. I. 1991. Interferon-gamma in the management of chronic granulomatous disease. Rev. Infect. Dis. 13:973-978. [DOI] [PubMed] [Google Scholar]
- 13.Gilligan, P. H., D. L. Kiska, and M. D. Appleman. 2006. Cumitech 43, cystic fibrosis microbiology. ASM Press, Washington, DC.
- 14.Goto, L., and K. Ohata. 1956. New bacterial diseases of rice (brown stripe and grain rot). Ann. Phytopath. Soc. Jpn. 21:46-47. [Google Scholar]
- 15.Heyworth, P. G., A. R. Cross, and J. T. Curnutte. 2003. Chronic granulomatous disease. Curr. Opin. Immunol. 15:578-584. [DOI] [PubMed] [Google Scholar]
- 16.Holmes, B., A. R. Page, and R. A. Good. 1967. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J. Clin. Investig. 46:1422-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong, Y., J. Kim, S. Kim, Y. Kang, T. Nagamatsu, and I. Hwang. 2003. Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis. 87:890-895. [DOI] [PubMed] [Google Scholar]
- 18.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. H. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacy, D. E., D. A. Spencer, A. Goldstein, P. H. Weller, and P. Darbyshire. 1993. Chronic granulomatous disease presenting in childhood with Pseudomonas cepacia septicaemia. J. Infect. 27:301-304. [DOI] [PubMed] [Google Scholar]
- 20.Lekstrom-Himes, J. A., and J. I. Gallin. 2000. Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 343:1703-1714. [DOI] [PubMed] [Google Scholar]
- 21.LiPuma, J., B. J. Currie, G. D. Lum, and P. A. R. Vandamme. Burkholderia, Stenotrophomonas, Ralstonia, Cupriavidus, Pandoraea, Brevundimonas, Comamonas, Delftia, and Acidovorax. In Manual of clinical microbiology, 9th ed., in press. ASM Press, Washington, DC.
- 22.LiPuma, J. J., B. J. Dulaney, J. D. McMenamin, P. W. Whitby, T. L. Stull, T. Coenye, and P. Vandamme. 1999. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 37:3167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In B. Pot, P. Vandamme, and K. Kersters (ed.), Modern microbial methods chemical methods in bacterial systematics. J. Wiley and Sons, Chichester, United Kingdom.
- 25.Quie, P. G., J. G. White, B. Holmes, and R. A. Good. 1967. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J. Clin. Investig. 46:668-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renella, R., J. M. Perez, S. Chollet-Martin, S. Sarnacki, A. Fischer, S. Blanche, J. L. Casanova, and C. Picard. 2006. Burkholderia pseudomallei infection in chronic granulomatous disease. Eur. J. Pediatr. 165:175-177. [DOI] [PubMed] [Google Scholar]
- 27.Rush, M. C., Q. M. Shao, S. Zhang, A. K. Shahjahan, K. O'Reilly, D. Shih, D. Groth, and S. D. Linscombe. 2003. Biotechnology and control of rice diseases. La. Agric. 46:20-23. [Google Scholar]
- 28.Sato, Z., Y. Koiso, S. Iwasaki, I. Matsuda, and A. Shirata. 1989. Toxins produced by Pseudomonas glumae. Ann. Phytopath. Soc. Jpn. 55:353-356. [Google Scholar]
- 29.Shahjahan, A. K., M. C. Rush, D. Groth, and C. Clark. 2000. Panicle blight. Rice J. 103:26-28. [Google Scholar]
- 30.Sieber, O. F., Jr., and V. A. Fulginiti. 1976. Pseudomonas cepacia pneumonia in a child with chronic granulomatous disease and selective IgA deficiency. Acta Paediatr. Scand. 65:519-520. [DOI] [PubMed] [Google Scholar]
- 31.Speert, D. P., M. Bond, R. C. Woodman, and J. T. Curnutte. 1994. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J. Infect. Dis. 170:1524-1531. [DOI] [PubMed] [Google Scholar]
- 32.Tsushima, S., H. Naito, and M. Koitabashi. 1996. Population dynamics of Pseudomonas glumae, the causal agent of bacterial grain rot of rice, on leaf sheafs of rice plants in relation to disease development in the field. Ann. Phytopath. Soc. Jpn. 62:108-113. [Google Scholar]
- 33.Uematsu, T., D. Yoshimura, K. Nishiyama, T. Ibaragi, and H. Fujii. 1976. Pathogenic bacterium causing seedling rot of rice. Ann. Phytopath. Soc. Jpn. 42:464-471. [Google Scholar]
- 34.Vandamme, P., B. Holmes, M. Vancanneyt, T. Coenye, B. Hoste, R. Coopman, H. Revets, S. Lauwers, M. Gillis, K. Kersters, and J. R. Govan. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188-1200. [DOI] [PubMed] [Google Scholar]
- 35.Vandamme, P., B. Pot, M. Gillis, P. de Vos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viallard, V., I. Poirier, B. Cournoyer, J. Haurat, S. Wiebkin, K. Ophel-Keller, and J. Balandreau. 1998. Burkholderia graminis sp. nov., a rhizospheric Burkholderia species, and reassessment of [Pseudomonas] phenazinium, [Pseudomonas] pyrrocinia and [Pseudomonas] glathei as Burkholderia. Int. J. Syst. Bacteriol. 48:549-563. [DOI] [PubMed] [Google Scholar]
- 37.Whitby, P. W., L. C. Pope, K. B. Carter, J. J. LiPuma, and T. L. Stull. 2000. Species specific PCR as a tool for the identification of Burkholderia gladioli. J. Clin. Microbiol. 38:282-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkelstein, J. A., M. C. Marino, R. B. Johnston, Jr., J. Boyle, J. Curnutte, J. I. Gallin, H. L. Malech, S. M. Holland, H. Ochs, P. Quie, R. H. Buckley, C. B. Foster, S. J. Chanock, and H. Dickler. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79:155-169. [DOI] [PubMed] [Google Scholar]