Abstract
Two new T-cell-based reporter cell lines were established to measure human immunodeficiency virus type 1 (HIV-1) infectivity. One cell line naturally expresses CD4 and CXCR4, making it susceptible to X4-tropic viruses, and the other cell line, in which a CCR5 expression vector was introduced, is susceptible to both X4- and R5-tropic viruses. Reporter cells were constructed by transfecting the human T-cell line HPB-Ma, which demonstrates high susceptibility to HIV-1, with genomes expressing two different luciferase reporters, HIV-1 long terminal repeat-driven firefly luciferase and cytomegalovirus promoter-driven renilla luciferase. Upon HIV infection, the cells expressed firefly luciferase at levels that were highly correlated (r2 = 0.91 to 0.98) with the production of the capsid antigen p24. The cells also constitutively expressed renilla luciferase, which was used to monitor cell numbers and viability. The reliability of the cell lines for two in vitro applications, drug resistance phenotyping and drug screening, was confirmed. As HIV-1 efficiently replicated in these cells, they could be used for multiple-round replication assays as an alternative method to a single-cycle replication protocol. Coefficients of variation for drug susceptibility evaluated with the cell lines ranged from 17 to 41%. The new cell lines were beneficial for evaluating antiretroviral drug resistance. Firefly luciferase gave a wider dynamic range for evaluating virus infectivity, and the introduction of renilla luciferase improved assay reproducibility. The cell lines were also beneficial for screening new antiretroviral agents, as false inhibition caused by the cytotoxicity of test compounds was easily detected by monitoring renilla luciferase activity.
Drug resistance assays have been accepted as standard clinical tests to guide the antiretroviral therapy of human immunodeficiency virus (HIV)-infected patients who have developed resistance to drug treatment or drug-naïve patients infected with drug-resistant virus. These tests have been shown to improve treatment outcomes by selecting the most effective drugs and by minimizing the risk of treatment failure (2, 5-7, 9, 34). Drug resistance has been determined by two approaches. One is drug resistance genotyping, in which drug resistance is evaluated by sequencing the viral genes targeted by the drug, such as the HIV-1 protease and reverse transcriptase (RT) genes. The level of drug resistance is estimated by using observed mutation patterns and interpretation algorithms (23). Several protocols have been used for drug resistance genotyping, including in-house sequencing (10, 13, 38). Although these protocols differ in some aspects, e.g., the design of primers, the length of analyses, and amplification procedures, all are based on the same technical approach, modified Sanger sequencing.
The other approach to drug resistance assays is phenotyping. In this method, the levels of drug resistance of patient-derived viral isolates are evaluated by using in vitro bioassays (17, 26). Two advantages of the phenotyping assay are its ability to directly evaluate the drug susceptibilities of patient-derived viruses and the ease of interpreting its results compared to those from genotyping. This assay is especially useful in cases with a high degree of exposure to antiretroviral drugs, therefore involving many mutations. In these cases, the evaluation of resistance levels by genotyping alone may be difficult (35). In addition, the resistance levels determined by phenotyping provide important information for updating interpretation algorithms used in genotyping.
Although peripheral blood mononuclear cells (PBMC) are the natural target of HIV type 1 (HIV-1) and hence are the best candidates for host cells in phenotyping assays, reporter cell systems are more commonly used in drug susceptibility assays (1, 12, 15, 31). Reporter systems are preferred because their susceptibility to HIV-1 is stable and their output is both rapidly measured and highly reproducible compared to that of PBMC assays. Several kinds of reporter cells have been used with different reporter proteins, such as MAGI cells with β-galactosidase (21), GHOST cells with enhanced green fluorescent protein (36), MOCHA cells with secreted alkaline phosphatase (24), and CEM.NKR-CCR5-Luc cells with luciferase (31). Although these systems use different cell lines, their basic strategies for evaluating HIV infectivity are similar (21, 36). The cell lines carry a reporter protein gene regulated by the HIV-1 long terminal repeat (LTR) promoter, inducing them to produce the reporter protein when they are infected with HIV-1. Which reporter system is used depends on the properties of the original cell line and the installed reporter protein.
Reporter systems using MAGI and GHOST cells have the advantages of high sensitivity and rapidity in determining infectivity. However, MAGI and GHOST cells have been established from HeLa cells (21) and human osteosarcoma cells (36), respectively, which are not naturally susceptible to HIV-1. Therefore, these cells cannot propagate viruses efficiently. On the other hand, MOCHA and CEM.NKR-CCR5-Luc cell lines were established from T-cell lines and secreted alkaline phosphatase and luciferase, respectively, were installed as reporters. These reporter systems allow for the evaluation of HIV-1 infectivity by using enzymatic reactions and demonstrate greater reproducibility with wider dynamic ranges of reporter proteins. However, for these cells to produce sufficient reporter protein for accurate determinations, they must be cultured for 5 to 7 days, longer than MAGI and GHOST cells. Longer culture periods allow reporter cells to divide, which may affect the accuracy of the quantification.
Given the advantages and limitations of previously constructed reporter cell lines, we designed and tested two new reporter cell lines with dual chemokine receptors for use in drug resistance phenotypic assays and other HIV infectivity assays. The cell lines we designed have unique characteristics in that they originate from the human T-cell line HPB-Ma (16, 29, 40) and were engineered to express the CCR5 receptor and two different marker proteins, firefly luciferase (FL) and renilla luciferase (RL). FL, which is under HIV-1 LTR promoter regulation, is produced upon HIV-1 infection. Therefore, firefly luciferase activity can be used as a marker for virus infectivity. RL, which is under cytomegalovirus (CMV) promoter control, is constitutively expressed in the cells. Therefore, renilla luciferase activity can be used as a marker for cell number and viability.
MATERIALS AND METHODS
Construction of luciferase and CCR5 expression vectors.
Two different luciferase expression vectors were constructed. The first luciferase construct comprised HIV-1 Tat-regulated FL and the red fluorescent protein (DsRed) construct 53LTRN-lucneor#1. The HIV-1 Tat-responsive reporter construct 53LTRN-lucneor#1 was constructed based on the expression vector pGEM-7Zf(+) (Promega, Madison, WI). Initially, a parent vector was constructed, 53LTRNCNS, which has a rabbit β-globin unit under the control of the HIV-1 LTR derived from strain HXB2. In this construct, a gene of interest can be cloned within the second exon of the β-globin gene and the polyadenylation signal is provided by the β-globin unit. A neomycin expression module was prepared by PCR and cloned upstream of the HIV-1 LTR region to generate 53LTRCNSneo. The reporter gene employed here was a fusion between an FL gene and a DsRed gene. The FL gene allows HIV-1 replication to be quantitatively evaluated by using luciferase activity when the LTR is activated by HIV-derived Tat, and the DsRed gene allows transfected and HIV-infected cells to be identified by red fluorescence. The FL portion was derived from pGLuc5 (Promega), and the DsRed portion was derived from pDsRed1N-1 (Clontech). Both genes were prepared by PCR, fused, and cloned into the β-globin unit by using NcoI and NotI restriction sites.
The second luciferase construct, pRenillaPac, was constructed using the plasmid pPUR (Clontech). The PCR-amplified RL gene, derived from phRL-CMV (Promega), was spliced into the upstream region of the pac gene. This hybrid gene manifests both RL activity and resistance to puromycin. Expression of the fusion gene was constitutive under the control of a CMV promoter.
A CCR5 expression vector, pCCR5/CEP4, was constructed based on the pCEP4 expression vector (Invitrogen), which possesses the Epstein-Barr nuclear antigen 1 episomal-expression gene. The CCR5 gene was inserted into the vector by using NotI and SnaB I restriction sites on the vector. Expression of the CCR5 gene was constitutive under the control of a CMV promoter.
Selection of host cell line and establishment of new reporter cell lines.
To design new reporter cell lines for quantifying HIV-1 replication, we selected the murine leukemia virus-transformed human T-cell line HPB-Ma, established by Y. K. Shimizu and H. Yoshikura (16, 29, 40), because of its high susceptibility to HIV-1 and its stable expression of CD4 and CXCR4. HPB-Ma cells were maintained at 37°C in 5% CO2 in complete RPMI 1640 medium (Sigma, Tokyo, Japan) supplemented with 10% fetal calf serum (HyClone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen, Tokyo, Japan). Cells were transfected by electroporation with the two luciferase expression vectors, 53LTRN-lucneor#1 and pRenillaPac. Plasmid DNA (10 μg) was mixed with HPB-Ma cells (5 × 106 cells in 500 μl phosphate-buffered saline), and the mixture was incubated for 5 min at 4°C and electropulsed with a Gene Pulser II apparatus (Bio-Rad, Hercules, CA) at 250 V and 950 μF. After electroporation, the cells were resuspended in complete medium and incubated at 37°C in 5% CO2. Subsequently, cells with incorporated plasmids were selected with 0.1 μg/ml puromycin (BD Biosciences, San Jose, CA) and 250 μg/ml Geneticin (Invitrogen), maintained in complete medium for several weeks, and enriched with cell populations expressing high levels of CD4 and CXCR4 by fluorescence-activated cell sorting with a FACSVantage system (BD Biosciences). Finally, clones were generated by limiting dilution and selected if they showed high sensitivity to HIV-1 and low spontaneous expression of FL and DsRed.
Since the parent HPB-Ma cell line expresses only the CXCR4 receptor, we extended the spectrum of the reporter cell lines to include R5-tropic viral isolates by transfecting cells by electroporation with a CCR5 expression plasmid. Clones were selected by incubating for several weeks with 0.1 μg/ml puromycin, 250 μg/ml Geneticin, and 150 μg/ml hygromycin B. Selected cells were recloned, and the expression of cell surface markers was confirmed by using FACSCaliber (Becton Dickinson, San Jose, CA). CD4, CXCR4, and CCR5 receptors were stained with SK-3-Cy5.5, 12G5-phycoerythrin, and 2D7-fluorescein isothiocyanate monoclonal antibodies, respectively (all from BD Biosciences, San Jose, CA).
Evaluation of introduced reporter gene functions.
To confirm the ability of FL activity to reliably measure virus titer and production, established cell lines were plated into 96-well plates at 105 cells per well and inoculated with 50 to 400 50% tissue culture infective doses (TCID50) of HXB2 or JRCSF. After 7 days of culture with the test viruses, cells were harvested and lysed in 75 μl of luciferase assay reagent. FL activity was quantified using a Dual-Glo luciferase reporter assay system (Promega, Madison, WI) and an LMax microplate luminometer (Molecular Devices, Sunnyvale, CA). Virus production was also quantified by using the p24 antigen enzyme-linked immunosorbent assay RETROtek kit (ZeproMetrix Co., Buffalo, NY) and compared with FL activity.
The validity of using RL activity to monitor MaRBLE cell numbers was evaluated by measuring RL activity in various numbers of cells and determining the correlation between RL activity and cell numbers. The correlation between RL activity and cell viability was also confirmed in cell killing assays with two anticancer drugs, hygromycin B (Invitrogen, Tokyo, Japan) and blasticidin S (Funakoshi, Tokyo, Japan). Target cells were plated into 96-well plates at 105 cells per well, and hygromycin B (15.6 to 500 μg/ml) and blasticidin S (1.25 to 20 μg/ml) were added. After 7 days of culture, cells were harvested and RL activity was measured by using the Dual-Glo luciferase reporter assay system (Promega) and the percentage of cell killing was determined by trypan blue staining.
Preparation of recombinant and patient-derived viruses.
Recombinant viruses with point mutations were constructed as described elsewhere (25). In brief, drug resistance mutations were introduced into the RT and protease genes of the HXB2 clone by site-directed mutagenesis (28). MT-2 cells (5 × 106 human T-lymphoblastoid cells) were then transfected by electroporation with the recombinant virus plasmids, and the cells were maintained in 10 ml of complete medium for 7 to 14 days. Half the culture supernatant was harvested and replaced with fresh medium every other day. Viral replication was monitored by measuring RT activity in the supernatant, and the sample with the highest RT activity was used in subsequent studies.
Eight clinical samples were selected randomly from patient blood specimens sent for routine HIV-1 drug resistance testing to the AIDS Research Center, National Institute of Infectious Diseases, Tokyo, Japan. Patient viruses were isolated by a standard coculture method described elsewhere (18). In brief, 2 × 107 patient PBMC were mixed with the same number of phytohemagglutinin-stimulated normal human PBMC and the mixture was cultured for 2 weeks. Half the culture supernatant was collected and replaced with the same amount of fresh culture medium every other day. Viral replication was monitored by measuring RT activity in the supernatant, and the sample with peak RT activity was selected and used for infection experiments afterward. RT assays were performed as previously described (37). Viral RNAs in collected supernatants were sequenced, and drug resistance mutation patterns were determined.
For the reconstructed virus, viral RNA was extracted from 200 μl of patient plasma by using a High Pure viral RNA kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. Subsequently, a 1.8-kb gag-pol fragment, encoding the region from p2gag to whole protease, and the 5′ half of the RT gene fragment were amplified and inserted into the HXB2 backbone. MT-2 cells (5 × 106) were then transfected by electroporation with the plasmid, and the cells were maintained in 10 ml of complete medium for 7 to 14 days. Half the culture supernatant was harvested and replaced with fresh medium every other day. Viral replication was monitored by measuring RT activity in the supernatant, and the sample with the highest RT activity was selected for use in subsequent studies. Viral RNAs in collected supernatants were sequenced, and the drug resistance mutation patterns were confirmed. For both the patient-derived and reconstructed viruses, HIV infectivity (TCID50) in the target cell lines was assayed by the Reed-Muench method (27).
Drug resistance genotyping.
HIV-1 RNA was extracted from 200 μl of patient plasma using a High Pure viral RNA kit according to the manufacturer's instructions. For amplification of the 500-bp protease gene fragment, DRPRO5 (5′-AGA-CAG-GYT-AAT-TTT-TTA-GGG-A) and DRPRO2L (5′-TAT-GGA-TTT-TCA-GGC-CCA-ATT-TTT-GA) were used for reverse transcription and the first PCR and DRPRO1M (5′-AGA-GCC-AAC-AGC-CCC-ACC-AG) and DRPRO6 (5′-ACT-TTT-GGG-CCA-TCC-ATT-CC) were used for the second PCR. For amplification of the 800-bp RT gene fragment, DRRT1L (5′-ATG-ATA-GGG-GGA-ATT-GGA-GGT-TT) and DRRT4L (5′-TAC-TTC-TGT-TAG-TGC-TTT-GGT-TCC) were used for reverse transcription and the first PCR and DRRT7L (5′-GAC-CTA-CAC-CTG-TCA-ACA-TAA-TTG-G) and DRRT6L (5′-TAA-TCC-CTG-CAT-AAA-TCT-GAC-TTG-C) were used for the second PCR. The amplicons were purified by using a MultiScreen PCR filter plate (Millipore), and sequence reactions were performed by using the BigDye Terminator v3.1 cycle sequencing kit, followed by electrophoresis using an ABI-3730 auto sequencer (Applied Biosystems, Foster City, CA).
HIV-1 replication kinetics analyses and drug susceptibility assays.
To analyze the replication kinetics of clinically derived HIV-1 isolates, target cells were plated into 96-well plates at 105 cells per well and infected with 100 TCID50 of test viruses per well. At days 3, 5, and 7, the culture supernatant of each well was collected and RT activity was measured as previously described (37).
To evaluate anti-HIV-1 drug susceptibility, 107 cells were infected with 10,000 TCID50 of wild-type control or test viruses in 50-ml tubes and incubated for 2 h at 37°C. Infected cells were resuspended in culture medium and plated into 96-well plates at 105 cells per well. At 2 and 48 h after infection, serial RT inhibitor dilutions and serial protease inhibitor (PI) dilutions were added, respectively. Each drug was prepared in a fivefold serial dilution and tested over different dose ranges, as follows. Didanosine, abacavir, and nevirapine were tested at concentrations from 25.0 × 101 μm to 3.2 × 10−4 μM. Lamivudine and stavudine were tested at concentrations from 5.0 × 101 μm to 6.4 × 10−5 μM. Zidovudine, zalcitabine, and the five PIs (saquinavir, indinavir, nelfinavir, lopinavir, and amprenavir) were tested at concentrations from 1.0 × 101 μm to 12.8 × 10−6 μM. Efavirenz was tested at concentrations from 0.2 × 101 μm to 25.6 × 10−7 μM. All samples were tested in triplicate. The following manufacturers kindly supplied anti-HIV drugs: GlaxoSmithKline, Middlesex, United Kingdom (zidovudine, lamivudine, and abacavir); Bristol-Myers Squibb, New York, NY (didanosine, stavudine, and efavirenz); Roche, Basel, Switzerland (zalcitabine and saquinavir); Boehringer Ingelheim, Ingelheim, Germany (nevirapine); Merck Research Laboratories, Rahway, NJ (indinavir); Japan Tobacco, Tokyo, Japan (nelfinavir); Vertex Pharmaceuticals, Cambridge, MA (amprenavir); and Abbott Laboratories, Abbott Park, IL (lopinavir).
After 7 days of culture with test drugs and test viruses, cells were harvested and lysed in 75 μl of luciferase assay reagent. Firefly and RL activities were sequentially quantified using a dual-luciferase reporter assay system (Promega) and an LMax microplate luminometer (Molecular Devices). Data were displayed by plotting the percentage of luciferase activity versus the log10 drug concentration. The concentration at which 50% of viral replication was inhibited (IC50) was determined by plotting curves defined by the four-parametric sigmoidal equation f(x) = A + ([B − A])/(1 + [C/x]D) using XLfit4 software (CTC Laboratory Systems Corporation, Tokyo, Japan). To determine susceptibility or resistance, results for test viruses were compared to those for wild-type HIV-1 and evaluated by Student's t test.
RESULTS
Establishment of new T-cell-based cell lines with two luciferase reporter proteins.
Two luciferase expression vectors were successfully constructed and used for transfection of the HPB-Ma cell line. These vectors were 53LTRN-lucneor#1, with FL under HIV-1 LTR regulation, and pRenillaPac, with RL under CMV promoter regulation. HPB-Ma cells with these vectors were subjected to several rounds of selection for cells resistant to Geneticin and puromycin and were enriched by flow cytometry with populations expressing high levels of CD4 and CXCR4 to establish the new cell line HPB-Ma/LTR-FL/CMV-RL (X4-MaRBLE).
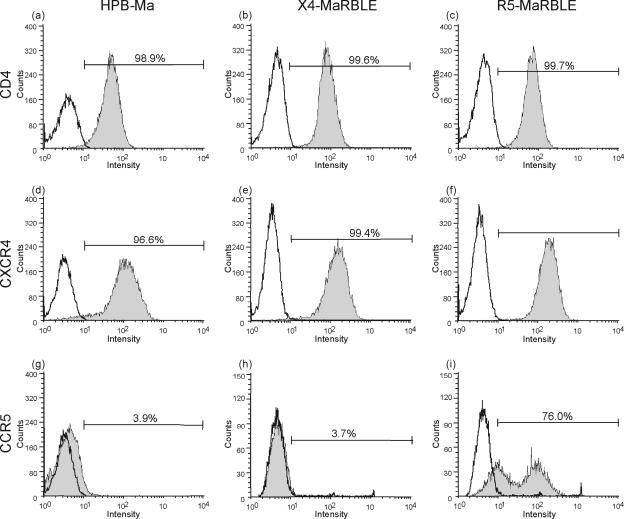
Since the parent HPB-Ma cell line expresses only CXCR4, the spectrum of the X4-MaRBLE cell line was extended to include R5-tropic viruses by transfection with a CCR5 expression plasmid, thus establishing the R5-MaRBLE cell line. Expression levels of CD4 were comparable among the parent HPB-Ma, X4-MaRBLE, and R5-MaRBLE cell lines (Fig. 1a to c), whereas the proportion of CXCR4-positive cell populations and CXCR4 expression levels were slightly higher in X4- and R5-MaRBLE cells than in the parent HPB-Ma cell line (Fig. 1d to f). This difference is due to the cell sorter's selecting for populations expressing high levels of CXCR4. As for CCR5 expression, HPB-Ma and X4-MaRBLE cells did not significantly express the receptor (Fig. 1g and h). On the other hand, more than 76% of the R5-MaRBLE cell population expressed CCR5 (Fig. 1i).
FIG. 1.
Levels of expression of CD4, CXCR4, and CCR5 in parent HPB-Ma cells and X4-MaRBLE and R5-MaRBLE cells. Parent HPB-Ma (a, d, g), X4-MaRBLE (b, e, h), and R5-MaRBLE (c, f, i) cells were stained with monoclonal antibodies to CD4 (a, b, c), CXCR4 (d, e, f), and CCR5 (g, h, i). To calculate the percentage of each population positive for the expression of cytokine receptors (bars), 2,000 to 5,000 cells were analyzed by fluorescence-activated cell sorting. To calculate the percentage of each population positive for expression of CD4 and cytokine receptors (bar), 2,000 to 5,000 cells were analyzed by FACSCalibur and compared with fluorescence-negative control cells. Histograms with gray shading indicate cell populations stained with each monoclonal antibody; histograms without shading indicate negative control populations.
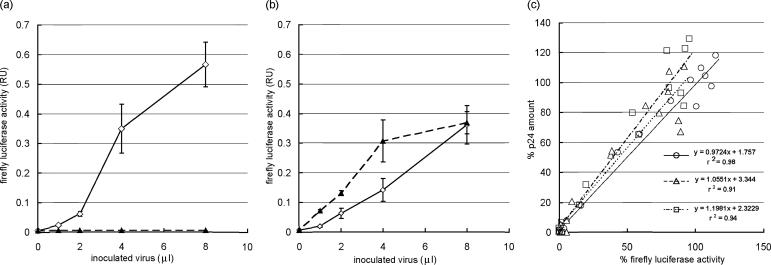
To confirm the susceptibility of cell lines to X4- and R5-tropic viruses, each cell line was inoculated with HXB2 (X4-tropic) and JRCSF (R5-tropic) viruses. X4-MaRBLE cells inoculated with HXB2 expressed FL activity in a dose-dependent manner but did not show any FL activity after inoculation with JRCSF (Fig. 2a). On the other hand, R5-MaRBLE cells were susceptible to both HXB2 and JRCSF, which induced FL activity in a dose-dependent manner (Fig. 2b). To validate the use of FL activity to evaluate viral production, FL levels were compared to amounts of the viral capsid antigen, p24, in both X4- and R5-MaRBLE cell lines, and the correlation between FL levels and the amounts of p24 was determined. As shown in Fig. 2c, FL activity in cell lysates and the amount of p24 antigen in the culture supernatant were positively and linearly correlated in X4-MaRBLE cells infected with HXB2 (r2 = 0.98), in R5-MaRBLE cells infected with HXB2 (r2 = 0.91), and in R5-MaRBLE cells infected with JRCSF (r2 = 0.94). These results verify that FL activity expressed by both X4- and R5-MaRBLE cell lines accurately represents the levels of viral replication and production. These good correlations also indicate a small likelihood of interference between the two LTRs in infected cells, the one driving luciferase and the other contained in the infecting virus.
FIG. 2.
FL reporter activity in MaRBLE cells accurately represents viral production\chatn. (a) X4-MaRBLE cells are susceptible to HXB2 (X4-tropic) but not JRCSF (R5-tropic) viruses. FL activity was confirmed as a reliable measure of X4-tropic HIV-1 in X4-MaRBLE cells by inoculating the cells with various amounts of HXB2 or JRCSF and reading FL activity 7 days later. Solid and dashed lines indicate HXB2 and JRCSF, respectively. (b) R5-MaRBLE cells are susceptible to both HXB2 (X4-tropic) and JRCSF (R5-tropic) viruses. FL activity was confirmed as a reliable measure of X4- and R5-tropic HIV-1 in R5-MaRBLE cells by inoculating the cells with various amounts of HXB2 or JRCSF and reading FL activity 7 days later. Solid and dashed lines indicate HXB2 and JRCSF, respectively. (c) FL activity and the amount of capsid antigen p24 are correlated in HIV-infected MaRBLE cells. The reliability of using FL activity instead of the amount of p24 to quantify HIV-1 production was evaluated by measuring intracellular FL activity and the amount of p24 antigen in the supernatant from the same culture. Solid, dashed, and dotted lines indicate HXB2-infected X4-MaRBLE cells, HXB2-infected R5-MaRBLE cells, and JRCSF-infected R5-MaRBLE cells, respectively. Percentages of FL activity and of p24 production were calculated from the following formula: percentage = (observed value with the drug − background value)/(observed value without the drug − background value) × 100. RU, relative units.
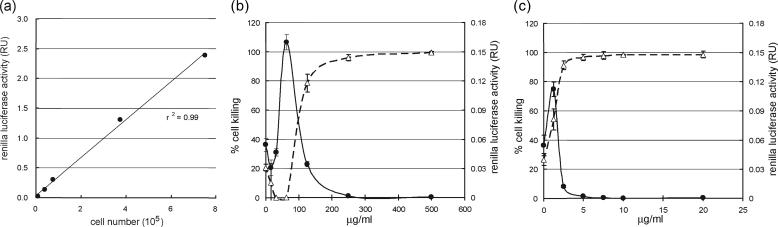
The second type of luciferase, RL, was inserted into MaRBLE cells to monitor and evaluate their number and viability. As shown in Fig. 3a, RL activity demonstrated a positive, linear correlation with cell number (r2 = 0.99). Thus, RL activity can be used to assess cell number in culture. Another useful parameter evaluated by RL activity was the cytotoxicity of test compounds added to cultures. To confirm the relationship between RL activity and cell viability, cell killing assays were performed with two anticancer drugs, hygromycin B (Fig. 3b) and blasticidin S (Fig. 3c). As the percentages of cells killed by both test chemicals increased, RL activity declined (Fig. 3b and c). The concentrations of hygromycin B and blasticidin S needed to kill 50% of the cells were 100 μg/ml and 2 μg/ml, respectively, in agreement with data from previous reports (3, 32). Thus, RL activity can be used to measure cytotoxicity.
FIG. 3.
Constitutively expressed RL in MaRBLE cells provides a reliable measure of cell number and viability. (a) RL activity accurately indicates MaRBLE cell numbers. The validity of using RL activity to monitor MaRBLE cell numbers was evaluated by measuring RL activity in various numbers of cells and plotting the corresponding values. RL activity and cell number were positively and linearly correlated (r2 = 0.99). (b and c) RL activity reliably indicates hygromycin B and blasticidin S cytotoxicity in MaRBLE cells. The reliability of RL activity as a marker of cytotoxicity was evaluated for hygromycin B (b) and blasticidin S HCl (c). Cells were cultured for 1 week with serial dilutions of each drug and lysed, and their RL activities were determined. In graphs in both panels b and c, solid lines represent the RL activities of cell lysates and dashed lines indicate percentages of dead cells as determined by trypan blue staining. RU, relative units.
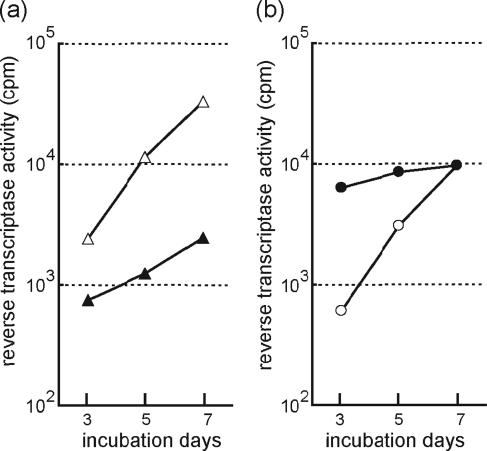
In addition, the replication of two patient-derived viral isolates, 8 and 9, in R5-MaRBLE cells was compared to that in PBMC. As shown in Fig. 4, the two clinical isolates efficiently replicated in R5-MaRBLE cells. Isolate 8 replicated more efficiently in R5-MaRBLE cells than in PBMC, as indicated by the 10-fold-higher RT activity at day 7 in R5-MaRBLE cells (Fig. 4a). Isolate 9 had comparable day 7 RT activities in R5-MaRBLE cells and PBMC (Fig. 4b). These data clearly show that R5-MaRBLE cells can efficiently propagate clinical isolates.
FIG. 4.
Clinically derived isolates replicate in R5-MaRBLE cells as efficiently as in PBMC. The replication kinetics of two clinical isolates, 8 and 9, were compared after inoculation into both R5-MaRBLE cells and human PBMC. (a) Replication kinetics of isolate 8. Open and closed triangles indicate kinetics in R5-MaRBLE cells and PBMC, respectively. (b) Replication kinetics of isolate 9. Open and closed circles indicate kinetics in R5-MaRBLE cells and PBMC, respectively. cpm, counts per minute.
Evaluation of HIV-1 drug susceptibility using X4- and R5-MaRBLE cells is highly reproducible.
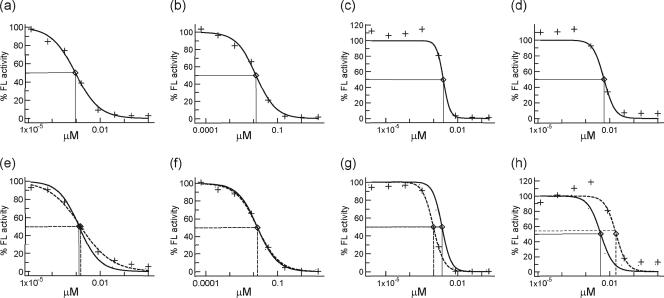
Having confirmed the quantitative reliability of FL expressed by HIV-infected X4- and R5-MaRBLE cell lines, we next used the cell lines to evaluate HIV-1 susceptibility to antiretroviral drugs. First, we evaluated the precision of phenotyping using the X4- and R5-MaRBLE cell lines. By using the wild-type HXB2 strain as a target virus for both X4- and R5-MaRBLE cells and JRCSF as a target virus for R5-MaRBLE cells, the IC50s of four representative drugs from three classes of antiretroviral agents (zidovudine, lamivudine, efavirenz, and lopinavir) were determined by measuring FL activity. As shown in Fig. 5, well-characterized dose-response curves were obtained for the four drugs. The mean IC50s, standard deviations (SD), and coefficients of variation (CV) for each cell line are summarized in Table 1. The CV ranged from 17 to 41%, demonstrating high reproducibility for drug susceptibility assays using both HIV-1-inoculated X4- and R5-MaRBLE cells. Interestingly, while the efavirenz and lopinavir susceptibilities of wild-type HXB2 were identical in evaluations with both X4- and R5-MaRBLE cells, the IC50s for HXB2 and JRCSF were significantly different (P < 0.001) in the R5-MaRBLE cell line. Thus, in our assay, JRCSF appeared to be more susceptible than the HXB2 HIV-1 strain to efavirenz and slightly less resistant to lopinavir.
FIG. 5.
Results of assays for HIV-1 drug susceptibility with MaRBLE reporter cell lines are highly reproducible. Dose-response curves for four representative agents against wild-type HXB2 and JRCSF are shown. Solid and dotted lines indicate HXB2 and JRCSF, respectively. (a to d) Results of assays for susceptibility to zidovudine, lamivudine, efavirenz, and lopinavir, respectively, using X4-MaRBLE cells. (e to h) Results of assays for susceptibility to zidovudine, lamivudine, efavirenz, and lopinavir, respectively, using R5-MaRBLE cells. The percentage of inhibition was calculated as follows: percentage = (observed FL activity with the drug − background FL activity)/(FL activity without the drug − background FL activity) × 100.
TABLE 1.
Susceptibility of wild-type HXB2 and JRCSF to representative antiretrovirals as determined using X4- and R5-MaRBLE cells
| Cell line | Agent | Mean IC50 (nM) ± SD (CV [%]) fora:
|
||
|---|---|---|---|---|
| HXB2 (n = 18) | HXB2 (n = 21) | JRCSF (n = 24) | ||
| X4-MaRBLE | Zidovudine | 0.9 ± 0.4 (41) | ||
| Lamivudine | 12.3 ± 3.9 (32) | |||
| Efavirenz | 2.7 ± 1.0 (37) | |||
| Lopinavir | 6.0 ± 1.0 (17) | |||
| R5-MaRBLE | Zidovudine | 1.3 ± 0.4 (31) | 1.5 ± 0.6 (40) | |
| Lamivudine | 13.6 ± 4.8 (35) | 13.4 ± 3.6 (27) | ||
| Efavirenz | 2.1 ± 0.5b (24) | 1.0 ± 0.3b (30) | ||
| Lopinavir | 4.2 ± 1.4b (33) | 17.6 ± 6.9b (39) | ||
n, number of isolates of the indicated virus strain.
IC50s of efavirenz and lopinavir were significantly different for HXB2 and JRCSF in R5-MaRBLE cells (P < 0.001).
Drug susceptibility of drug-resistant HIV-1 can be evaluated using X4- and R5-MaRBLE cell lines.
Given the accuracy and reproducibility of assays using MaRBLE cells to determine the drug susceptibilities of wild-type HXB2 and JRCSF, we then evaluated the reliability of using the cell lines for drug resistance phenotyping. Recombinant viruses with representative drug resistance mutations were constructed, and the drug resistance levels of the viruses were determined using X4-MaRBLE cells. The drug resistance levels associated with five patterns of nucleoside RT inhibitor (NRTI) resistance mutations are summarized in Table 2. Of the five mutant viral clones tested, four showed significant resistance to zidovudine, with resistance levels in the following order from lowest to highest: M41L/M184V/T215Y ≤ D67N/K70R < M41L/T215Y < M41L/D67N/K70R/T215Y. Thus, zidovudine resistance increased with the accumulation of thymidine analogue mutations (TAMs), and the M184V mutation caused reversion to the zidovudine resistance phenotype in the M41L/T215Y mutant, with a change in the susceptibility level of 12.5- to 3.5-fold relative to that of the wild-type virus, similar to results in previous reports (11, 19). Two clones with the M184V mutation demonstrated over 500-fold (>533.7- and >1,339.3-fold)-greater resistance to lamivudine but no significant resistance to didanosine and zalcitabine, although M184V has been reported to confer a three- to fivefold increase in the level of resistance (14). Our data confirm the recent revalidation of using didanosine for cases involving the M184V mutation (39). Low-level but significant stavudine resistance in M41L/T215Y (change, 2.2-fold), M41L/M184V/T215Y (change, 2.9-fold), and M41L/D67N/K70R/T215Y (change, 3.8-fold) was found, consistent with data from previous reports (22, 33). All five clones demonstrated significant resistance to abacavir. The highest resistance was observed in the M41L/M184V/T215Y mutant, consistent with findings in previous reports that TAMs with M184V reduce susceptibility to abacavir 10-fold (1).
TABLE 2.
Drug resistance levels associated with NRTI resistance mutations as determined using X4-MaRBLE cells infected with HIV-1 clones
| Agent | IC50 (nM) ± SD (change, n-fold)a for:
|
|||||
|---|---|---|---|---|---|---|
| Wild type | M41L/T215Y | M184V | M41L/M184V/T215Y | D67N/K70R | M41L/D67N/K70R/T215Y | |
| Zidovudine | 0.8 ± 0.4 | 10.6 ± 6.9c (12.5 ± 1.9) | 0.4 ± 0.1 (0.5 ± 0.01) | 1.9 ± 1.0b (3.5 ± 0.5) | 4.6 ± 1.0c (4.6 ± 1.2) | 54.0 ± 32.3c,d,e (48.1 ± 16.0) |
| Didanosine | 2,097.7 ± 1,101.4 | 2,899.8 ± 1,627.9 (1.4 ± 0.4) | 2,164.6 ± 1,019.8 (1.3 ± 0.2) | 2,893.7 ± 1519.0 (2.1 ± 0.8) | 3,754.2 ± 1,468.3 (1.4 ± 0.3) | 5,082.9 ± 2,397.5b (1.8 ± 0.5) |
| Zalcitabine | 2.5 ± 1.1 | 3.8 ± 3.3 (1.5 ± 0.8) | 3.4 ± 0.6 (1.3 ± 0.4) | 3.9 ± 2.0 (2.2 ± 0.8) | 3.0 ± 0.8 (1.2 ± 0.3) | 3.5 ± 2.3 (1.2 ± 0.6) |
| Lamivudine | 8.7 ± 4.5 | 20.9 ± 14.5b (3.8 ± 3.2) | >5,000c (>533.7) | >5,000c (>1,339.3) | 17.8 ± 6.9b (2.4 ± 1.3) | 44.3 ± 27.7c (6.8 ± 7.4) |
| Stavudine | 14.4 ± 5.2 | 29.9 ± 14.0b (2.2 ± 0.4) | 17.5 ± 7.6 (0.9 ± 0.3) | 40.7 ± 23.8b (2.9 ± 1.1) | 31.3 ± 24.9 (2.3 ± 1.7) | 50.3 ± 24.2c (3.8 ± 1.6) |
| Abacavir | 348.7 ± 122.8 | 985.5 ± 306.3c (3.2 ± 1.1) | 1,497.5 ± 589.4c (3.9 ± 1.2) | 3,300.0 ± 1,986.9c,d (10.6 ± 3.1) | 836.2 ± 522.5b (2.1 ± 0.7) | 2,015.1 ± 842.8c,d (5.2 ± 0.9) |
Change (n-fold) = (observed IC50 for strain)/(IC50 for wild type).
P, <0.05 for comparison with wild-type virus.
P, <0.005 for comparison with wild-type virus.
P, <0.05 for comparison with M41L/T215Y virus.
P, <0.05 for comparison with D67N/K70R virus.
The drug resistance levels associated with the two most common nonnucleoside RT inhibitor (NNRTI) resistance mutations (K103N and Y181C) are summarized in Table 3. The K103N mutant virus demonstrated reduced susceptibility to both nevirapine (change, 78.6-fold) and efavirenz (change, 54.7-fold), whereas the Y181C virus was resistant only to nevirapine (change, 47.5-fold) but remained susceptible to efavirenz (change, 1.5-fold).
TABLE 3.
Drug resistance levels associated with NNRTI resistance mutations as determined using X4-MaRBLE cells infected with HIV-1 clones
| Agent | IC50 (nM) ± SD (change, n-fold)a for:
|
||
|---|---|---|---|
| Wild type | K103N | Y181C | |
| Nevirapine | 206.3 ± 68.2 | 16,110.7 ± 6,445.7b (78.6 ± 19.7) | 9,586.3 ± 6,396.4c (47.5 ± 23.8) |
| Efavirenz | 1.4 ± 0.4 | 79.3 ± 33.3b (54.7 ± 15.6) | 2.3 ± 0.6c (1.5 ± 0.1) |
Change (n-fold) = (observed IC50 for strain)/(IC50 for wild type).
P, <0.005 for comparison with wild-type virus.
P, <0.05 for comparison with wild-type virus.
The drug resistance levels of three PI-resistant mutant clones (M46I, V82T, and L90M) are summarized in Table 4. Clones with M46I and L90M mutations did not demonstrate significant resistance to any PI tested, except for nelfinavir, to which the L90M clone demonstrated low-level (change, 3.3-fold) resistance. The clone with the V82T mutation demonstrated low-level resistance to indinavir (change, 3.8-fold), nelfinavir (change, 5.4-fold), amprenavir (change, 2.9-fold), and lopinavir (change, 5.0-fold), consistent with results in previous reports (8). Thus, the drug susceptibilities of viruses with resistance mutations evaluated with the MaRBLE cell lines matched those from previous reports of drug resistance, indicating the reliability of using the new cell lines to evaluate drug resistance.
TABLE 4.
Drug resistance levels of PI-resistant mutants analyzed using HIV-1-infected X4-MaRBLE cells
| Agent | IC50 (nM) ± SD (change, n-fold)a for:
|
|||
|---|---|---|---|---|
| Wild type | M46I | V82T | L90M | |
| Indinavir | 11.3 ± 4.3 | 19.5 ± 17.0 (2.2 ± 2.7) | 38.0 ± 6.8b (3.8 ± 1.6) | 11.5 ± 1.5 (1.1 ± 0.3) |
| Saquinavir | 7.4 ± 3.4 | 5.0 ± 2.4 (0.7 ± 0.3) | 6.5 ± 1.3 (1.0 ± 0.4) | 6.9 ± 1.1 (1.0 ± 0.4) |
| Nelfinavir | 4.9 ± 2.3 | 7.4 ± 5.3 (1.9 ± 1.5) | 20.0 ± 5.5b (5.4 ± 3.7) | 11.5 ± 0.7d (3.3 ± 2.2) |
| Amprenavir | 6.7 ± 3.1 | 9.7 ± 5.4 (2.0 ± 2.0) | 17.2 ± 5.2c (2.9 ± 1.0) | 10.7 ± 2.1 (2.0 ± 1.3) |
| Lopinavir | 6.6 ± 3.8 | 6.3 ± 2.9 (1.1 ± 0.7) | 29.1 ± 9.0c (5.0 ± 1.8) | 5.6 ± 0.9 (1.1 ± 0.7) |
Change (n-fold) = (observed IC50 for strain)/(IC50 for wild type).
P, <0.005 for comparison with wild-type virus.
P, <0.05 for comparison with wild-type virus.
P, <0.01 for comparison with wild-type virus.
To assess the reliability of using R5-MaRBLE cells to evaluate the levels of drug resistance of viral isolates from patients for whom treatment failed, seven cases were selected and viruses were isolated by coculture with normal human PBMC. Among the isolates from these seven cases, isolate 7 did not yield measurable virus by coculture. Therefore, a protease-RT gene fragment was amplified by RT-PCR and inserted into the HXB2 backbone. As shown in Table 5, isolate 1 had three minor mutations in the protease region and the virus was susceptible to all four inhibitors tested. The increase in resistance, calculated by comparison to the drug resistance of JRCSF, was <1.0-fold for zidovudine, lamivudine, and lopinavir. The patient in case 2 had been heavily treated with antiretroviral agents, and isolate 2 had a high accumulation of NRTI and PI resistance mutations but no NNRTI resistance mutation. This isolate had six TAMs and an M184V mutation in the RT region and demonstrated resistance to zidovudine (change, 32.9-fold) and lamivudine (change, >380.7-fold) but not to efavirenz. As for the protease region, 11 mutations were detected, including three major mutations (M46I, V82F, and L90M). Of these 11 mutations, 10 matched known lopinavir resistance mutations (International AIDS Society—USA drug resistance chart) (20). Indeed, high resistance to lopinavir (change, >76.5-fold) was observed.
TABLE 5.
Susceptibilities of seven patient-derived viral isolates to representative drugs assayed using R5-MaRBLE cells
| Strain or isolate (subtype) | Tropismb | RT mutation(s) | Protease mutation(s) | IC50 (nM) ± SD (change, n-fold) of:
|
|||
|---|---|---|---|---|---|---|---|
| Zidovudine | Lamivudine | Efavirenz | Lopinavir | ||||
| JRCSF (B) | R5 | None | L63P | 1.4 ± 0.6 (1) | 13.6 ± 4.6 (1) | 0.9 ± 0.1 (1) | 19.3 ± 8.4 (1) |
| 1 (B) | R5 | None | L63P/T, A71A/V, V77I | 1.2 (0.7) | 4.2 (0.3) | 1.5 (1.8) | 6.8 (0.5) |
| 2 (B) | X4, R5 | M41L, D67N, K70R, K101Q, M184V, L210L/W, T215F, K219Q | L10I, L33F, M46I, F53L, I54V, L63P, A71V, G73S/T, V77I, V82F, L90M | 56.1 (32.9) | >5,000 (>380.7) | 1.1 (1.3) | >1,000 (>76.5) |
| 3 (F) | X4, R5 | M41L, E44E/D, D67N, K101K/E, V118I, L210W, T215Y | K20T, D30N, M36I, M46M/L, L63P, A71V, N88D | 195.1 (114.5) | 34.5 (2.6) | 0.1 (0.2) | NDc (ND) |
| 4 (B) | X4, R5 | M41L, E44A, D67N, V118I, L210W, T215Y | L10V, K20T, D30N, M36I, I54V, L63T, A71V, V77V/I, N88D, L90M | 326.5 (191.7) | 13.5 (1.0) | 0.2 (0.2) | 123.4 (9.4) |
| 5 (B) | X4, R5 | M41L, E44D, D67N, V118I, M184V, L210W, T215Y | L10V, K20R, V32I, M36I, M46L, F53F/L, I54V, L63P, A71V, V82A, L90M | 177.4 (104.1) | >5,000 (>380.7) | 0.5 (0.6) | 986.8 (75.5) |
| 6 (F) | R5 | M41L, E44D, D67N, V118I, L210W, T215Y | L10I, K20T, M36I, M46I, F53L, L63L/I/T/P, A71V, I84V, L90M | 381.7 (224.0) | 88.0 (6.7) | 0.3 (0.4) | 190.6 (14.6) |
| 7Ra (B) | X4 | K103N | L63C, V77I | 2.1 (1.5) | 22.3 (1.6) | 60.2 (66.8) | 7.6 (0.4) |
Recombinant HXB2 with patient-derived protease and RT sequences.
The tropism of each virus was determined by using X4-GHOST and R5-GHOST cells.
ND, not determined.
Isolate 3 had 6 TAMs (M41L, E44D, D67N, V118I, L210W, and T215Y), and a high level of resistance to zidovudine (change, 114.5-fold) was observed. As the virus in this isolate had all four known NRTI resistance mutations responsible for hypersusceptibility to NNRTIs, it was hypersusceptible to efavirenz (change, 0.2-fold). Efavirenz hypersusceptibility was defined as a change in resistance of <0.4-fold compared to that of the wild type by statistical analysis (mean value minus 2 SD) and by data from a previous report (4). Isolate 4 had accumulated five TAMs and demonstrated 191.7-fold-higher resistance to zidovudine. No lamivudine resistance and NNRTI resistance mutations were observed, and the virus was susceptible to lamivudine. Isolate 4 also had M41L, V118I, L210W, and T215Y mutations, and the virus demonstrated hypersusceptibility to efavirenz (change, 0.2-fold). Two major mutations, D30N and L90M, and eight secondary mutations in the protease region were observed. Of these eight secondary mutations, five matched lopinavir resistance mutations, with our assay indicating a 9.4-fold increase in resistance to lopinavir. Isolate 5 had an RT inhibitor resistance pattern similar to that of isolate 2, having accumulated six TAMs and the M184V lamivudine resistance mutation in RT and demonstrating 104.1-fold-higher resistance to zidovudine and >380.7-fold-higher resistance to lamivudine. This virus also had M41L, V118I, L210W, and T215Y mutations. Though this virus appeared to be slightly more susceptible to efavirenz (change, 0.6-fold), this effect was not statistically significant. Isolate 5 had accumulated 11 lopinavir resistance mutations in the protease region and demonstrated 75.5-fold-higher resistance to lopinavir in our assay.
Isolate 6 had accumulated six TAMs in RT and showed 224.0-fold-higher resistance to zidovudine. This isolate had E44D and V118I mutations (low-level-lamivudine-resistance mutations) and showed 6.7-fold-higher resistance to lamivudine. Similar to those in cases 3 to 5, the virus in case 6 had M41L, V118I, L210W, and T215Y mutations and demonstrated slight hypersusceptibility to efavirenz (change, 0.4-fold). As for the protease region, isolate 6 had three major mutations, M46I, I84V, and L90M, and six minor resistance mutations. Of these nine mutations, eight were listed as lopinavir resistance mutations, and intermediate-level resistance to lopinavir (change, 14.6-fold) was observed. Isolate 7R had K103N and showed high-level resistance to efavirenz (change, 66.8-fold). No other drug resistance mutations in the RT region were found, and thus the isolate was susceptible to zidovudine and lamivudine. The protease region did show two minor mutations, L63C and V77I, and the isolate was susceptible to lopinavir.
Thus, the increases observed in levels of resistance to zidovudine and lopinavir were associated with the accumulation of known resistance mutations associated with those drugs. Similarly, hypersusceptibility to efavirenz was associated with the accumulation of M41L, V118I, L210W, and T215Y mutations in four out of six clinical isolates (30). Taken together, these results confirm the reliability of using X4- and R5-MaRBLE cells in drug resistance phenotyping.
DISCUSSION
The development of reliable methodologies to evaluate drug susceptibility in vitro has been a major thrust of drug resistance research. Although several phenotypic assays are commercially available for clinical usage, they are expensive and may not be readily available either in developing or developed countries. As for use in research laboratories, these commercial assays target only the protease and reverse transcriptase gene regions of the HIV-1 genome, limiting their flexibility as a tool for basic research. Thus, there is still a need for easy-to-use assay systems with high reproducibility, for both clinical and research usage. To fill this gap, we drew a blueprint for a new type of reporter cell line by considering the advantages and drawbacks of several previously reported cell lines (12, 15, 17, 21, 24, 26, 31, 36). Based on this blueprint, we chose the T-cell-based cell line HPB-Ma (16, 29, 40) as the parent cell line to introduce reporter genes and to establish new reporter cell lines. HPB-Ma is a murine leukemia virus-transformed human T-cell line demonstrating high susceptibility to HIV-1, which can replicate efficiently in these host cells. As HPB-Ma cells naturally express CD4 and CXCR4, but not CCR5, we introduced the CCR5 expression gene to widen the susceptibility spectrum of the cell line to include R5-tropic viruses. The reporter protein chosen to measure HIV infectivity was FL, as it has a wider dynamic range than other known reporter candidates. Another type of luciferase, RL, was introduced as a marker of cell number and viability.
Finally, two types of new reporter cell lines were established, X4-MaRBLE and R5-MaRBLE. As expected, these new cell lines had several advantages over previously described cell lines. First, viruses efficiently propagated in these cell lines, making multiple-round replication assays possible. In addition, viruses could be isolated from patient plasma and PBMC by using the cell lines. Since other reporter cells may not allow replication-competent viruses to be efficiently produced, their use is largely limited to single-cycle replication assays. These assays are currently accepted as the major method for measuring viral infectivity due to their rapid readout of results. However, single-cycle replication assays cannot evaluate the postintegration late phase of the viral life cycle. To evaluate late-phase inhibitors, such as PIs, by using single-cycle replication assays, an additional step is required prior to the assay to produce viruses under test drug pressure. On the other hand, multiple-round replication assays allow late-phase inhibitors to be directly evaluated, just as early-phase inhibitors, without additional culture. Furthermore, multiple-round replication assays allow for a clearer readout of drug susceptibility, as the differences in drug susceptibilities between the reference and test viruses may be amplified by each round of replication.
Second, the cell lines were successfully transfected with RL to broaden their application. The constitutive expression of this second luciferase in the cell line has made it possible to easily and accurately evaluate cell number and the cytotoxicities of test compounds. As we planned to conduct multiple rounds of assays, the cells were cultured for at least a week, long enough for them to propagate and increase the background level of FL. The extent of this increase depended on the amount of viral inocula or the level of inhibition by antiretroviral agents. By monitoring RL activity, we could easily evaluate culture conditions and their effects on assay results.
These two characteristics confer a great advantage to using the MaRBLE cell lines for screening new antiretroviral agents. They allow both early- and late-phase inhibitor candidates to be evaluated under the same protocol, as the assay permits multiple viral replications. Moreover, monitoring of RL activity allows false-positive results (inhibition by test drugs due to cytotoxicity) to be detected and eliminated. Finally, the use of RL activity greatly improved the efficacy of screening.
The MaRBLE cell lines stably expressed the transfected genes, as confirmed by the stable expression of CD4, CXCR4, and CCR5 on the surfaces of cells maintained in culture for up to 6 months with continuous passage. We also confirmed that the two reporter genes were stably expressed and that IC50s were identical for both newly plated and 6-month-old cultures (data not shown).
In conclusion, we successfully established two unique cell lines, X4-MaRBLE and R5-MaRBLE, which are useful for assaying viral drug resistance and for screening new antiretroviral compounds. Although the cost of phenotypic assays using our cell lines may be less than that of commercial systems, the assays require a biosafety level 3 laboratory, general culture equipment, and a luminometer for readout. Since these are all expensive items, the assay price should be reduced and the assay protocol should be simplified for wider usage of the assay.
Acknowledgments
We thank Hiroshi Yoshikura, Mari Takizawa, and Mitsuo Honda for their help and discussions. We also thank Claire Baldwin for her help in preparing the manuscript.
This study was supported by a grant from the Human Sciences Foundation and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO).
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Ait-Khaled, M., A. Rakik, P. Griffin, A. Cutrell, M. A. Fischl, N. Clumeck, S. B. Greenberg, R. Rubio, B. S. Peters, F. Pulido, J. Gould, G. Pearce, W. Spreen, M. Tisdale, and S. Lafon. 2002. Mutations in HIV-1 reverse transcriptase during therapy with abacavir, lamivudine and zidovudine in HIV-1-infected adults with no prior antiretroviral therapy. Antivir. Ther. 7:43-51. [PubMed] [Google Scholar]
- 2.Baxter, J. D., D. L. Mayers, D. N. Wentworth, J. D. Neaton, M. L. Hoover, M. A. Winters, S. B. Mannheimer, M. A. Thompson, D. I. Abrams, B. J. Brizz, J. P. Ioannidis, T. C. Merigan, et al. 2000. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. AIDS 14:F83-93. [DOI] [PubMed] [Google Scholar]
- 3.Bento, F. M., D. Takeshita, C. B. Sacramento, T. R. Machado, M. B. Mathor, A. K. Carmona, and S. W. Han. 2004. Over expression of the selectable marker blasticidin S deaminase gene is toxic to human keratinocytes and murine BALB/MK cells. BMC Biotechnol. 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch, R. J., G. F. Downey, D. A. Katzenstein, N. Hellmann, L. Bacheler, and M. A. Albrecht. 2003. Evaluation of cutpoints for phenotypic hypersusceptibility to efavirenz. AIDS 17:2395-2396. [DOI] [PubMed] [Google Scholar]
- 5.Cingolani, A., A. Antinori, M. G. Rizzo, R. Murri, A. Ammassari, F. Baldini, S. Di Giambenedetto, R. Cauda, and A. De Luca. 2002. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: a randomized study (ARGENTA). AIDS 16:369-379. [DOI] [PubMed] [Google Scholar]
- 6.Clevenbergh, P., J. Durant, P. Halfon, P. del Giudice, V. Mondain, N. Montagne, J. M. Schapiro, C. A. Boucher, and P. Dellamonica. 2000. Persisting long-term benefit of genotype-guided treatment for HIV-infected patients failing HAART. The Viradapt Study: week 48 follow-up. Antivir. Ther. 5:65-70. [PubMed] [Google Scholar]
- 7.Cohen, C. J., S. Hunt, M. Sension, C. Farthing, M. Conant, S. Jacobson, J. Nadler, W. Verbiest, K. Hertogs, M. Ames, A. R. Rinehart, and N. M. Graham. 2002. A randomized trial assessing the impact of phenotypic resistance testing on antiretroviral therapy. AIDS 16:579-588. [DOI] [PubMed] [Google Scholar]
- 8.Condra, J. H., W. A. Schleif, O. M. Blahy, L. J. Gabryelski, D. J. Graham, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, D. Titus, T. Yang, H. Teppler, K. E. Squires, P. J. Deutsch, and E. A. Emini. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374:569-571. [DOI] [PubMed] [Google Scholar]
- 9.Durant, J., P. Clevenbergh, P. Halfon, P. Delgiudice, S. Porsin, P. Simonet, N. Montagne, C. A. Boucher, J. M. Schapiro, and P. Dellamonica. 1999. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet 353:2195-2199. [DOI] [PubMed] [Google Scholar]
- 10.Eshleman, S. H., G. Crutcher, O. Petrauskene, K. Kunstman, S. P. Cunningham, C. Trevino, C. Davis, J. Kennedy, J. Fairman, B. Foley, and J. Kop. 2005. Sensitivity and specificity of the ViroSeq human immunodeficiency virus type 1 (HIV-1) genotyping system for detection of HIV-1 drug resistance mutations by use of an ABI PRISM 3100 genetic analyzer. J. Clin. Microbiol. 43:813-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fumero, E., and D. Podzamczer. 2003. New patterns of HIV-1 resistance during HAART. Clin. Microbiol. Infect. 9:1077-1084. [DOI] [PubMed] [Google Scholar]
- 12.Gervaix, A., D. West, L. M. Leoni, D. D. Richman, F. Wong-Staal, and J. Corbeil. 1997. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. USA 94:4653-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant, R. M., D. R. Kuritzkes, V. A. Johnson, J. W. Mellors, J. L. Sullivan, R. Swanstrom, R. T. D'Aquila, M. Van Gorder, M. Holodniy, R. M. Lloyd Jr., C. Reid, G. F. Morgan, and D. L. Winslow. 2003. Accuracy of the TRUGENE HIV-1 genotyping kit. J. Clin. Microbiol. 41:1586-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, Z., Q. Gao, X. Li, M. A. Parniak, and M. A. Wainberg. 1992. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2′,3′- dideoxyinosine and 2′,3′-dideoxycytidine. J. Virol. 66:7128-7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hachiya, A., S. Aizawa-Matsuoka, M. Tanaka, Y. Takahashi, S. Ida, H. Gatanaga, Y. Hirabayashi, A. Kojima, M. Tatsumi, and S. Oka. 2001. Rapid and simple phenotypic assay for drug susceptibility of human immunodeficiency virus type 1 using CCR5-expressing HeLa/CD4(+) cell clone 1-10 (MAGIC-5). Antimicrob. Agents Chemother. 45:495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartley, J. W., and W. P. Rowe. 1976. Naturally occurring murine leukemia viruses in wild mice: characterization of a new “amphotropic” class. J. Virol. 19:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hertogs, K., M. P. de Bethune, V. Miller, T. Ivens, P. Schel, A. Van Cauwenberge, C. Van Den Eynde, V. Van Gerwen, H. Azijn, M. Van Houtte, F. Peeters, S. Staszewski, M. Conant, S. Bloor, S. Kemp, B. Larder, and R. Pauwels. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob. Agents Chemother. 42:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollinger, F. B., J. W. Bremer, L. E. Myers, J. W. Gold, L. McQuay, and the NIH/NIAID/DAIDS/ACTG Virology Laboratories. 1992. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS Clinical Trials Group. J. Clin. Microbiol. 30:1787-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamichi, T. 2004. Action of anti-HIV drugs and resistance: reverse transcriptase inhibitors and protease inhibitors. Curr. Pharm. Des. 10:4039-4053. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, V. A., F. Brun-Vezinet, B. Clotet, B. Conway, D. R. Kuritzkes, D. Pillay, J. M. Schapiro, A. Telenti, and D. D. Richman. 2005. Update of the drug resistance mutations in HIV-1: fall 2005. Top. HIV Med. 13:125-131. [PubMed] [Google Scholar]
- 21.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 23.Larder, B. A., A. Kohli, P. Kellam, S. D. Kemp, M. Kronick, and R. D. Henfrey. 1993. Quantitative detection of HIV-1 drug resistance mutations by automated DNA sequencing. Nature 365:671-673. [DOI] [PubMed] [Google Scholar]
- 24.Miyake, H., Y. Iizawa, and M. Baba. 2003. Novel reporter T-cell line highly susceptible to both CCR5- and CXCR4-using human immunodeficiency virus type 1 and its application to drug susceptibility tests. J. Clin. Microbiol. 41:2515-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myint, L., M. Matsuda, Z. Matsuda, Y. Yokomaku, T. Chiba, A. Okano, K. Yamada, and W. Sugiura. 2004. Gag non-cleavage site mutations contribute to full recovery of viral fitness in protease inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed, L., and H. Muench. 1938. A simple method of estimating fifty percent endpoint. Am. J. Hyg. 27:493-497. [Google Scholar]
- 28.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 29.Shimizu, Y. K., R. H. Purcell, and H. Yoshikura. 1993. Correlation between the infectivity of hepatitis C virus in vivo and its infectivity in vitro. Proc. Natl. Acad. Sci. USA 90:6037-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shulman, N. S., R. J. Bosch, J. W. Mellors, M. A. Albrecht, and D. A. Katzenstein. 2004. Genetic correlates of efavirenz hypersusceptibility. AIDS 18:1781-1785. [DOI] [PubMed] [Google Scholar]
- 31.Spenlehauer, C., C. A. Gordon, A. Trkola, and J. P. Moore. 2001. A luciferase-reporter gene-expressing T-cell line facilitates neutralization and drug-sensitivity assays that use either R5 or X4 strains of human immunodeficiency virus type 1. Virology 280:292-300. [DOI] [PubMed] [Google Scholar]
- 32.Sugden, B., K. Marsh, and J. Yates. 1985. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol. Cell. Biol. 5:410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tural, C., L. Ruiz, C. Holtzer, J. Schapiro, P. Viciana, J. Gonzalez, P. Domingo, C. Boucher, C. Rey-Joly, and B. Clotet. 2002. Clinical utility of HIV-1 genotyping and expert advice: the Havana trial. AIDS 16:209-218. [DOI] [PubMed] [Google Scholar]
- 35.Vandamme, A. M., F. Houyez, D. Banhegyi, B. Clotet, G. De Schrijver, K. A. De Smet, W. W. Hall, R. Harrigan, N. Hellmann, K. Hertogs, C. Holtzer, B. Larder, D. Pillay, E. Race, J. C. Schmit, R. Schuurman, E. Schulse, A. Sonnerborg, and V. Miller. 2001. Laboratory guidelines for the practical use of HIV drug resistance tests in patient follow-up. Antivir. Ther. 6:21-39. [PubMed] [Google Scholar]
- 36.Vodros, D., C. Tscherning-Casper, L. Navea, D. Schols, E. De Clercq, and E. M. Fenyo. 2001. Quantitative evaluation of HIV-1 coreceptor use in the GHOST3 cell assay. Virology 291:1-11. [DOI] [PubMed] [Google Scholar]
- 37.Willey, R. L., R. Shibata, E. O. Freed, M. W. Cho, and M. A. Martin. 1996. Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J. Virol. 70:6431-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson, J. W. 2003. Update on antiretroviral drug resistance testing: combining laboratory technology with patient care. AIDS Read. 13:25-30, 35-38. [PubMed] [Google Scholar]
- 39.Winters, M. A., R. J. Bosch, M. A. Albrecht, and D. A. Katzenstein. 2003. Clinical impact of the M184V mutation on switching to didanosine or maintaining lamivudine treatment in nucleoside reverse-transcriptase inhibitor-experienced patients. J. Infect. Dis. 188:537-540. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikura, H. 1989. Thermostability of human immunodeficiency virus (HIV-1) in a liquid matrix is far higher than that of an ecotropic murine leukemia virus. Jpn. J. Cancer Res. 80:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]