Abstract
Benign papular eruption on the left leg of a 72-year-old diabetic man developed into rapidly spreading necrotizing fasciitis despite antimicrobial therapy and surgical debridements. This led to eventual amputation to control the infection. The etiological agent was a Staphylococcus aureus isolate harboring the enterotoxin gene cluster seg, sei, sem, sen, and seo but lacked all common toxin genes, including Panton-Valentine leukocidin.
CASE REPORT
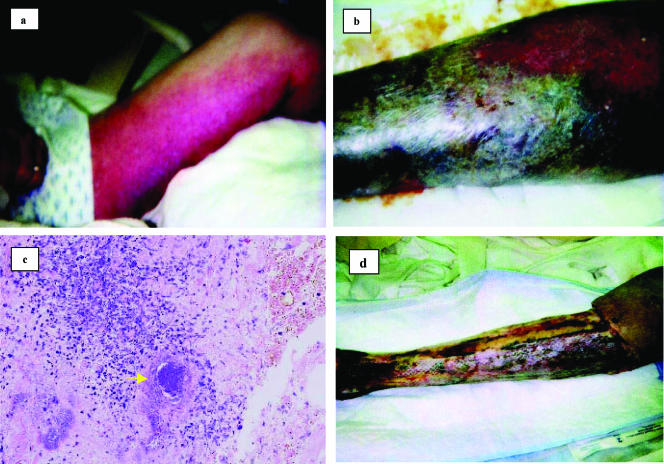
A 72-year-old man presented to his local physician with a 1-day history of swelling and erythematous papular eruption on his left ankle. Despite doing yard work when he noticed the rash, he did not remember trauma to the area. At the initial visit he was febrile and was diagnosed with cellulitis. The patient was treated with intravenous ciprofloxacin and clindamycin for 5 days with no improvement. Two blood cultures drawn at the time of presentation yielded methicillin-sensitive Staphylococcus aureus (MSSA). The MSSA strain was susceptible to all classes of antimicrobial agents tested, including penicillin. The patient was transferred to the Marshfield center of the Marshfield Clinic with continued worsening erythema and edema on his left leg (Fig. 1A). The leg was purpuric, and there was necrosis along the medial aspect of the left leg and thigh (Fig. 1B). Edema was present outside the area of erythema and purpura. His admission temperature was 101.4°F. He had a leucocytosis of 14,400/mm3. His past medical history was significant for type 2 diabetes mellitus, coronary artery disease, hyperlipidemia, hypertension, and thrombocytopenia. The patient was allergic to penicillin. He denied smoking and drank four to five alcoholic beverages a week. After vancomycin was added to his intravenous antibiotic regimen, the decision was made to emergently take the patient to the operating room for aggressive debridement of the skin and soft tissue of his left thigh and leg with the presumptive diagnosis of necrotizing fasciitis. Intraoperative histological tissue examination indicated focal areas of necrosis with acute inflammation in the skin and subcutaneous tissue (Fig. 1C). Fascial biopsy showed mild acute inflammation. The underlying skeletal muscle was involved with what appeared to be reactive inflammatory changes. Microbiological culture of the tissue sample taken from the wound grew a clonally related MSSA. The ciprofloxacin was discontinued and rifampin was started. The patient subsequently underwent two more operative debridements (Fig. 1D). On hospital day 7 his wound cultures were negative. On hospital day 10, his wounds were grafted with porcine skin. For social reasons he was transferred to a hospital closer to his home on hospitalization day 16. He continued to receive intravenous antibiotics, but unfortunately his clinical picture worsened and he underwent an above-the-knee amputation. He recovered from his surgery and was later discharged to home.
FIG. 1.
(a) Appearance of the left leg on admission to the hospital. There is extensive erythema and purpura involving the entire left leg. Edema is present outside the area of erythema, and necrosis is developing along the medial aspect of the thigh. (b) Extensive necrosis of the leg. The infection rapidly progressed to frank necrosis of the skin and subcutaneous tissues, necessitating surgical debridement. (c) Histological appearance of deep subcutaneous tissue from surgical debridement. There is a dense inflammatory infiltrate of neutrophils, lymphocytes, and macrophages. A bacterial colony is present within the tissue (arrow). (d) Appearance of the left leg after two surgical debridement procedures. After extensive debridement the wounds were grafted with porcine skin. Despite continued antibiotic therapy his condition worsened and the patient required an above-the-knee amputation.
Staphylococcus aureus is a hardy and versatile human pathogen. It is capable of causing a variety of human diseases ranging from mild skin and soft-tissue infections to more severe diseases, such as osteomyelitis, pneumonia, bacteremia, and endocarditis. Necrotizing fasciitis is a rare soft-tissue infection that primarily involves superficial fascia and results in the extensive undermining and necrosis of the surrounding tissue. S. aureus is thought to be an uncommon agent for this disease. However, rare cases of rapidly progressing, necrotizing fasciitis have been described in diabetic patients (16, 21) and patients with chronic use of steroid-containing medications (21). Miller et al. recently described 14 cases of necrotizing fasciitis disease due to Panton-Valentine leukocidin (PVL)-producing community-associated methicillin-resistant S. aureus (CA-MRSA) in a retrospective study of 843 MRSA cases from 2003 to 2004 in Los Angeles (18). In the few cases reported so far, virulence profiles and genotypes of the S. aureus strains have not been described in detail. With the global epidemic of CA-MRSA, a better understanding of how virulence factors of S. aureus relate to specific clinical syndromes would be useful. Here we describe a severe case of necrotizing fasciitis in a diabetic patient due to a highly virulent MSSA strain that originated in the community. The strain lacked lukSF-PV but harbored other virulence gene sets, notably an enterotoxin gene cluster (egc).
Microbiology.
A series of blood and tissue samples was obtained from the patient for microbiological culture during the course of treatment for necrotizing fasciitis. Blood samples were incubated in the BacT Alert System (bioMérieux, Durham, NC) to isolate the etiological agent. A small piece of tissue culture was directly processed for microbiological cultures. Bacterial identification and characterization of the suspected etiological agent were done by routine microbiological methods and 16S rRNA gene PCR and sequencing. Antibiogram susceptibilities were determined by the Vitek System (bioMérieux). The 16S rRNA and mecA gene PCR and sequencing were done by the method previously described (19, 20). PVL gene PCR was done by the method of Gillet et al. (8). The strain was genotyped by pulsed-field gel electrophoresis, multilocus sequence typing (MLST), and spa typing (3, 6, 13). Because this was a very unusual and persistent strain, a number of known toxin and virulence genes were screened for their presence in this strain. Staphylococcal enterotoxin genes sea, seb, sec, sed, see, seh, sej, sek, sel, egc, seg, sei, sem, sen, and seo; exfoliative toxin genes eta and etb; toxic shock syndrome toxin (TSST) gene tst; PVL gene lukSF-PV; and α-, β-, δ-, and γ-hemolysin genes hla, hlb, hld, and hlg were tested. We also screened for seven newer virulence genes, bsa, ear, seg2, sel2, sec4, set16, and lpl10, that were reported to be uniquely associated with a hypervirulent Midwestern strain, MW2 (also known as USA400 MRSA clone) (2, 17). In addition, fibronectin binding protein genes fnaA and fnaB, collagen binding protein gene cna, intracellular adhesion gene icaA, clumping factor genes clfA and clfB, and adhesion factor genes sdrD and sdrE were also tested. All these virulence genes were tested by a series of multiplex and singleplex PCR assays (J. M. Brady and S. K. Shukla, unpublished data).
S. aureus was identified by colony morphology, Gram stain, and positive coagulase test and was confirmed by 16S rRNA gene PCR and sequencing. The bacterial isolate WI-MSSA184, recovered from the blood sample collected on the day of clinical presentation, was identified as S. aureus. A 734-bp sequence of the 16S rRNA gene of this isolate had 100% sequence identity with S. aureus strains, including a known virulent MSSA strain, MSSA476 (9).
The isolate showed susceptibility to the following antimicrobial agents: cefazolin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, tetracycline, trimethoprim-sulfamethoxazole, levofloxacin, linezolid, and amoxicillin-sulbactum. The SmaI macrorestricted pulsed-field gel electrophoresis pattern of the isolate was closely related to that of the type strain of the USA600 MRSA clone, except for the absence of the DNA fragment containing the mecA element (17). The MLST allelic profile (10-14-8-6-10-3-2) identified it as sequence type 45, which was consistent with the sequence type determined for the type strain USA600 clonal group. Typing by the surface protein A gene showed it to be spa type t917. As expected, the isolate was negative for the mecA gene and other genetic elements of staphylococcal cassette chromosome mec (SCCmec). The strain was also negative for leukocidin genes (lukSF-PV, lukE, and lukD), fnbB, exfoliative toxin genes (eta and etb), as well as enterotoxin genes (sea, seb, sec, sed, see, seh, sej, sek, and sel). However, the strain harbored α-, β-, δ-, and γ-toxin genes and was positive for the egc genes (seg, sei, sem, sen, and seo) and the newer toxin genes bsa, set16, and lpl10. The strain was positive for clumping factors genes clfA and clfB, fibronectin binding protein gene fnbA, collagen binding adhesion gene cna, and intracellular adhesion gene icaA. The deep subcutaneous tissue obtained during surgical debridement showed a dense inflammatory infiltrate of neutrophils, lymphocytes, and macrophages as well as bacterial colonies within the tissue (Fig. 1C).
Necrotizing fasciitis is a syndrome predominantly due to polymicrobial infection and includes infection by pathogens such as Streptococcus pyogenes and species of Bacteroides, Clostridium, Peptostreptococcus, and members of Enterobacteriaceae. There have been rare reports of monobacterial necrotizing fasciitis caused by S. aureus. In all these reports, the clinical course was indolent. In this report, the course of infection was slow during the first few days but progressed rapidly thereafter to frank fascial necrosis. Despite proper antibiotic coverage, the patient required an above-the-knee amputation to prevent further necrosis. Usually when a patient presents with a soft-tissue infection and cultures due to S. aureus, the patient is more likely to have cellulitis than a necrotizing process. This case, along with the recently described cases, provides evidence that S. aureus should be added to the list of pathogens that can cause severe necrotizing fasciitis (16, 18, 21).
Severity of skin and soft-tissue infection caused by S. aureus, especially CA-MRSA, is dictated by its ability to produce tissue-necrotizing toxins and other virulence factors. One of the nearly ubiquitous toxins present in CA-MRSA is PVL, a two-component pore-forming toxin with the ability to cause necrotizing pneumonia (8). Besides the PVL genes, several other exotoxins and enterotoxins have been reported on the genomes of virulent strains of MRSA, including MW2, which caused a fatal illness in four children in 1997 to 1999 (5).
Kravitz et al. recently reported five cases (three of them fatal) of purpura fulminans, which were due to toxin-producing strains of S. aureus (15). One of these fatal cases was due to a PVL and staphylococcal enterotoxin C (SEC)-producing CA-MRSA strain of clone USA400. The four remaining cases were caused by other toxin (SEB, SEC, PVL, or TSST-1)-producing strains of CA-MSSA. Despite the numbers being relatively small, necrotizing fasciitis and purpura fulminans diseases could be viewed as examples of S. aureus-associated emerging illnesses. S. aureus that was reported to cause fatal cases of sepsis and Waterhouse-Friderichsen syndrome also produced PVL (1). Virulence profile results from these cases suggest that at least PVL, SEA, SEC, and TSST, either alone or in combination, play some role in diseases such as necrotizing pneumonia, necrotizing fasciitis, purpura fulminans, and Waterhouse-Friderichsen syndrome (1, 8, 15, 18).
S. aureus strains that caused purpura fulminans and Waterhouse-Friderichsen syndrome included both MSSA and MRSA phenotypes (1, 15). The severity of the disease due to a methicillin-sensitive strain described in this report prompted us to genotype this strain to see if it had molecular traits of known virulent strains of community-associated and/or health care-associated MRSA. Genotyping showed it to be related to a clonal type (USA600) observed in nosocomial settings (17). This strain belonged to ST45, a clone not previously reported to be hypervirulent. A search of the MLST database showed that ST45 has been found among nasal carriers and is associated with nosocomial infections. Both MRSA and MSSA ST45 phenotypes have been reported from several European countries and at least one case from New York (www.saureus.mlst.net). Even though the strain in this case was an ST45 type, it should be considered uncommon due to its susceptibility to penicillin. Given the severity of the necrotizing fasciitis, it was surprising to see that it lacked leukocidin genes lukD and lukE, tissue-necrotizing factor PVL, and genes for several superantigens (SEA, SEB, SEC, SED, SEE, SEH, SEJ, SEK, and SEL). The MSSA strain, WI-MSSA184, was positive for staphylococcal adhesin genes, clfA and clfB, that are capable of contributing to the initial attachment of the fascia followed by FnbA acting in the necrosis of fascia.
This strain's virulence likely came from a cluster of genes that belong to the superantigen family, the family of proteins capable of triggering a massive toxic shock response (14). We speculate that products of superantigen genes seg, sei, sem, sen, and seo played a role in the severity of the syndrome. Our understanding of staphylococcal egc is far from complete given the limited literature available on their role in pathogens. The egc genes belong to an operon and have been hypothesized to be a putative nursery of superantigens in S. aureus (12). However, toxins made by egc appear to be fewer in quantity than the classical enterotoxins (10), but EGC toxins are capable of evading immune response due to lack of neutralization by the human sera (7). In one study, egc was found to be less commonly present than sea in S. aureus strains that caused sepsis with or without septic shock (11). However, in another study, seg and sei, two egc genes, were implicated in causing staphylococcal toxic shock syndrome and staphylococcal scarlet fever (10). Interestingly, allelic variants of seg and sei have been recently described by Blaiotta et al. (4). It is possible that the proteins made by the allelic variant of egc could be more toxigenic than previously thought. In this context it seems plausible that EGC toxins could add to the severity of infections in certain compromised individuals. We speculate that the diabetic patient's immune response to the EGC toxins may have been inadequate. This case report serves as a sobering reminder to clinicians that initially benign-appearing soft-tissue infections caused by an MSSA harboring the appropriate virulence arsenal can progress to more severe disease, especially in compromised individuals. It also underscores the need for a better understanding of how virulence factors, especially egc, are associated with specific clinical syndromes.
Acknowledgments
The work presented here was funded in part by grant AI061385 to S.K.S. from the National Institute of Allergy and Infectious Diseases.
We thank the Marshfield Clinic Research Foundation for its support through the assistance of Linda Weis and Alice Stargardt in the preparation of the manuscript.
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Adem, P. V., C. P. Montgomery, A. N. Husain, T. K. Koogler, V. Arangelovich, M. Humilier, S. Boyle-Vavra, and R. S. Daum. 2005. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N. Engl. J. Med. 353:1245-1251. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 3.Bannerman, T. L., G. A. Hancock, F. C. Tenover, and J. M. Miller. 1995. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J. Clin. Microbiol. 33:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaiotta, G., V. Fusco, C. von Eiff, F. Villani, and K. Becker. 2006. Biotyping of enterotoxigenic Staphylococcus aureus by enterotoxin gene cluster (egc) polymorphism and spa typing analyses. Appl. Environ. Microbiol. 72:6117-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997-1999. Morb. Mortal. Wkly. Rep. 48:707-710. [Online.] http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4832a2.htm. [PubMed] [Google Scholar]
- 6.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferry, T., D. Thomas, A. L. Genestier, M. Bes, G. Lina, F. Vandenesch, and J. Etienne. 2005. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin. Infect. Dis. 41:771-777. [DOI] [PubMed] [Google Scholar]
- 8.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 9.Holden, M. T., E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Spratt, and J. Parkhill. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 101:9786-9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtfreter, S., K. Bauer, D. Thomas, C. Feig, V. Lorenz, K. Roschack, E. Friebe, K. Selleng, S. Lovenich, T. Greve, A. Greinacher, B. Panzig, S. Engelmann, G. Lina, and B. M. Broker. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 72:4061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarraud, S., G. Cozon, F. Vandenesch, M. Bes, J. Etienne, and G. Lina. 1999. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J. Clin. Microbiol. 37:2446-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 13.Koreen, L., S. V. Ramaswamy, E. A. Graviss, S. Naidich, J. M. Musser, and B. N. Kreiswirth. 2004. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J. Clin. Microbiol. 42:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotzin, B. L., D. Y. Leung, J. Kappler, and P. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 15.Kravitz, G. R., D. J. Dries, M. L. Peterson, and P. M. Schlievert. 2005. Purpura fulminans due to Staphylococcus aureus. Clin. Infect. Dis. 40:941-947. [DOI] [PubMed] [Google Scholar]
- 16.Lee, Y. T., T. D. Chou, M. Y. Peng, and F. Y. Chang. 2005. Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus. J. Microbiol. Immunol. Infect. 38:361-364. [PubMed] [Google Scholar]
- 17.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 19.Poitout, F. M., J. K. Shinozaki, P. J. Stockwell, C. J. Holland, and S. K. Shukla. 2005. Genetic variants of Anaplasma phagocytophilum infecting dogs in Western Washington State. J. Clin. Microbiol. 43:796-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla, S. K., S. V. Ramaswamy, J. Conradt, M. E. Stemper, R. Reich, K. D. Reed, and E. A. Graviss. 2004. Novel polymorphisms in mec genes and a new mec complex type in methicillin-resistant Staphylococcus aureus isolates obtained in rural Wisconsin. Antimicrob. Agents Chemother. 48:3080-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong, C. H., S. H. Tan, A. Kurup, and A. B. Tan. 2004. Recurrent necrotizing fasciitis caused by methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 23:909-911. [DOI] [PubMed] [Google Scholar]