Shigellosis is an important cause of morbidity and mortality in India. In contrast to what was reported for the developed world and Iran (1), where Shigella sonnei is common, the occurrence of nalidixic acid resistance is low, and no isolates have been determined to be resistant to fluoroquinolones, for the Indian subcontinent, Shigella flexneri continues to be the most common serogroup isolated, antibiotic resistance to nalidixic acid is high, and ciprofloxacin resistance is emerging. New disease burden estimates of shigellosis in Asia from the ICCDRB, Bangladesh, indicate only a modest reduction in the number of infections (114 million cases) but a 90% decrease in mortality compared to the previous estimate (D. A. Sack, P. Bardhan, and A. Naheed, Abstr. 11th Asian Conf. Diarrheal Dis. Nutr., p. 15, 2006). At our 1,358-bed tertiary care referral center in north India, Shigella spp. were the second most common bacterial agents causing diarrhea after Escherichia coli. From January 2000 to October 2006, 199 shigella strains were isolated from 5,558 stool samples (3.58%). Shigellae were identified by standard biochemical methods (6) and confirmed by serotyping using Denka-Seiken antisera (Japan). Antibiotic susceptibility was determined per NCCLS guidelines. The MICs of nalidixic acid and ciprofloxacin were determined by the agar dilution method of the NCCLS and the Etest, respectively. Patients' clinical and treatment details were noted.
Out of 199 culture-confirmed episodes of shigellosis, 122 (61.3%) occurred in children; 152 (76.3%) children were under the age of 5 years, and 75 (37.6%) were under the age of 2 years. Male children were predominately affected (88 [72%] male, 72 [28%] female); 47.6% of children presented with acute gastroenteritis, whereas 52.4% presented with frank dysentery.
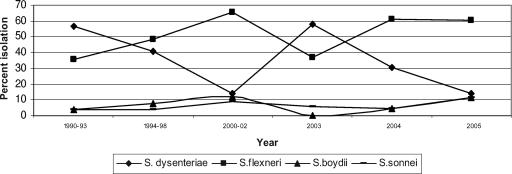
We have observed not only an increasing incidence of multidrug resistance among shigellae but also shifts in the prevalent serogroups (Fig. 1). At our center, Shigella flexneri had been the predominant species isolated from 1994 to 2002. From May to November 2003, a sudden increase in the number of patients admitted with dysentery was noted. Ciprofloxacin-resistant S. dysenteriae serotype 1 was isolated from stool samples. Isolates were also resistant to other commonly used drugs. S. dysenteriae serotype 1 emerged as the predominant serogroup after a decade (5). Cyclical serogroup changes have been reported in many parts of the world. Epidemic dysentery caused by multidrug-resistant S. dysenteriae serotype 1 has been a recurrent challenge in many parts of developing world. This organism caused an extensive epidemic of shigellosis in eastern India in 1984 (3). After a lapse of about 18 years, an outbreak of bacillary dysentery with high morbidity and mortality was reported in April 2002 among the laborers of tea gardens in the same region (2). The strains were resistant to ampicillin, co-trimoxazole, nalidixic acid, and norfloxacin. Similar strains have also been reported from sporadic cases in Nepal and Bangladesh, leading to a regional alert in south Asia (4). Pulsed-field gel electrophoresis of the isolates performed at the National Institute of Cholera and Enteric Diseases, Kolkata, India, showed these strains to be same clones as those causing outbreaks in northeast India and those isolated from sporadic cases of shigellosis in Nepal and Bangladesh (unpublished data). A serogroup switch occurred in 2004, when S. flexneri again became the predominant serogroup. However, ciprofloxacin resistance emerged in S. flexneri also. Table 1 shows the levels of resistance to nalidixic acid and ciprofloxacin in various serogroups of shigellae. Out of 73 S. flexneri strains isolated from 2004 to October 2006, 46 (63%) showed MICs of 6 or more by Etest (6 isolates had MICs of ≥32, 10 had a MIC of 16, 19 had a MIC of 8, 4 had a MIC of 12, and 7 had a MIC of 6). Resistance to other commonly used agents is also very high (there is uniform resistance to amoxicillin and nalidixic acid, and 87% of isolates are resistant to co-trimoxazole). Therapeutic failures with ciprofloxacin occurred with both ciprofloxacin-resistant S. dysenteriae and ciprofloxacin-resistant S. flexneri. The severity of illness was more with ciprofloxacin-resistant strains. However, there was no mortality. Varied results were obtained by ciprofloxacin disc diffusion and the Etest. Susceptibility to norfloxacin as determined by disc diffusion was a good predictor of a high MIC (>8) for ciprofloxacin. Ciprofloxacin resistance in S. flexneri is sporadic and uncommon, though resistance to co-trimoxazole and ampicillin is common and in some areas resistance to nalidixic acid has also emerged. Fluoroquinolones are being extensively used and misused for many other illnesses in our region. However, S. boydii and S. sonnei remain susceptible to fluoroquinolones. To conclude, due to indiscriminate use, fluoroquinolones will soon be ineffective in our region for the treatment of shigellosis. The emergence of a clone of ciprofloxacin-resistant S. dysenteriae serotype 1 in north India and its clonal similarity with isolates from East India indicate clonal spread and is a cause of serious concern. We are also worried about the emergence of high-level ciprofloxacin resistance in S. flexneri.
FIG. 1.
Changing serogroup distributions of patients with shigellosis at PGIMER, Chandigarh, India.
TABLE 1.
Overall resistance to nalidixic acid and ciprofloxacin from 2000 to 2006 in Indiaa
| Organism (total no. of isolates) | No. (%) of isolates resistant to:
|
|
|---|---|---|
| Nalidixic acid | Ciprofloxacin | |
| S. dysenteriae (44) | 36 (81.8%) | 24 (54.5%) |
| S. flexneri (116) | 86 (74.13%) | 53 (45.6%) |
| S. boydii (14) | 8 (57.1%) | 0 (0%) |
| S. sonnei (14) | 14 (100%) | 0 (0%) |
Results are not shown for 11 isolates that were nonagglutinable shigellae.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Farshad, S., R. Sheikhi, A. Japoni, E. Basiri, and A. Alborzi. 2006. Characterization of Shigella strains in Iran by plasmid profile analysis and PCR amplification of ipa genes. J. Clin. Microbiol. 44:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pazhani, G. P., B. Sarkar, T. Ramamurthy, S. K. Bhattacharya, Y. Takeda, and S. K. Niyogi. 2004. Clonal multidrug-resistant Shigella dysenteriae type 1 strains associated with epidemic and sporadic dysenteries in eastern India. Antimicrobial. Agents Chemother. 48:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal, S. C. 1984. Epidemic bacillary dysentery in West Bengal. Lancet i:1462. [DOI] [PubMed] [Google Scholar]
- 4.Talukdar, K. A., B. K. Khajanchi, M. A. Islam, K. Dutta, J. Islam, A. Safe, et al. 2004. Genetic relatedness of ciprofloxacin-resistant Shigella dysenteriae type 1 strains isolated in south Asia. J. Antimicrob. Chemother. 54:730-734. [DOI] [PubMed] [Google Scholar]
- 5.Taneja, N., V. Lyngdoh, A. Vermani, B. Mohan, P. Rao, M. Singh, et al. 2005. Re-emergence of multi-drug resistant Shigella dysenteriae with added resistance to ciprofloxacin in north India and their plasmid profiles. Indian J. Med. Res. 122:348-354. [PubMed] [Google Scholar]
- 6.World Health Organization. 1983. Manual for laboratory investigation of acute enteric infections. Programme for Control of Diarrhoeal Diseases. WHO CDD/83.3. World Health Organization, Geneva, Switzerland.