Abstract
PCR has greatly facilitated pertussis diagnosis due to the speed, sensitivity, and specificity of this assay compared to other detection methods. Various single-target PCR assays are currently utilized, but none is universally considered to be the “gold standard.” Our aim was to assess the use of multitarget versus single-target PCR for the diagnosis of pertussis in clinical samples. PCR assays targeting insertion sequence IS481 (IS), pertussis toxin ptxA promoter region (PT), and outer membrane porin (PO), or recA (RA) were evaluated in respiratory specimens collected from 4,442 patients with suspected pertussis. The diagnosis of pertussis was confirmed in 309 (6.96%) patients by the 3-target IS-PT-PO/RA PCR versus 247 (5.56%) by the conventional single-target IS (P = 0.007). Compared to single-target IS, the three-target combination increased the proportion of positive specimens by 1.25-fold, and two-target combinations increased the proportion of positive specimens by 1.10- to 1.24-fold. In addition, nine cases of B. parapertussis infection were also confirmed by using the discriminative features of this multitarget PCR. Of the 89 culture-proven pertussis cases, 17 (19.1%) and 5 of the 16 patients (31.3%) admitted to intensive care unit would have been missed had only the single-target IS PCR been applied. Patients with mild disease (P = 0.004) and shorter hospitalization (P = 0.006) were less likely to have positive cultures. This consensus generating real-time PCR approach permits a sensitive detection, as well as an accurate species identification of the causative Bordetella pathogens for the timely management of patients.
The timely and reliable diagnosis of pertussis, a highly contagious respiratory tract infection caused by Bordetella pertussis and, less frequently, by B. parapertussis, is crucial in instituting specific therapy and preventing transmission of disease (16, 19, 28, 40). Clinical manifestations, such as prolonged cough, have been used to define disease, but it is now clear that these definitions have not captured culture-positive, symptomatic patients with a shorter duration of cough (3, 7, 13, 31). A variety of diagnostic methods have been developed for the detection of pertussis disease, but all have limitations in terms of sensitivity, specificity, and practicality (9, 10). Recovery of the organism by culture or direct fluorescent antibody (DFA) methods is highly specific, but the sensitivity is low and the results are not rapidly available (2, 3, 16, 29, 40). Serologic studies, although not practical for a rapid diagnosis, have been used to measure immunoglobulin G antibodies to pertussis toxin successfully in outbreak investigations involving adolescents and adults, as well as vaccine trials (6, 25, 34). Detection of pertussis-specific antibodies in serum has never been widely accepted in clinical settings, since serologic results are not rapidly available, may be confusing early in the disease course especially in infancy, and cannot always differentiate host immunities acquired after infection or vaccination (1, 3, 16, 35).
PCR assays have substantially facilitated the diagnosis of pertussis. PCR assays can be applied directly to specimens, can detect just a few or even nonviable Bordetella organisms, provide results rapidly, and perform well in infants. These assays have been shown to be more sensitive than culture, with sensitivity and specificity rates up to 61 and 88%, respectively (1, 2, 9-12, 14, 16, 19, 24, 32, 35, 40).
Although PCR for the detection of pertussis was introduced in 1989, standardization of methods has been problematic (1, 3, 11, 14, 19, 24, 32, 34). PCR methods by far amplify a single gene sequence, usually within the insertion sequence IS481 (11, 18, 24, 32, 34, 35). Unfortunately, both false-positive and false-negative results have been reported (14, 24, 37). Pseudo outbreaks have been reported as a result of improper laboratory handling and suboptimal testing procedures (24, 29). No specific recommendation using more than one pertussis genetic target for laboratory diagnosis has been made by regulatory agencies in the United States or in Europe (EUpertstrain [34]), although several laboratories, including ours, have previously suggested the use of two-target PCR for pertussis diagnosis (14, 32). We present here the retrospective results of two- and three-target PCR compared to the detection of a single target, including the conventional IS481 PCR, as well as the correlation of these with culture results, and clinical variables.
MATERIALS AND METHODS
Specimen collection and preparation.
All specimens obtained from patients with suspected pertussis from King County, Washington, that were referred to the Microbiology Laboratory at Children's Hospital and Regional Medical Center (CHRMC), Seattle, WA, between January 2002 and December 2005 were studied. Cultures were performed only on PCR-positive and PCR-indeterminate specimens. Two dacron or rayon nasopharyngeal swabs were generally obtained; one was set aside in Regan-Lowe transport medium for culture pending the PCR results, and the other was stored at −20°C in a sterile tube with no transport additives. The latter swab was processed by adding 1 ml of sterile saline, followed by 30 s of vigorous vortex mixing. Saline suspensions were transferred to a sterile 1.5-ml Eppendorf tube and centrifuged at 16,110 × g for 5 min, and the pellet suspended in 100 μl of molecular-grade water (Fischer Scientific, Fairlawn, NJ) and then heated at 95°C for 5 min and cooled to 4°C. Amplification in-run positive controls consisted of fresh-grown organisms diluted to 1:10 and 1:100 from 0.5 McFarland suspensions of B. pertussis ATCC 9340 and B. parapertussis ATCC 15237. The PCR sensitivity for IS, PT, and PO/RA was validated at the lower detection limit of ∼20 CFU. The final inoculum of the heated B. pertussis ATCC 9340 control material using its far-end dilution would ensure a genome copy number of as few as 50 per reaction in each test run. Molecular-grade water was used for the negative controls, and human β-actin marker was used to control for specimen quality and PCR inhibition.
Culture.
If a positive or indeterminate PCR assay was noted, the second swab (or the only swab retained after a saline wash) was inoculated onto a Regan-Lowe agar plate and incubated in a humidity chamber at 35°C for a maximum of 15 days. The plates were examined on a daily basis for colonies typical of B. pertussis. Colonies were further evaluated by Gram stain for bacterial morphology, and B. pertussis was confirmed by direct fluorescent antibody assay (Difco Laboratories, Detroit, MI).
Real-time amplification.
The real-time PCR assays were performed by using fluorescence resonance energy transfer SYBR green chemistry that measured the fluorescent SYBR green signal increase as a result of growing amplicon concentration. The amplicons were analyzed at the end of the 45th cycle for their specific melting-point temperatures. The commercial master mix iQ SYBR green Supermix (Bio-Rad, Hercules, CA) was used according to the manufacturer's recommendations. Uracil-N-glycosylase was incorporated into the master mix for the amplicon carryover contamination control. The master mix was made fresh daily with primer concentrations at a final concentration of 0.1 μM. The master mix (36 μl) was transferred into premapped wells in a 96-well plate; 4-μl samples (an undiluted 1:1 and the 1:4 dilution) were then each inoculated into two sets of four reactions. A total of eight wells (IS, PT, PO/RA, and BA) were used for each sample. The thermocycling conditions were 20°C for 5 min and 95°C for 2 min, followed by 45 cycles of 94°C for 30 s, 68°C for 30 s, and 72°C for 30 s, followed in turn by 5 min at 72°C as the extension step before a final melting-peak analysis in an iCycler (Bio-Rad) instrument. The specific melting-peak temperatures with ≤±0.5°C were accepted.
PCR interpretation.
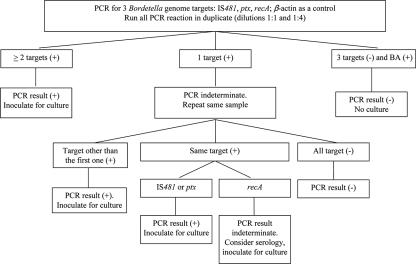
The interpretation criteria are depicted in Fig. 1. The PCR result was reported as “positive” when ≥2 positive targets or a single reproducible IS, PO, or PT target was positive; as “negative” when no target was positive or a single target was initially positive but not reproducible; and as indeterminate when RA was the only reproducible target detected or BA was negative for specimen concentrations of 1:1, 1:4, and 1:8 upon repeat. With the indeterminate results, the original specimen was cultured for Bordetella spp., and the physician was contacted to suggest recollection of the clinical specimen.
FIG. 1.
Flow diagram of evaluating three-target PCR for the diagnosis of pertussis when β-actin was tested positive. In cases of β-actin negative result, the retaining unheated specimen was inoculated for culture.
Clinical variables.
Clinical data were collected from the medical records of children receiving care at only CHRMC, Seattle, WA, following approval by the Institutional Human Subject Review Board. Clinical variables included gender and age, immunization history and contact with a confirmed case or an individual with prolonged cough illness, characteristics and duration of coughing, severe manifestations (including apnea and cyanosis), fever, administration of antibiotics prior to sampling, and duration of hospitalization.
Statistics.
Statistical analysis was based on contingency tables, including two-sided Fisher exact test and odds ratio (OR) calculations, and on calculation of the proportions and of the lower and upper limits of the 95% confidence interval (95% CI) with correction for continuity. Additional analyses included nonparametric evaluations, including the Kruskal-Wallis and two-tailed Mann-Whitney tests. A P value of <0.05 was considered to be significant.
RESULTS
Population.
A single sample from each of 4,442 patients with a clinical suspicion of pertussis was investigated. In 309 (6.96%) samples, pertussis was detected based on the diagnostic algorithm presented in Fig. 1. The patients diagnosed with pertussis were between the ages of 18 days to 84 years (median, 2.50 years); 157 were male, and 152 were female. Pertussis was detected more often during the late summer and autumn months, with 98 (31.7%) cases clustering in the 3-month period from August to October. Nine cases were attributed to B. parapertussis, all occurring in November, December, and January but in different years. A total of 70 patients were hospitalized at CHRMC, 42 outpatients aged less than 15 years were seen in the emergency department or clinics, and 197 were referral patients whose swabs were sent to our laboratory for pertussis evaluation. The median ages of hospitalized patients was 0.25 years (range, 0.06 to 13.8 years), of outpatients was 0.70 years (range, 0.05 to 14.1 years), and of referral patients was 12.5 years (range, 0.05 to 84 years), a difference that was significant (P < 0.0001).
PCR primers designed for specific targets.
The primers used in the present study are shown in Table 1. The PCR assay was designed to detect three independent targets in the Bordetella genome: chromosomal repeated insertion sequence IS481 (IS), the polymorphic pertussis toxin ptxA promoter region (PT), and the recA (RA) gene coding region (3, 15-18, 20-24, 26-30); during the first period of the study, the outer membrane porin gene (PO) was used before the RA sequences became available. The sequence IS481 is present at a rate of approximately 200 copies in the genome of B. pertussis but is also found in B. holmesii, an uncommon respiratory tract colonizer, and possibly in other species of Bordetella such as B. bronchiseptica (11, 14, 19, 31, 34). Taking advantage of intra- and interspecies polymorphism, primers for PT amplification were designed to cover both B. pertussis and B. parapertussis but not B. bronchiseptica. Differentiation between B. pertussis and B. parapertussis was achieved by distinct melting peaks at 89 and 91°C, respectively.
TABLE 1.
Primers used as PCR targets in this study
| Primer type and name | Sequence | Amplicon length (bp) |
|---|---|---|
| IS481 (IS) | 182 | |
| IS-F | GATTCAATAGGTTGTATGCATGGTTC | |
| IS-R | TTCAGGCACACAAACTTGATGGGCG | |
| ptx promoter (PT) | 189 | |
| PT-F1 | CCAACGCGCATGCGTGCAGATTCG | |
| PT-F2 | CCAACGCGTATGCGTGCGGATGCG | |
| PT-R1 | CTCTGCGTTTTGATGGTGCCTATT | |
| PT-R2 | CTCTGCGTTTCGGTGGTGCCTATT | |
| Outer membrane porin (PO) | 148 | |
| PO-F | GGCCGGGCTCCTTGAGTGAACTGG | |
| PO-R | GTTGGTAAGTTGCAACATCCTGTCC | |
| recA gene (RA) | 204 | |
| RA-F | CGCGCTCAAGTTCTATTCCTCG | |
| RA-R | TTGCACGCCCAGGTCGATGATTTC | |
| Human β-actin (BA) | 331 | |
| BA-F | AAAGACCTGTACGCCAACACAGTGCTGTCTGG | |
| BA-R | CGTCATACTCCTGCTTGCTGATCCACATCTGC |
The recA gene was used as a common target for detecting B. pertussis, B. parapertussis, and B. bronchiseptica. The RA primer pair was chosen based on multisequence alignment of the recA coding regions (Fig. 2) from B. pertussis (NC_002929), B. parapertussis (NC_002928), B. bronchiseptica (NC_002927), B. holmesii (AF399664), B. hinzii (AY124331), and B. avium (AY124330). Amplification of β-actin (BA) was designed as a control for specimen quality and for false-negative results due to Taq polymerase inhibition in unextracted human specimen materials or inadequate samples.
FIG. 2.
Sequence alignment of the recA coding region of pathogen-specific primers. The dotted lines represent 156-bp intervening sequences; the dashes represent bases identical to those of the three-pathogen consensus; the question marks represent unknown nucleotides. BP, B. pertussis; BPP, B. parapertussis; BB, B. bronchiseptica; BHI, B. hinzii; BHO, B. holmesii; BAV, B. avium.
PCR findings.
Pertussis was confirmed in 309 of 4,442 (6.96%) specimens by the three-target PCR and in 247 (5.56%) by the conventional single-target IS PCR (P = 0.007). The three-target PCR detected pertussis in 62 more samples (25.1% [95% CI = 20 to 31%]) than single-target IS PCR. Single targets IS, PT, PO, and RA demonstrated detection rates (95% CI) of 0.80 (0.75 to 0.84), 0.76 (0.71 to 0.81), 0.80 (0.73 to 0.86), and 0.68 (0.59 to 0.75), respectively, compared to the three-target PCR algorithm (Table 2). Compared to the conventional single-target IS PCR, the three-target combination IS-PT-PO/RA increased detection by 1.25-fold, and the two-target combinations IS-PT, IS-PO/RA, and PT-PO/RA increased detection by 1.24-, 1.21-, and 1.10-fold, respectively. Single-target reproducible positive results were generated from 47 of the 309 (15%) pertussis-positive specimens (or 1% of the total 4,442 specimens) with 34 IS only, 9 PT only, and 4 PO only, respectively. Nonreproducible single-target positive results were obtained for 223 specimens (i.e., 5% of the total number of specimens, excluding the 47 [1%] samples giving reproducible results by single-target PCR), suggesting potential carryover contamination, nonspecific amplification, or pathogen levels below the detection limit. All nine samples attributed to B. parapertussis were positive for PT sequences with a melting point of 91°C and negative for IS. Four of these were tested by PO as a third target, and all were found negative: the remaining five were tested with RA, and all were found to be positive. Nine specimens testing positive only for RA and 32 BA-negative specimens were considered indeterminate.
TABLE 2.
Diagnostic sensitivity of various PCR targets in 309 B. pertussis-positive samples compared to the detection of three-target PCR algorithms
| Target | No. (%)
|
|||
|---|---|---|---|---|
| All patients | Inpatients | Outpatients | Referrals | |
| Positive specimens | 309 | 70 | 42 | 197 |
| Single target | ||||
| IS | 247 (79.9) | 58 (82.9) | 35 (83.3) | 154 (78.2) |
| PT | 236 (76.4) | 56 (80.0) | 32 (76.2) | 148 (75.1) |
| PO/RA | 228 (74.5) | 63 (90.0) | 31 (73.8) | 134 (69.1) |
| Two-target combinations | ||||
| IS and/or PT | 305 (98.7) | 69 (98.6) | 42 (100) | 194 (98.5) |
| IS and/or PO/RA | 300 (97.1) | 70 (100) | 42 (100) | 188 (95.4) |
| PT and/or PO/RA | 271 (88.6) | 67 (95.7) | 38 (90.5) | 166 (84.3) |
No relationship between patient gender and PCR results was observed. Age was not correlated with rates of detection of IS or PO, but detection of PT and RA was more frequent in younger than in older patients (P = 0.003 and P = 0.014; median ages of 1.84 versus 7.36 and 2.77 versus 12.9, respectively). No relationship between inpatients, outpatients, or referral patients and the detection of IS, PT, or RA was documented, but PO was detected more frequently in hospitalized patients than in outpatients (P = 0.031) or referral patients (P < 0.0001).
PCR as a predictor of culture results.
A total of 93 (29.2%) Bordetella isolates were grown from the 318 PCR-positive specimens. Eighty-nine strains were B. pertussis, and four were B. parapertussis. Patients with a positive culture had a younger median age than those with a negative culture (median ages of 0.67 versus 3.24 years [P = 0.003]), a strong indication of potential lack or incompletion of vaccination. Those with positive cultures were more frequently inpatients compared to referral patients (P < 0.0001, OR 3.19 [95% CI = 1.80 to 5.67]). Detection of IS, PT, PO, or RA predicted culture growth with sensitivities (95% CI) of 0.82 (0.72 to 0.89), 0.90 (0.82 to 0.95), 0.93 (0.83 to 0.97), and 0.91 (0.70 to 0.98), respectively. Combinations of IS and either PT or PO/RA predicted growth by culture with a sensitivity of 1.0 (95% CI = 0.95 to 1.0). Of the 89 samples from which pertussis was detected by culture, 17 (19%) were not detected by the single-target IS PCR, including 3 of the 10 culture-positive (noticeably 5 in total 16 PCR-positive) in the intensive care unit (ICU) patients. Isolates were retrospectively tested for the presence of IS, PT, and RA, and all targets were detected in all isolates after culture. Among the 32 indeterminate BA-negative specimens, 1 grew B. pertussis, but no growth was observed among the 9 indeterminate RA specimens.
The overall culture sensitivity of the B. pertussis PCR-positive cases was 28.8% (89 of 309). The sensitivity of culture increased when the child was hospitalized (34 of 70 [49%]) and ICU (10 of 16 [63%]). The increasing number of positive PCR targets also predicted a higher rate of positive pertussis cultures, since 43.3% (61 of 141) of the three-target PCR positive specimens yielded positive growth, compared to 27.4% (24 of 111) of the two-target positive specimens and 8.5% (4 of 47) the single-target positive specimens.
Clinical variables and positivity of PCR and culture.
Clinical variables and the PCR and/or culture results were studied in the 112 children receiving care at CHRMC. Seventy (62.5%) children were hospitalized, of whom sixteen (22.9%) were admitted to the ICU. No association was noted between any single positive PCR target (IS, PT, or PO/RA) any of the following variables: a positive culture result, duration of cough, the presence of cough paroxysms or fever, or a history of having received antibiotics prior to sampling. Patients with mild disease as defined by the absence of apnea or cyanosis were more likely to have negative cultures (P = 0.004) or require shorter periods of hospitalization (median, 2 versus 4 days, P = 0.006).
DISCUSSION
PCR assays have greatly facilitated pertussis diagnosis, but problems with this assay persist: sensitivity is not yet as high as with serologic tests, false-positive results remain a problem, and differentiation between Bordetella species is not usually feasible with the single target assays (24, 28). Furthermore, no single-target PCR assay is universally considered to be a “gold standard” for pertussis diagnosis. Our findings were obtained on samples collected from a large cohort of patients of all ages and disease severity and confirm that individual targets may not perform consistently in all patient groups and demonstrate higher detection rates with multitarget PCR. For analytical accuracy, the use of multitarget PCR can minimize the incidence of the “pseudo-outbreaks” given that the potential error of any single-target approach may be recognized or counterbalanced by the use of additional target(s). Because of the increased sensitivity and the analytical precision permitted by this approach, culturing all clinical samples for B. pertussis becomes unnecessary.
The choice of target is critical for the specificity of any PCR assay, and combinations of primers may allow for the simultaneous detection of, and discrimination between, Bordetella species in the same assay (8, 18, 21, 27, 32, 33). Pertussis detection based on the single-target IS would have missed nearly 20% (17 of 89) culture-positive pertussis cases, including 3 of the 10 culture-positive ICU patients, or 5 of the 16 PCR-positive ICU patients. Because combinations of IS with either PT or RA provided significantly enhanced diagnostic sensitivity, we believe testing of clinical specimens for more than one pertussis target should be routinely conducted in clinical laboratories.
PT-PCR has been used to confirm the diagnosis of B. pertussis and B. parapertussis by IS-PCR (11, 14, 17, 30). Although ptxA is present as a single-copy gene, and IS is present in multiple copies, detection of PT has been reported to be equivalent to that of IS (14, 36). The present study did not find substantial differences between the detection of PT and IS for pertussis diagnosis: of the 309 PCR positive specimens, the detection rate was 76% by PT alone versus 80% by IS alone. This does not reflect the marked difference in their genome copy numbers at the 1:200 ratio (31). Nevertheless, the IS single-target reproducible positive cases (n = 34) outnumbered the PT (n = 9) and PO (n = 4) positive cases, suggesting the copy number theory. Limited by the availability of patient information for further analysis, we suspect that the multicopy IS may be more sensitive during early onset of illness when the organism load was low but the genome integrity was high. Despite its high copy number, IS481 is considered a nonessential genomic element. We therefore speculate that the underperformance of IS481 overall may be a result of nonrandom genome degradation during the course of host-pathogen interaction further into the disease.
PT was efficient in differentiating between B. pertussis and B. parapertussis using melting-point analysis. A comparable assay using IS1001 in a dual-target system also provides such bases for species distinction (36). The detection of PO by PCR has been suggested to allow an accurate approach to the diagnosis of pertussis (14). We replaced the detection of the noncoding PO sequence with RA, a stable coding region, following the availability of the three whole-genome sequencing data in 2003 (31). RA target was specifically chosen in the recA protein coding region where phylogenetic divergence of the three pathogens of interest (B. pertussis, B. parapertussis, and B. bronchiseptica) from the other Bordetella species can be found. Although not as many recA copies as IS481 copies exist in the B. pertussis genome (14, 19, 39), RA was a reliable target, performed better than PO in diagnosing B. parapertussis infection, and provided both a biologically stable marker for diagnosis and an analytical control for species confirmation.
All three targets were retrospectively detected in all culture-grown B. pertussis organisms in our study. Thus, the presence of a major sequence polymorphism in the targeted regions was unlikely, and the unequal detection of targets seemed to reflect a varying degree of the integrity of the organisms during disease. In other real-time PCR studies, fewer threshold cycles (CT < 25) have been used to predict positive cultures (38). In the present study, an increasing number of positive targets detected was increasingly predictive of a culture being positive. This is important in clinical practice, since positive cultures are more frequently associated with specimens from the much younger patients who were hospitalized.
The cost savings can be considerable when fluorescence resonance energy transfer techniques using CYBR green and melting-point analysis are compared to the commercially available single- or dual-target probe assay systems. Elimination of the DNA extraction step provided additional cost savings and additional specimen protection from crossover contamination. In the present study, only 32 of the 4,442 specimens (0.007%) tested were β-actin negative, indicating potentially poor sampling or PCR inhibition. DNA extraction therefore can be safely replaced by heat lysis after a rigorous saline wash. Further, employing a number of consensus-generating reactions per sample is far more effective than relying on a single stringent reaction. In our experience, an overall 6% rate of repeat testing due to those single-target positive results suggests that the analytical factors intrinsic to this type of molecular assay can be better controlled by multitarget reference parameters. In multitarget analysis, each independent reaction serves as an in-run control for potential false-positive or false-negative results. Thus, from both analytical and operational standpoints, substantial labor and time savings can be achieved by reducing repeat testing due to ambiguous results.
A limitation in our study was the lack of cultures and serological testing in all patients. Specimens that are culture positive and PCR negative have been reported, although only rarely (19, 35), and in our study only one PCR-negative specimen (BA negative) was culture positive, presumably due to polymerization inhibition. Despite the advantages of PCR for the identification of Bordetella species, culture techniques remain important for epidemiologic analysis or antibiotic susceptibility (4, 19, 20). In our study, however, only 30% of PCR-confirmed cases were culture positive, a finding in accordance with previous reports (35), and PCR identified many pertussis cases that would have escaped diagnosis by culture alone.
A better understanding of genomic targets of Bordetella has provided insight into new approaches for the detection of pertussis (34). Earlier studies have proposed the use of two PCR targets for pertussis diagnosis (9, 11), and we have refined our previous experience (32) to further enhance the reliable PCR detection of a pathogen that is becoming increasingly important in the clinical setting. We demonstrated here the application of the three-target PCR approach under routine diagnostic conditions in a hospital laboratory. Our findings suggest that multitarget PCR, beginning with IS481, increases sensitivity, discriminates false-positive and false-negative results, and allows for a specific identification of the causative Bordetella organism. Our findings further suggest that two-target approaches may also be worth considering. It is well known that pertussis is under-recognized, both in the community and in hospitalized patients; in the latter setting, missed diagnoses may lead to nosocomial outbreaks (1, 3, 5, 9, 10, 29, 34). The increasing use of vaccination in older children, adolescents, and adults may ultimately decrease the incidence of pertussis, but advances in diagnostic assays that contribute to early and reliable identification will enable treatment to be initiated and infection control measures to be implemented.
Acknowledgments
We thank Lynn Stapp, Patrick Abe, Scott Anderson, Joan Guzzo, Treva Tsosie, Dona DeGroat, and Jenny Stapp for technical assistance and Jane Kuypers and Joe Rutledge for reviewing the manuscript.
The research was conducted while Emmanouil Galanakis was on sabbatical from the University of Crete, Chania, Greece.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Allen, C. W., and H. E. Jeffery. Pertussis in the neonatal nursery. 2005. J. Paediatr. Child Health 41:140-142. [DOI] [PubMed] [Google Scholar]
- 2.Bamberger, E., N. Lahat, V. Gershtein, R. Gershtein, D. Benilevi, S. Shapiro, I. Kassis, L. Rubin, and I. Srugo. 2005. Diagnosing pertussis: the role of polymerase chain reaction. Isr. Med. Assoc. J. 7:351-354. [PubMed] [Google Scholar]
- 3.Baughman, A. L., K. M. Bisgard, K. M. Edwards, D. Guris, M. D. Decker, K. Holland, B. D. Meade, and F. Lynn. 2004. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin. Diagn. Lab. Immunol. 11:1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne, S., and A. T. Slack. 2006. Analysis of Bordetella pertussis pertactin and pertussis toxin types from Queensland, Australia, 1999-2003. BMC Infect. Dis. 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calugar, A., I. R. Ortega-Sanchez, T. Tiwari, L. Oakes, J. A. Jahre, and T. V. Murphy. 2006. Nosocomial pertussis: costs of an outbreak and benefits of vaccinating health care workers. Clin. Infect. Dis. 42:981-988. [DOI] [PubMed] [Google Scholar]
- 6.CDC. 2000. Guidelines for the control of pertussis outbreaks. U.S. Department of Health and Human Services, CDC, Atlanta, GA. [Online.] http://www.cdc.gov/nip/publications/pertussis/guide.htm.
- 7.Cherry, J. D. 2005. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 115:1422-1427. [DOI] [PubMed] [Google Scholar]
- 8.Cloud, J. L., W. C. Hymas, A. Turlak, A. Croft, U. Reischl, J. A. Daly, and K. C. Carroll. 2003. Description of a multiplex Bordetella pertussis and Bordetella parapertussis LightCycler PCR assay with inhibition control. Diagn. Microbiol. Infect. Dis. 46:189-195. [DOI] [PubMed] [Google Scholar]
- 9.Crowcroft, N. S., R. Booy, T. Harrison, L. Spicer, J. Britto, Q. Mok, P. Heath, I. Murdoch, M. Zambon, R. George, and E. Miller. 2003. Severe and unrecognized: pertussis in UK infants. Arch. Dis. Child. 88:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks, S., G. De Serres, N. Boulianne, B. Duval, L. Rochette, P. Dery, and S. Halperin. 1999. Failure of physicians to consider the diagnosis of pertussis in children. Clin. Infect. Dis. 28:840-846. [DOI] [PubMed] [Google Scholar]
- 11.Dragsted, D. M., B. Dohn, J. Madsen, and J. S. Jensen. 2004. Comparison of culture and PCR for detection of Bordetella pertussis and Bordetella parapertussis under routine laboratory conditions. J. Med. Microbiol. 53:749-754. [DOI] [PubMed] [Google Scholar]
- 12.Edelman, K., S. Nikkari, O. Ruuskanen, Q. He, M. Viljanen, and J. Mertsola. 1996. Detection of Bordetella pertussis by polymerase chain reaction and culture in the nasopharynx of erythromycin-treated infants with pertussis. Pediatr. Infect. Dis. J. 15:54-57. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, K. M. 2005. Overview of pertussis: focus on epidemiology, sources of infection, and long-term protection after infant vaccination. Pediatr. Infect. Dis. J. 24:S104-S108. [DOI] [PubMed] [Google Scholar]
- 14.Farrell, D. J., M. McKeon, G. Daggard, M. J. Loeffelholz, C. J. Thompson, and T. K. Mukkur. 2000. Rapid-cycle PCR method to detect Bordetella pertussis that fulfills all consensus recommendations for use of PCR in diagnosis of pertussis. J. Clin. Microbiol. 38:4499-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallander, H. O. 1999. Microbiological and serological diagnosis of pertussis. Clin. Infect. Dis. 28:S99-S106. [DOI] [PubMed] [Google Scholar]
- 16.Heininger, U., G. Schmidt-Schlapfer, J. D. Cherry, and K. Stehr. 2000. Clinical validation of a polymerase chain reaction assay for the diagnosis of pertussis by comparison with serology, culture, and symptoms during a large pertussis vaccine efficacy trial. Pediatrics 105:E31. [DOI] [PubMed] [Google Scholar]
- 17.Herwegh, S., C. Carnoy, F. Wallet, C. Loiez, and R. J. Courcol. 2005. Development and use of an internal positive control for detection of Bordetella pertussis by PCR. J. Clin. Microbiol. 43:2462-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houard, S., C. Hackel, A. Herzog, and A. Bollen. 1989. Specific identification of Bordetella pertussis by the polymerase chain reaction. Res. Microbiol. 140:477-487. [DOI] [PubMed] [Google Scholar]
- 19.Knorr, L., J. D. Fox, P. A. Tilley, and J. Ahmed-Bentley. 2006. Evaluation of real-time PCR for diagnosis of Bordetella pertussis infection. BMC Infect. Dis. 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korgenski, E. K., and J. A. Daly. 1997. Surveillance and detection of erythromycin resistance in Bordetella pertussis isolates recovered from a pediatric population in the Intermountain West region of the United States. J. Clin. Microbiol. 35:2989-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosters K., U. Reischl, J. Schmetz, M. Riffelmann, and C. H. Wirsing von Konig. 2002. Real-time LightCycler PCR for detection and discrimination of Bordetella pertussis and Bordetella parapertussis. J. Clin. Microbiol. 40:1719-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Z., D. L. Jansen, T. M. Finn, S. A. Halperin, A. Kasina, S. P. O'Connor, T. Aoyama, C. R. Manclark, and M. J. Brennan. 1994. Identification of Bordetella pertussis infection by shared-primer PCR. J. Clin. Microbiol. 32:783-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Z. M., J. H. Hannah, S. Stibitz, N. Y. Nguyen, C. R. Manclark, and M. J. Brennan. 1991. Cloning and sequencing of the structural gene for the porin protein of Bordetella pertussis. Mol. Microbiol. 5:1649-1656. [DOI] [PubMed] [Google Scholar]
- 24.Lievano, F. A., M. A. Reynolds, A. L. Waring, J. Ackelsberg, K. M. Bisgard, G. N. Sanden, D. Guris, A. Golaz, D. J. Bopp, R. J. Limberger, and P. F. Smith. 2002. Issues associated with and recommendations for using PCR to detect outbreaks of pertussis. J. Clin. Microbiol. 40:2801-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchant, C. D., A. M. Loughlin, S. M. Lett, C. W. Todd, L. H. Wetterlow, R. Bicchieri, S. Higham, P. Etkind, E. Silva, and G. R. Siber. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 169:1297-1305. [DOI] [PubMed] [Google Scholar]
- 26.Melton, A. R., and A. A. Weiss. 1989. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J. Bacteriol. 171:6206-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mooi, F. R., H. Hallander, C. H. Wirsing von Konig, B. Hoet, and N. Guiso. 2000. Epidemiological typing of Bordetella pertussis isolates: recommendations for a standard methodology. Eur. J. Clin. Microbiol. Infect. Dis. 19:174-181. [DOI] [PubMed] [Google Scholar]
- 28.Muller F. M., J. E. Hoppe, and C. H. Wirsing von Konig. 1997. Laboratory diagnosis of pertussis: state of the art in 1997. J. Clin. Microbiol. 35:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muyldermans, G., O. Soetens, M. Antoine, S. Bruisten, B. Vincart, F. Doucet-Populaire, N. K. Fry, P. Olcen, J. M. Scheftel, J. M. Senterre, A. van der Zee, M. Riffelmann, D. Pierard, and S. Lauwers. 2005. External quality assessment for molecular detection of Bordetella pertussis in European laboratories. J. Clin. Microbiol. 43:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nygren, M., E. Reizenstein, M. Ronaghi, and J. Lundeberg. 2000. Polymorphism in the pertussis toxin promoter region affecting the DNA-based diagnosis of Bordetella infection. J. Clin. Microbiol. 38:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, Harris, D. E., et al. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 32.Qin, X., D. K. Turgeon, B. P. Ingersoll, P. W. Monsaas, C. J. Lemoine, T. Tsosie, L. O. Stapp, and P. M. Abe. 2002. Bordetella pertussis PCR: simultaneous targeting of signature sequences. Diagn. Microbiol. Infect. Dis. 43:269-275. [DOI] [PubMed] [Google Scholar]
- 33.Reizenstein, E., B. Johansson, L. Mardin, J. Abens, R. Mollby, and H. O. Hallander. 1993. Diagnostic evaluation of polymerase chain reaction discriminative for Bordetella pertussis, B. parapertussis, and B. bronchiseptica. Diagn. Microbiol. Infect. Dis. 17:185-191. [DOI] [PubMed] [Google Scholar]
- 34.Riffelmann M., C. H. Wirsing von Konig, V. Caro, N. Guiso, and the Pertussis PCR Consensus Group. 2005. Nucleic Acid amplification tests for diagnosis of Bordetella infections. J. Clin. Microbiol. 43:4925-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlapfer, G., J. D. Cherry, U. Heininger, M. Uberall, S. Schmitt-Grohe, S. Laussucq, M. Just, and K. Stehr. 1995. Polymerase chain reaction identification of Bordetella pertussis infections in vaccinees and family members in a pertussis vaccine efficacy trial in Germany. Pediatr. Infect. Dis. J. 14:209-214. [DOI] [PubMed] [Google Scholar]
- 36.Sloan, L. M., M. K. Hopkins, P. S. Mitchell, E. A. Vetter, J. E., Rosenblatt, W. S. Harmsen, F. R. Cockerill, and R. Patel. 2002. Multiplex LightCycler PCR assay for detection and differentiation of Bordetella pertussis and Bordetella parapertussis in nasopharyngeal specimens. J. Clin. Microbiol. 40:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taranger, J., B. Trollfors, L. Lind, G. Zackrisson, and K. Beling-Holmquist. 1994. Environmental contamination leading to false-positive polymerase chain reaction for pertussis. Pediatr. Infect. Dis. J. 13:936-937. [DOI] [PubMed] [Google Scholar]
- 38.Templeton, K. E., S. A. Scheltinga, A. van der Zee, B. M. Diederen, A. M. van Kruijssen, H. Goossens, E. Kuijper, and E. C. Claas. 2003. Evaluation of real-time PCR for detection of and discrimination between Bordetella pertussis, Bordetella parapertussis, and Bordetella holmesii for clinical diagnosis. J. Clin. Microbiol. 41:4121-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vielemeyer, O., J. Y. Crouch, S. C. Edberg, and J. G. Howe. 2004. Identification of Bordetella pertussis in a critically ill human immunodeficiency virus-infected patient by direct genotypical analysis of Gram stained material and discrimination from B. holmesii by using a unique recA gene restriction enzyme site. J. Clin. Microbiol. 42:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Konig, C. H., S. Halperin, M. Riffelmann, and N. Guiso. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2:744-750. [DOI] [PubMed] [Google Scholar]