Abstract
The correlation and the level of agreement between the standardized agar dilution and the agar disk diffusion methods for antimicrobial susceptibility testing of Campylobacter were investigated. A high-level agreement between the two methods was evident for aminoglycosides and fluoroquinolones, while a low-level agreement was observed for other antibiotics.
Campylobacter species, particularly Campylobacter jejuni, have been recognized as an important cause of food-borne bacterial diarrhea in humans worldwide (2). As enteric organisms, Campylobacter spp. are carried in the intestinal tracts of food animals, especially poultry, and they are often present in food of animal origin through fecal contamination during processing (17). Although most patients with Campylobacter infections do not require antibiotic treatment, antimicrobial therapy is necessary for patients with severe or prolonged systemic diseases (2, 4). In this circumstance, macrolides (e.g., erythromycin) and fluoroquinolones (e.g., ciprofloxacin) are considered the drugs of choice (2, 5). However, other antibiotics such as gentamicin, tetracycline, clindamycin, and ampicillin may be listed as alternative drugs for the treatment of systemic Campylobacter infections (5). Usually, antimicrobial susceptibility testing prior to treatment of Campylobacter infections is unnecessary; however, it may be useful, especially with the increase of resistant Campylobacter organisms. Several antimicrobial susceptibility testing methods, including agar dilution, broth microdilution, epsilometer test (E-test), and disk diffusion test, have been used to measure antimicrobial resistance in Campylobacter species (1, 9, 10, 11, 12, 13, 14, 15, 16, 18, 19, 20, 24). Recently, the agar dilution method has been considered a standard antimicrobial susceptibility testing method for thermophilic Campylobacter species (19, 22). Although the agar dilution method is reliable and highly reproducible and also provides quantitative MICs, it is a labor-intensive, time-consuming, and costly test (6, 20). Alternatively, the agar diffusion test, such as the disk diffusion method, is simple and inexpensive and can provide reproducible results if it is conducted carefully with appropriate standardization and quality controls (6, 25). Over the years, several comparisons of the agreement between different antimicrobial susceptibility testing methods for Campylobacter species have been conducted (1, 9, 10, 11, 12, 13, 15, 16, 18, 24). However, these studies were performed prior to the establishment of a standardized antimicrobial susceptibility test for Campylobacter. Since a standardized test is proposed for Campylobacter (19), no information has been reported on the agreement between the standardized agar dilution method and the agar disk diffusion method. Hence, the aim of this study was to determine whether the agar disk diffusion test could be used as a reliable alternative method for antimicrobial susceptibility testing of Campylobacter species.
Six hundred sixty-eight Campylobacter isolates (431 C. jejuni and 237 Campylobacter coli), obtained from the intestinal tracts of poultry with different histories of antibiotic exposure, were evaluated for resistance to nine antimicrobial agents, including ampicillin, tetracycline, gentamicin, kanamycin, clindamycin, erythromycin, ciprofloxacin, norfloxacin, and nalidixic acid, by both the standardized agar dilution method and the disk diffusion method according to the guideline established by the CLSI (formerly NCCLS) (22). All antimicrobial agents for the agar dilution method were obtained from Sigma Chemical Co., St. Louis, MO, except ciprofloxacin (Serologicals Proteins, Inc., Kankakee, IL), and antibiotic disks for the disk diffusion method were obtained from Becton Dickinson and Company, Sparks, MD. The concentrations of antimicrobial agents tested in this study are shown in Table 1. For the agar dilution method, after Campylobacter suspensions were adjusted to a turbidity equivalent to a 0.5 McFarland standard, approximately 104 CFU of these suspensions was inoculated onto Mueller-Hinton agar containing a twofold dilution series of antibiotics and supplemented with 5% defibrinated sheep blood using a multipoint inoculator (a Cathra replicator system) with 1-mm pins (Oxoid, Inc., Ogdensburg, NY). For the disk diffusion method, sterile cotton-tipped swabs were used to transfer the inoculum onto Mueller-Hinton plates to produce a confluent lawn of bacterial growth. After the inoculum on the plates was dried, antibiotic disks were distributed over the inoculated plates using a BBL Sensi-disc dispenser (BBL Becton Dickinson Microbiology Systems, Cockeysville, MD). These plates were then incubated at 42°C for 24 h under microaerobic conditions (5% O2, 10% CO2, and 85% N2). C. jejuni ATCC 33560 was used as a quality control organism in this study. The MIC breakpoints and the zone diameter breakpoints of each antimicrobial agent were determined according to the breakpoints used by the National Antimicrobial Resistance Monitoring System (NARMS) and the CLSI-established guideline for bacteria isolated from animals (7, 22, 23) (Table 1). To measure the correlation and the level of agreement between the standardized agar dilution method and the agar disk diffusion method, the scatter plot, the correlation coefficient, the percent agreement, and the kappa statistic were calculated as previously described (8, 18).
TABLE 1.
Breakpoints of the agar dilution and disk diffusion methods used to determine antimicrobial susceptibility of Campylobacter isolates
| Antimicrobial agent | Result for method:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Agar dilution
|
Disk diffusion
|
|||||||
| Test range (μg/ml) | MIC breakpoint (μg/ml)a
|
Disk concn (μg) | Zone diam breakpoint (mm)b
|
|||||
| S | I | R | S | I | R | |||
| Ampicillin | 0.06-128 | ≤8 | 16 | ≥32 | 10 | ≥17 | 14-16 | ≤13 |
| Tetracycline | 0.06-128 | ≤4 | 8 | ≥16 | 30 | ≥19 | 15-18 | ≤14 |
| Gentamicin | 0.06-128 | ≤4 | 8 | ≥16 | 10 | ≥15 | 13-14 | ≤12 |
| Kanamycin | 0.25-128 | ≤16 | 32 | ≥64 | 30 | ≥18 | 14-17 | ≤13 |
| Clindamycin | 0.06-128 | ≤0.5 | 1-2 | ≥4 | 2 | ≥21 | 15-20 | ≤14 |
| Erythromycin | 0.06-128 | ≤0.5 | 1-4 | ≥8 | 15 | ≥23 | 14-22 | ≤13 |
| Ciprofloxacin | 0.008-128 | ≤1 | 2 | ≥4 | 5 | ≥21 | 16-20 | ≤15 |
| Norfloxacin | 0.06-128 | ≤4 | 8 | ≥16 | 10 | ≥17 | 13-16 | ≤12 |
| Nalidixic acid | 0.25-128 | ≤16 | ≥32 | 30 | ≥19 | 14-18 | ≤13 | |
MIC breakpoints for enteric bacteria from the NARMS were used for all antimicrobial agents except norfloxacin. MIC breakpoints for Enterobacteriaceae for norfloxacin were recommended by the CLSI (formerly NCCLS). S, susceptible; I, intermediate; R, resistant.
Zone diameter breakpoints of ampicillin, tetracycline, gentamicin, kanamycin, clindamycin, and erythromycin for bacteria isolated from animals were recommended by the CLSI. Zone diameter breakpoints of ciprofloxacin, norfloxacin, and nalidixic acid for Enterobacteriaceae were recommended by the CLSI. S, susceptible; I, intermediate; R, resistant.
Since there are no antimicrobial resistance breakpoints specific for Campylobacter currently available, the resistance breakpoints of enteric bacteria in the family Enterobacteriaceae have been used to determine antimicrobial resistance of Campylobacter spp. (13, 18, 21). According to these resistance breakpoints, a majority of Campylobacter isolates were classified as either susceptible or resistant to ciprofloxacin, norfloxacin, nalidixic acid, gentamicin, and kanamycin by both the agar dilution and the agar disk diffusion methods (Table 2). For erythromycin, clindamycin, and ampicillin, a large number of Campylobacter isolates were classified as intermediate to these antimicrobial agents when the current NARMS resistance breakpoints were used (Table 2). The agar dilution method and the disk diffusion method agreed well in identifying aminoglycoside and quinolone/fluoroquinolone resistance in Campylobacter. The percent agreements between these methods for gentamicin, kanamycin, ciprofloxacin, norfloxacin, and nalidixic acid were 99.85%, 97.46%, 94.46%, 95.81%, and 91.02%, respectively (Table 3). In terms of the kappa, an almost perfect agreement (kappa > 0.8) was also observed between the agar dilution and the disk diffusion methods for aminoglycosides and quinolone/fluoroquinolones (Table 3). In addition, the correlation coefficient and the scatter plot of the MICs and the zone diameters of each antimicrobial agent evaluated in this study also demonstrated a correlation between the standardized agar dilution and the agar disk diffusion methods for aminoglycosides and quinolone/fluoroquinolones as well as for erythromycin, clindamycin, and tetracycline. The correlation coefficients of kanamycin, ciprofloxacin, erythromycin, clindamycin, and tetracycline were 0.937, −0.86, −0.885, −0.8, and −0.863, respectively, whereas the correlation coefficient between the MICs and the zone diameters of ampicillin was −0.588. When the numbers of falsely susceptible and falsely resistant Campylobacter isolates were investigated, the numbers of isolates that were classified as resistant by the agar dilution method but susceptible by the disk diffusion method (falsely susceptible) were less than 1.5% of the isolates tested for resistance to every antimicrobial agent except ampicillin and tetracycline (Table 3). Likewise, the numbers of Campylobacter isolates that were classified as susceptible by the agar dilution method but resistant by the disk diffusion method (falsely resistant) were less than 3% of the isolates tested for resistance to every antimicrobial agent except ampicillin (Table 3).
TABLE 2.
Antimicrobial susceptibility patterns of Campylobacter spp. identified by the agar dilution and the disk diffusion methodsa
| Antimicrobial agent | Agar dilution method
|
Disk diffusion method
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Campylobacter isolatesb
|
% of resistant isolates | No. of Campylobacter isolatesb
|
% of resistant isolates | |||||
| S | I | R | S | I | R | |||
| Ampicillin | 434 | 154 | 75 | 11.31 | 416 | 138 | 109 | 16.44 |
| Tetracycline | 158 | 4 | 505 | 75.71 | 195 | 49 | 423 | 63.42 |
| Gentamicin | 668 | 0 | 0 | 0 | 667 | 0 | 1 | 0.15 |
| Kanamycin | 418 | 2 | 248 | 37.13 | 415 | 10 | 243 | 36.38 |
| Clindamycin | 159 | 366 | 143 | 21.41 | 329 | 178 | 161 | 24.10 |
| Erythromycin | 76 | 413 | 179 | 26.80 | 449 | 62 | 157 | 23.50 |
| Ciprofloxacin | 444 | 10 | 214 | 32.04 | 437 | 2 | 229 | 34.28 |
| Norfloxacin | 451 | 4 | 213 | 31.89 | 439 | 0 | 229 | 34.28 |
| Nalidixic acid | 454 | 0 | 214 | 32.04 | 434 | 31 | 203 | 30.39 |
The total number of Campylobacter isolates tested for antimicrobial resistance was 668 for all antimicrobials except ampicillin (663 isolates) and tetracycline (667 isolates).
Number of susceptible (S), intermediate (I), and resistant (R) Campylobacter isolates identified by the agar dilution method or by the disk diffusion method.
TABLE 3.
Agreement between the agar dilution and disk diffusion methods for antimicrobial susceptibility testing of Campylobacter speciesa
| Antimicrobial agent | No. of Campylobacter isolates
|
Total no. of isolates tested by both AD and DD | Total no. of isolates with agreement of results for both AD and DD | % agreement between AD and DD | Kappab | |||
|---|---|---|---|---|---|---|---|---|
| Susceptible by both AD and DD | Susceptible by AD but resistant by DD | Susceptible by DD but resistant by AD | Resistant by both AD and DD | |||||
| Ampicillin | 336 | 39 | 14 | 45 | 663 | 444 | 66.97 | 0.37 |
| Tetracycline | 143 | 14 | 50 | 408 | 667 | 552 | 82.76 | 0.62 |
| Gentamicin | 667 | 1 | 0 | 0 | 668 | 667 | 99.85 | NAc |
| Kanamycin | 408 | 1 | 6 | 243 | 668 | 651 | 97.46 | 0.95 |
| Clindamycin | 127 | 0 | 7 | 129 | 668 | 397 | 59.43 | 0.41 |
| Erythromycin | 74 | 0 | 9 | 150 | 668 | 264 | 39.52 | 0.25 |
| Ciprofloxacin | 422 | 20 | 5 | 209 | 668 | 631 | 94.46 | 0.88 |
| Norfloxacin | 431 | 20 | 4 | 209 | 668 | 640 | 95.81 | 0.91 |
| Nalidixic acid | 425 | 20 | 9 | 183 | 668 | 608 | 91.02 | 0.81 |
AD, agar dilution; DD, disk diffusion.
The magnitude of kappa indicates the level of agreement between the two tests as follows: <0.2, slight agreement; 0.2 to 0.4, fair agreement; 0.4 to 0.6, moderate agreement; 0.6 to 0.8, substantial agreement; >0.8, almost perfect agreement.
The kappa value for gentamicin could not be calculated because none of the isolates was classified as resistant to this antibiotic by both methods.
In this study, Campylobacter strains that were resistant to fluoroquinolones and aminoglycosides by the standardized agar dilution method were also resistant to these antimicrobial agents by the disk diffusion method. No zones of inhibition around these antibiotic disks were observed among the resistant Campylobacter strains, while large clear zones of inhibition averaging more than 37 mm in diameter were observed around the disks of the susceptible Campylobacter strains. The drastic difference in the zone of inhibition diameters, a high correlation coefficient, a high percent agreement, and a high kappa value between the standardized agar dilution and the agar disk diffusion methods indicate that the disk diffusion test is a reliable screening method for antimicrobial susceptibility testing of thermophilic Campylobacter to quinolone/fluoroquinolone and aminoglycoside antibiotics. This finding is correlated well with the previous study by Gaudreau and Gilbert, who reported a complete agreement between the agar dilution method and the disk diffusion method for susceptibility testing of C. jejuni and C. coli to ciprofloxacin (12). In addition, Frediani-Wolf and Stephan also suggested that the disk diffusion method can be used as a reliable and easy tool for monitoring the prevalence of ciprofloxacin-resistant C. jejuni strains, although they found a weak correlation between the MIC and zone diameter results for ciprofloxacin-susceptible strains (11).
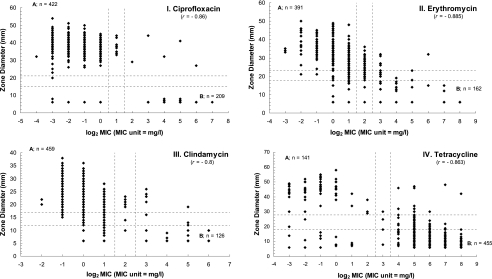
When the current NARMS resistance breakpoints were used, a large number of Campylobacter isolates were classified as intermediate to erythromycin and clindamycin by the agar dilution method. Since the MICs of erythromycin for C. jejuni ATCC 33560, the quality control organism, and the majority of Campylobacter isolates tested in this study fell consistently in a range between 1 and 4 μg/ml, this information, plus the findings from other studies (3, 12, 14, 16), warrants a change of the MIC breakpoints of erythromycin for susceptible Campylobacter isolates. Our data suggest that the breakpoint for erythromycin-susceptible Campylobacter strains may be more appropriately set at ≤2 μg/ml instead of ≤0.5 μg/ml. If the MIC breakpoints of erythromycin for thermophilic Campylobacter are changed to ≤2 μg/ml for susceptible isolates and ≥8 μg/ml for resistant isolates and the zone diameter breakpoints of the disk diffusion method are set at ≥23 mm for susceptible isolates and ≤18 mm for resistant isolates (Fig. 1), the percent agreement as well as the kappa value between the agar dilution method and the disk diffusion method for erythromycin would significantly improve, while the numbers of falsely susceptible and falsely resistant isolates would still be less than 1.5% and 3%, respectively. Likewise, if the MIC breakpoints for clindamycin are changed to ≤2 μg/ml for susceptible isolates and ≥8 μg/ml for resistant isolates and the zone diameter breakpoints are changed to ≥17 mm for susceptible isolates and ≤12 mm for resistant isolates (Fig. 1), the percent agreement and the kappa value between the agar dilution and the disk diffusion methods for clindamycin would increase dramatically. Also, the numbers of falsely susceptible and falsely resistant Campylobacter isolates would still be in an acceptable range.
FIG. 1.
Scatter plot of the MICs in the agar dilution method and the zone diameters in the disk diffusion method with tentative breakpoints (dashed line) for ciprofloxacin (I), erythromycin (II), clindamycin (III), and tetracycline (IV) against Campylobacter species. A, the area where Campylobacter isolates were susceptible to the antimicrobial agent by both the agar dilution and the disk diffusion methods. B, the area where Campylobacter isolates were resistant to the antimicrobial agent by both the agar dilution and the disk diffusion methods. The values of −4, −3, −2, −1, 0, 1, 2, 3, 4, 5, 6, and 7 on the x axis represents the log2 MIC of each MIC, which are equivalent to 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, and 128 μ/ml, respectively.
Some Campylobacter isolates that were determined to be tetracycline resistant by the agar dilution method were classified as susceptible or intermediate by the disk diffusion method. This may explain why the percent agreement and the kappa between the agar dilution method and the disk diffusion method for tetracycline were not as high as those of the quinolone/fluoroquinolones and aminoglycosides. Although the kappa value for tetracycline demonstrated moderate agreement (kappa = 0.62) between the two methods, the correlation coefficient revealed a strong correlation (r = −0.863) between the agar dilution and disk diffusion methods. Likewise, Alfredson et al. also reported that the disk diffusion method correlated well with the agar dilution method when they were used for the screening of tetracycline-resistant Campylobacter strains (1). Although the current breakpoints used for tetracycline for enteric bacteria belonging to the family Enterobacteriaceae may be used for Campylobacter spp., it will be better if the zone diameter breakpoints are modified to ≥28 mm for susceptible strains and ≤18 mm for resistant strains (Fig. 1). These new zone diameter breakpoints for tetracycline will help reduce the numbers of falsely susceptible Campylobacter strains by the agar disk diffusion test from 50 isolates to 16 isolates while increasing the percent agreement between the agar dilution method and the disk diffusion method to 89.51%.
In this study, a weak agreement (kappa = 0.37) and a weak correlation (r = −0.58) between the agar dilution and the disk diffusion methods was observed for ampicillin. Similar to our finding, Gaudreau and Gilbert also reported that the correlation coefficient between the two methods for ampicillin was only 0.57 (12). Since the scatter plot of the MICs and the zone diameters for ampicillin were widely distributed in this study and because the correlation between the two methods was quite poor, the tentative breakpoints of ampicillin for thermophilic Campylobacter cannot be provided by this study.
Although the disk diffusion method is not as complicated to perform as the agar dilution method and provides reliable results for several classes of antimicrobials, only qualitative data can be obtained from this method. Moreover, the poor growth of Campylobacter isolates on the plates, which was observed sometimes with the disk diffusion method, can also cause difficulty in interpreting the antimicrobial resistance results. Nevertheless, this method is very useful, especially when several antimicrobial agents need to be tested against a few isolates. If the quantitative data are required, other methods, such as the agar dilution method or the E-test, should be used.
In conclusion, this study reveals a high-level correlation between the standardized agar dilution method and the agar disk diffusion method for aminoglycosides, quinolone/fluoroquinolones, erythromycin, clindamycin, and tetracycline in evaluating the resistance of Campylobacter spp. to these antimicrobial agents. This study also suggests that the disk diffusion method can be used as a reliable alternative method for susceptibility testing of thermophilic Campylobacter to several classes of antimicrobial agents, particularly to quinolone/fluoroquinolones and aminoglycosides. Based on the data obtained in this study, we proposed some changes for the breakpoints of erythromycin, clindamycin, and tetracycline. However, until the standard breakpoints specific for Campylobacter are established and validated, the agar dilution method is likely to be a preferable method for determining antimicrobial resistance of Campylobacter species.
Acknowledgments
We are indebted to Yulong Zhang and Dongmei Li at the Statistical Consulting Service (SCS) at The Ohio State University for their assistance on statistical analysis of this study. We thank Benjamas Promsopone, Elisabeth J. Angrick, Lori Martin, Nicole Fehribach, Andew M. Krieger, and fellow colleagues at the Avian Disease Investigation Laboratory at The Ohio State University for their help, advice, and technical support. In addition, the assistance of Nuttha Thongchul at Chulalongkorn University, Audrey Torres at The Ohio State University, and Sonya M. Bodeis at the Center for Veterinary Medicine, Food and Drug Administration is also gratefully acknowledged.
This work is supported by U.S. Department of Agriculture grants 2003-35212-13316 and 2005-511110-03273.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Alfredson, D. A., R. J. Akhurst, and V. Korolik. 2003. Antimicrobial resistance and genomic screening of clinical isolates of thermophilic Campylobacter spp. from south-east Queensland, Australia. J. Appl. Microbiol. 94:495-500. [DOI] [PubMed] [Google Scholar]
- 2.Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 3.Baker, C. N. 1992. The E-test and Campylobacter jejuni. Diagn. Microbiol. Infect. Dis. 15:469-472. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, M. J. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176(Suppl. 2):S103-S105. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 2000. Campylobacter jejuni and related species, p. 2276-2285. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, vol. 2, 5th ed. Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 6.Caprioli, A., L. Busani, J. L. Martel, and R. Helmuth. 2000. Monitoring of antibiotic resistance in bacteria of animal origin: epidemiological and microbiological methodologies. Int. J. Antimicrob. Agents 14:295-301. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2003. National antimicrobial resistance monitoring system: enteric bacteria, 2001 annual report. National Antimicrobial Resistance Monitoring System, Atlanta, GA.
- 8.Dohoo, I., W. Martin, and H. Stryhn. 2003. Veterinary epidemiologic research, p. 85-120. AVC, Inc., Charlottetown, Prince Edward Island, Canada.
- 9.Engberg, J., S. Andersen, R. Skov, F. M. Aarestrup, and P. Gerner-Smidt. 1999. Comparison of two agar dilution methods and three agar diffusion methods, including the Etest, for antibiotic susceptibility testing of thermophilic Campylobacter species. Clin. Microbiol. Infect. 5:580-584. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez, H., M. Mansilla, and V. Gonzalez. 2000. Antimicrobial susceptibility of Campylobacter jejuni subsp. jejuni assessed by E-test and double dilution agar method in Southern Chile. Mem. Inst. Oswaldo Cruz 95:247-249. [DOI] [PubMed] [Google Scholar]
- 11.Frediani-Wolf, V., and R. Stephan. 2003. Resistance patterns of Campylobacter spp. strains isolated from poultry carcasses in a big Swiss poultry slaughterhouse. Int. J. Food Microbiol. 89:233-240. [DOI] [PubMed] [Google Scholar]
- 12.Gaudreau, C., and H. Gilbert. 1997. Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. J. Antimicrob. Chemother. 39:707-712. [DOI] [PubMed] [Google Scholar]
- 13.Ge, B., S. Bodeis, R. D. Walker, D. G. White, S. Zhao, P. F. McDermott, and J. Meng. 2002. Comparison of the E test and agar dilution method for in vitro antimicrobial susceptibility testing of Campylobacter. J. Antimicrob. Chemother. 50:487-494. [DOI] [PubMed] [Google Scholar]
- 14.Halbert, L. W., J. B. Kaneene, L. S. Mansfield, P. L. Ruegg, L. D. Warnick, S. J. Wells, C. P. Fossler, A. M. Campbell, and A. M. Geiger-Zwald. 2005. Comparison of automated microbroth dilution and agar dilution for antimicrobial susceptibility of Campylobacter jejuni isolated from dairy sources. J. Antimicrob. Chemother. 56:686-691. [DOI] [PubMed] [Google Scholar]
- 15.Huang, M. B., C. N. Baker, S. Banerjee, and F. C. Tenover. 1992. Accuracy of the E test for determining antimicrobial susceptibility of staphylococci, enterococci, Campylobacter jejuni, and gram-negative bacteria resistant to antimicrobial agents. J. Clin. Microbiol. 30:3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huysmans, M. B., and J. D. Turnidge. 1997. Disc susceptibility testing for thermophilic campylobacters. Pathology 29:209-216. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs-Reitsma, W. 2000. Campylobacter in the food supply, p. 467-481. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. American Society for Microbiology, Washington, DC.
- 18.Luber, P., E. Bartelt, E. Genschow, J. Wagner, and H. Hahn. 2003. Comparison of broth microdilution, E test, and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 41:1062-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott, P. F., S. M. Bodeis, F. M. Aarestrup, S. Brown, M. Traczewski, P. Fedorka-Cray, M. Wallace, I. A. Critchley, C. Thornsberry, S. Graff, R. Flamm, J. Beyer, D. Shortridge, L. J. Piddock, V. Ricci, M. M. Johnson, R. N. Jones, B. Reller, S. Mirrett, J. Aldrobi, R. Rennie, C. Brosnikoff, L. Turnbull, G. Stein, S. Schooley, R. A. Hanson, and R. D. Walker. 2004. Development of a standardized susceptibility test for Campylobacter with quality-control ranges for ciprofloxacin, doxycycline, erythromycin, gentamicin, and meropenem. Microb. Drug Resist. 10:124-131. [DOI] [PubMed] [Google Scholar]
- 20.McDermott, P. F., S. M. Bodeis-Jones, T. R. Fritsche, R. N. Jones, R. D. Walker, and the Campylobacter Susceptibility Testing Group. 2005. Broth microdilution susceptibility testing of Campylobacter jejuni and the determination of quality control ranges for fourteen antimicrobial agents. J. Clin. Microbiol. 43:6136-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachamkin, I., J. Engberg, and F. M. Aarestrup. 2000. Diagnosis and antimicrobial susceptibility of Campylobacter species, p. 45-66. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. American Society for Microbiology, Washington, DC.
- 22.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, approved standard M31-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 23.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing, 12th informational supplement M100-S12. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 24.Oncul, O., P. Zarakolu, O. Oncul, and D. Gur. 2003. Antimicrobial susceptibility testing of Campylobacter jejuni: a comparison between E test and agar dilution method. Diagn. Microbiol. Infect. Dis. 45:69-71. [DOI] [PubMed] [Google Scholar]
- 25.Potz, N. A. C., S. Mushtaq, A. P. Johnson, C. J. Henwood, R. A. Walker, E. Varey, M. Warner, D. James, and D. M. Livermore. 2004. Reliability of routine disc susceptibility testing by the British Society for Antimicrobial Chemotherapy (BSAC) method. J. Antimicrob. Chemother. 53:729-738. [DOI] [PubMed] [Google Scholar]