Abstract
Dalbavancin is a lipoglycopeptide antimicrobial agent with a potency significantly better than that of vancomycin when tested against staphylococci and streptococci. These two pathogens are common causes of skin and skin structure infections (SSSIs), and dalbavancin has been approved for the treatment of moderate to severe SSSIs. This study generated susceptibility data for staphylococci and β-hemolytic streptococci from 52 U.S. medical centers that locally tested dalbavancin, vancomycin, and other antimicrobial class representatives to assess the potency of dalbavancin and the overall contemporary activities of commonly prescribed agents. Locally generated data showed that oxacillin-resistant staphylococci (57.0% overall) had slightly higher dalbavancin MIC90 values (0.19 μg/ml) than oxacillin-susceptible strains (0.125 μg/ml). This potency was 8- to 16-fold greater than that for vancomycin. β-Hemolytic streptococci had MIC90 values ranging between ≤0.016 and 0.064 μg/ml (highest for group B isolates). Levofloxacin, gentamicin, and tetracycline were active against oxacillin-susceptible staphylococci (82 to 99% susceptible), with lower susceptibility rates seen for the oxacillin-resistant strains. Linezolid coverage was >98% against staphylococci. Erythromycin resistance was high for staphylococci (30.6 to 94.1%) with inducible clindamycin resistance rates of 26.0% and 55.0% for oxacillin-susceptible and -resistant Staphylococcus aureus, respectively. β-Hemolytic streptococci had a 20.2% erythromycin resistance rate and a 60% inducible clindamycin resistance rate but were highly susceptible to other tested agents. Etest reading errors were apparent and skewed results towards slightly higher dalbavancin MICs, requiring further education on how to interpret Etest method results for this compound. Current disk diffusion breakpoint criteria for oxacillin susceptibility for S. aureus showed a very-major-error rate of 2.3% and only a 0.9% minor-error rate when cefoxitin was used to predict oxacillin susceptibility. Dalbavancin demonstrated excellent potency and activity (100% susceptibility at proposed breakpoints) against common causes of SSSI pathogens in this U.S. multicenter study sample.
Dalbavancin is a novel, long-acting lipoglycopeptide antimicrobial agent indicated for the treatment of moderate to severe skin and skin structure infections (SSSIs) caused by gram-positive organisms (12, 18, 19, 21, 22). The most common and important gram-positive pathogens that are associated with SSSIs are Staphylococcus spp. and Streptococcus spp. Of these two organism groups, staphylococci are much more clinically significant due to their higher prevalence and their ability to display an adept resistance profile involving many antimicrobial classes. A report from the SENTRY Antimicrobial Surveillance Program during a 7-year period (1998 to 2004) showed that Staphylococcus aureus was the leading cause of SSSIs in North America, Latin America, and Europe, with prevalence rates of 44.6, 33.5, and 37.5%, respectively (20). Resistance to oxacillin among these collected S. aureus isolates ranged from 22.8 to 35.9%. This same study (20) also showed that β-hemolytic streptococci accounted for only 2.2 to 4.7% of SSSIs during that surveillance period, with similar isolation frequency rates noted for coagulase-negative staphylococci (CoNS) (2.8 to 5.1%). High staphylococcal prevalence and elevated oxacillin resistance rates were detected in dalbavancin clinical trials studying SSSIs. A phase II proof-of-concept study conducted in the United States showed that S. aureus was the most commonly isolated pathogen (83%) and had an oxacillin resistance rate of 38%, which was most similar to the surveillance data generated from the SENTRY Program in the United States (20, 22). A subsequent larger phase III noninferiority SSSI trial demonstrated that oxacillin-resistant strains were recovered from 51% of the enrolled patients (12). These results and those generated from other published reports have established that staphylococci and streptococci are the principal causes of cutaneous infections, and that emerging antimicrobial resistance among these species requires alternative empirical or directed treatment options.
Dalbavancin has an extremely long elimination half-life and is administered intravenously at 1 gram on day 1 followed by a 500-mg dose on day 8 (4, 12, 21, 22). This treatment regimen was shown to be clinically and microbiologically efficacious and comparable to twice-daily linezolid therapy administered over a 14-day period (12). Dalbavancin exhibits excellent in vitro activity against Staphylococcus spp. (MIC90, 0.06 to 0.12 μg/ml), including strains resistant to oxacillin, and has been shown to be bactericidal without a propensity towards the development of resistance during in vitro serial passage studies (15, 17, 19, 23). This compound also has proven activity against staphylococci that are resistant to other drug classes, including strains that are (i) macrolide-lincosamide-streptogramin (MLSB) resistant, (ii) intermediately resistant or tolerant to vancomycin and, (iii) resistant to linezolid or multidrug resistant (24). Dalbavancin is very potent against β-hemolytic streptococci (MIC90, 0.016 to 0.06 μg/ml), which are usually more susceptible to most antimicrobial agents than are staphylococci (2, 15). β-Hemolytic streptococci, however, are evolving towards reduced antimicrobial susceptibility profiles which include resistance to MLSB agents and tetracyclines, and they have developed tolerance or resistance to some beta-lactam agents, vancomycin, and fluoroquinolones, although this is rarely seen at present (3, 8, 11, 25). Dalbavancin has proven activity against nonindicated strains, which include gram-positive anaerobes isolated from diabetic foot ulcers and many other important gram-positive aerobes, including some antimicrobial-resistant strains (10). The U.S. Food and Drug Administration (FDA) has recently issued dalbavancin a marketable letter. Susceptible breakpoints have been proposed by the sponsor for staphylococci at ≤1 μg/ml and for streptococci at ≤2 μg/ml. By utilizing these tentative breakpoints and data generated by numerous published studies that have assessed the activity of dalbavancin in multiple geographic regions, it has been demonstrated that resistance to dalbavancin is extremely rare and does not readily develop in vitro (15, 17, 19, 23).
The objective of this study was to generate contemporary (immediately before clinical launch) quantitative MIC susceptibility testing data for dalbavancin and vancomycin (comparator agent), along with the susceptibility profiles of several other drug classes against staphylococci (oxacillin-susceptible and -resistant strains) and β-hemolytic streptococci. This multicenter study identified the current susceptibility profiles of these important pathogens and determined the comparative activity of dalbavancin tested against nearly 2,500 isolates collected from over 50 leading medical institutions geographically dispersed throughout the United States prior to the clinical availability of dalbavancin into clinical or hospital settings.
MATERIALS AND METHODS
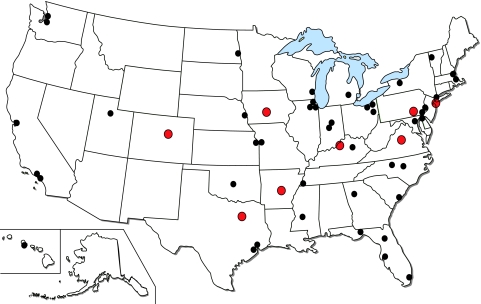
Each study site was recruited to test 50 locally collected clinical isolates, including a targeted number of oxacillin-resistant S. aureus (ORSA) strains (20 isolates), oxacillin-susceptible S. aureus (OSSA) strains, or CoNS (20 isolates) and β-hemolytic streptococci with an emphasis on S. pyogenes strains (10 isolates). A total of 52 sites from 30 states participated in the study (Fig. 1). Each site tested between 10 and 59 isolates of staphylococci and β-hemolytic streptococci, contributing a total of 2,538 isolate results (97.2% compliance). Among the tested isolates, 2,490 strains were considered to be valid for analysis after quality control (QC) results were taken into consideration. These included ORSA strains (1,009), OSSA strains (762), OR-CoNS (182), OS-CoNS (58), and β-hemolytic streptococci (479; 63.3% S. pyogenes strains). The medical centers were asked to provide a minimal amount of patient demographic information, including the sex and age of each patient, the source of infection, and whether the patient was hospitalized in an intensive care unit. Isolates were collected from the following specimen sources: skin/skin structure (41%), bloodstream (28%), respiratory tract (21%), and unknown sources (10%). For sites that sent demographic data, 58% of patients were male and 42% female, their ages ranged from 0 to 97 years (median age, 45 years), and nearly 300 patients (12%) were in the intensive care unit.
FIG. 1.
Geographic distribution of laboratories contributing data for the dalbavancin prelaunch susceptibility study. Centers highlighted in red indicate sites reporting elevated Etest MICs.
At the local site, isolates were tested for susceptibility using Etest (AB BIODISK, Solna, Sweden) for dalbavancin and vancomycin. Four other antimicrobial agents were tested against both staphylococci and streptococci by the disk diffusion method on the same Mueller-Hinton agar plate (supplemented with 5% sheep blood when testing streptococci). These antimicrobials included erythromycin, clindamycin, levofloxacin, and linezolid. Additionally, oxacillin, cefoxitin, gentamicin, and tetracycline were tested against the Staphylococcus spp., and ceftriaxone and penicillin were tested against the Streptococcus spp. Oxacillin and cefoxitin were tested against staphylococci only to differentiate susceptible (OSSA) and resistant (ORSA) populations of isolates for comparative purposes for dalbavancin and the other tested agents. Oxacillin-resistant (mecA phenotype-positive) strains were defined as those having resistance to oxacillin or cefoxitin or both. For comparison between disk diffusion test methods of oxacillin and cefoxitin, the latter was utilized as the reference method according to CLSI guidelines (7). Susceptibility test methods for disk diffusion followed recommendations from the Clinical and Laboratory Standards Institute (6) and the manufacturer's instructions for the Etest (AB BIODISK). Each site was provided Etest technical guides, including endpoint reading instructions; photographs were also provided on how to properly interpret disk approximation with erythromycin (D-test) results (7). Inducible clindamycin resistance was determined by D-testing as recommended by the CLSI (7). Reported D-test results were determined only on those isolates observed to be erythromycin resistant and susceptible to clindamycin (ER-CS phenotype). Concurrent QC was performed by each medical center using S. aureus ATCC 25923 (disk diffusion only), S. aureus ATCC 29213 (Etests only), and Streptococcus pneumoniae ATCC 49619 for disk diffusion and Etest reagents. Sites were instructed to perform QC on each day of testing, and QC failures resulted in the elimination of susceptibility data for that day and for the affected antimicrobial agent(s). Sites were provided access to a secure website to download data entry forms which when completed were uploaded and transferred to the study coordinator and QC manager (JMI Laboratories, North Liberty, IA) to minimize potential data entry errors.
RESULTS
Table 1 provides the direct MIC comparison of dalbavancin with vancomycin for the three species groups tested in this surveillance study. Overall, oxacillin-resistant staphylococci had slightly higher dalbavancin MICs than oxacillin-susceptible strains, with MIC90 values of 0.19 and 0.125 μg/ml, respectively. The potency of dalbavancin was 8- to 16-fold greater than that of vancomycin against the staphylococcal strains. All CoNS isolates were inhibited by ≤0.25 μg/ml of dalbavancin (100% susceptible using a proposed breakpoint criterion of ≤1 μg/ml). However, 1.2% of the ORSA and 1.1% of the OSSA isolates had dalbavancin MICs of ≥0.38 μg/ml and ≥0.5 μg/ml, respectively. It is suspected that many of the Etest MICs recorded at ≥0.19 μg/ml may have been erroneously read by sites that are unfamiliar with the potential reading problems associated with large-molecular-weight compounds (described in further detail below). A total of 165 strains (9.3%) of S. aureus had dalbavancin MICs of ≥0.19 μg/ml. A large majority of these (80.0%) were recorded by only 8 of the 52 participant laboratories located in geographically diverse areas. Figure 1 shows the locations of the participant sites, and those recording the higher dalbavancin MICs are highlighted in red. Overall, dalbavancin was also 16-fold more potent against β-hemolytic streptococci, with a MIC90 of 0.047 μg/ml, than vancomycin (MIC90 of 0.75 μg/ml; Table 1).
TABLE 1.
Dalbavancin activity directly compared to that of vancomycin when tested against 2,490 recent (2005 and 2006) gram-positive isolates from the United States
| Organism group (no. tested) | Antimicrobial agent | Cumulative % inhibited at indicated MIC (μg/ml)a
|
MIC50 (μg/ml)b | MIC90 (μg/ml)b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.002 | 0.004 | 0.008 | 0.016 | 0.032 | 0.064 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||||
| S. aureus | ||||||||||||||||
| Oxacillin-susceptible (762) | Dalbavancin | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 | 57.5 | 92.0 | 99.0 | 100.0 | 0.064 | 0.125 | ||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 | 65.9 | 100.0 | 1.0 | 1.5 | |||
| Oxacillin-resistant (1,009) | Dalbavancin | 0.0 | 0.0 | 0.0 | 0.0 | 2.4 | 59.0 | 89.7 | 98.8 | 100.0 | 0.064 | 0.19 | ||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.7 | 61.4 | 99.8 | 99.9 | 100.0 | 1.0 | 1.5 | |
| Coagulase-negative staphylococcic | ||||||||||||||||
| Oxacillin-susceptible (58) | Dalbavancin | 0.0 | 0.0 | 0.0 | 12.1 | 31.0 | 82.8 | 93.1 | 100.0 | 0.047 | 0.125 | |||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 8.1 | 38.7 | 100.0 | 1.5 | 2.0 | |||
| Oxacillin-resistant (182) | Dalbavancin | 0.0 | 0.0 | 0.0 | 0.5 | 13.7 | 67.0 | 89.6 | 100.0 | 0.064 | 0.19 | |||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 1.1 | 13.1 | 90.2 | 100.0 | 2.0 | 2.0 | ||
| β-Hemolytic streptococci (479)d | Dalbavancin | 0.2 | 1.3 | 8.1 | 73.5 | 86.6 | 98.7 | 99.8 | 100.0 | 0.016 | 0.047 | |||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 28.0 | 86.9 | 100.0 | 0.38 | 0.75 | ||||
Etest results (AB BIODISK, Solna, Sweden); results were rounded to log2 scale.
Etest results unrounded to the log2 scale, allowing MIC precision at the one-half log2 scale (15 total dilution steps).
CoNS included S. capitis (3 strains), S. epidermidis (59 strains), S. haemolyticus (4 strains), S. hominis (4 strains), S. lugdunensis (4 strains), S. saprophyticus (1 strain), S. simulans (2 strains), S. warneri (4 strains), and CoNS unidentified to the species level (159 strains).
β-Hemolytic serotypes of streptococci were group A (302 strains), group B (101 strains), group C (26 strains), group F (8 strains), and group G (42 strains).
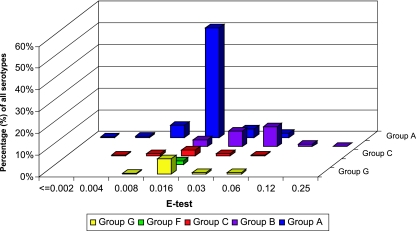
Variation in the antimicrobial potency of dalbavancin tested against the five different Lancefield groups of β-hemolytic streptococci was noted (Fig. 2). The MIC90 for group A (S. pyogenes) strains was 0.016 μg/ml, compared with 0.064 μg/ml for group B (S. agalactiae) strains. The remaining serogroups had MIC90 values ranging between those for the more commonly isolated group A and B streptococci and were 0.023 μg/ml for group G and 0.032 μg/ml for group C. Only eight group F strains were tested in this study, and dalbavancin was very potent against them, inhibiting all isolates at MICs of ≤0.016 μg/ml. Variability in the potency of vancomycin tested against the different groups of β-hemolytic streptococci was minimal (data not shown). The MIC90 values for vancomycin were 0.5 μg/ml, 0.75 μg/ml, and 1 μg/ml for group G, groups A and B, and group C, respectively. Serogroup F isolates had a vancomycin MIC50 result of 0.5 μg/ml and a MIC range of 0.19 to 1 μg/ml.
FIG. 2.
Variations in the antimicrobial activity of dalbavancin tested against different serotypes of β-hemolytic streptococci.
Table 2 shows the comparative activities of dalbavancin and seven other commonly used antimicrobial agents tested against the 2,490 strains of staphylococci and β-hemolytic streptococci. Although dalbavancin has been shown to be much more potent than vancomycin, similar breadths of activity were noted for these antimicrobial agents against all isolates tested. Susceptibility percentages were 100.0% for dalbavancin and ranged from 99.0 to 100.0% for vancomycin. No vancomycin-resistant isolates were recovered from this study sample. However, two strains of ORSA were found that had intermediate vancomycin MICs of 4 and 8 μg/ml (vancomycin-intermediate S. aureus). The MIC for dalbavancin was only slightly elevated in these two strains, at 0.25 μg/ml. These organisms came from the same institution in Pennsylvania from different patients, including a 47-year-old male with a bloodstream infection and a 58-year-old male with an SSSI. The isolates were unrelated based upon widely different antibiogram profiles.
TABLE 2.
Dalbavancin activity compared to those of seven other agents when tested against 2,490 gram-positive coccus isolates in 52 laboratories by Etest and disk diffusion methods (United States, 2005 and 2006)
| Organism group (no. tested) | Antimicrobial agent | % of strains
|
|
|---|---|---|---|
| Susceptiblea | Resistantb | ||
| S. aureus | |||
| Oxacillin-resistant (1,009) | Dalbavancin | 100.0 | - |
| Vancomycin | 99.8 | 0.0 | |
| Erythromycin | 5.4 | 94.1 | |
| Clindamycin | 55.2 | 41.7 | |
| Levofloxacin | 27.7 | 70.3 | |
| Gentamicin | 92.0 | 7.9 | |
| Tetracycline | 91.8 | 8.0 | |
| Linezolid | 99.7 | - | |
| Oxacillin-susceptible (762) | Dalbavancin | 100.0 | - |
| Vancomycin | 100.0 | 0.0 | |
| Erythromycin | 65.8 | 30.6 | |
| Clindamycin | 92.8 | 5.8 | |
| Levofloxacin | 91.1 | 7.6 | |
| Gentamicin | 98.7 | 1.3 | |
| Tetracycline | 95.1 | 4.4 | |
| Linezolid | 99.9 | - | |
| Coagulase-negative staphylococcic | |||
| Oxacillin-resistant (182) | Dalbavancin | 100.0 | - |
| Vancomycin | 100.0 | 0.0 | |
| Erythromycin | 18.6 | 81.4 | |
| Clindamycin | 41.5 | 53.6 | |
| Levofloxacin | 31.9 | 64.3 | |
| Gentamicin | 58.2 | 35.6 | |
| Tetracycline | 82.4 | 16.4 | |
| Linezolid | 100.0 | - | |
| Oxacillin-susceptible (58) | Dalbavancin | 100.0 | - |
| Vancomycin | 100.0 | 0.0 | |
| Erythromycin | 45.2 | 51.6 | |
| Clindamycin | 79.0 | 21.0 | |
| Levofloxacin | 82.3 | 9.7 | |
| Gentamicin | 95.0 | 5.0 | |
| Tetracycline | 95.0 | 5.0 | |
| Linezolid | 98.4 | - | |
| β-Hemolytic streptococci | Dalbavancin | 100.0 | - |
| (479)d | Vancomycin | 100.0 | 0.0 |
| Penicillin | 98.3 | - | |
| Ceftriaxone | 98.9 | - | |
| Erythromycin | 76.9 | 20.2 | |
| Clindamycin | 87.7 | 6.0 | |
| Levofloxacin | 98.7 | 0.2 | |
| Linezolid | 99.8 | - | |
Susceptibility criteria of the CLSI (M100-S16, 2006) were used where available (7). For dalbavancin, the sponsor-proposed susceptible-only breakpoints of ≤1 μg/ml (staphylococci) and ≤2 μg/ml (streptococci) were used for comparisons with vancomycin, with both drugs tested by Etest (AB BIODISK).
-, no resistance breakpoint criteria have been recommended.
CoNS included S. capitis (3 strains), S. epidermidis (59 strains), S. haemolyticus (4 strains), S. hominis (4 strains), S. lugdunensis (4 strains), S. saprophyticus (1 strain), S. simulans (2 strains), S. warneri (4 strains), and CoNS unidentified to the species level (159 strains).
β-Hemolytic serotypes of streptococci were group A (302 strains), group B (101 strains), group C (26 strains), group F (eight strains), and group G (42 strains).
The susceptibility profiles for the other antimicrobial classes varied significantly between the species groups, as did those for resistance to oxacillin among the staphylococci. ORSA was very refractory to erythromycin (94.1% resistance), and nearly one-half (41.7%) of these isolates were also constitutively resistant to clindamycin. The D-test results for inducible clindamycin resistance showed that 26.0% of ER-CS phenotype ORSA strains were inducibly resistant to clindamycin. Fluoroquinolone resistance assay using levofloxacin as a class marker showed that more than two-thirds (70.3%) of ORSA isolates were resistant. Resistance to gentamicin and tetracycline was 8%. Two isolates of ORSA, isolated in different hospitals, were determined to be nonsusceptible to linezolid (MIC, ≥8 μg/ml). Overall, the rate of S. aureus inducible clindamycin resistance ranged from 0.0 to 78.6% among the medical centers in this study, with typically higher rates noted for centers in the eastern portion of the United States and from some centers in the Midwest.
Erythromycin resistance among OSSA strains was 30.6%, with clindamycin resistance at only 5.8%. However, 55.0% of the ER-CS isolates were shown to be inducibly resistant to clindamycin. The remaining antimicrobial agents were very active against these strains, with susceptibility rates of 91.1, 98.7, and 95.1% for levofloxacin, gentamicin, and tetracycline, respectively. Only one isolate was nonsusceptible to linezolid among this species group.
The OR-CoNS were also much more resistant to the other tested agents than were oxacillin-susceptible strains. Erythromycin and clindamycin resistance levels were 81.4 and 53.6%, respectively, for the oxacillin-resistant strains and 51.6 and 21.0%, respectively, for the oxacillin-susceptible strains. Approximately 32% of the ER-CS phenotype strains showed inducible clindamycin resistance, regardless of oxacillin susceptibility. Resistance to the other agents was detected for oxacillin-resistant/oxacillin-susceptible strains at rates of 64.3/9.7%, 35.6/5.0%, and 16.4/5.0% for levofloxacin, gentamicin, and tetracycline, respectively. Only one isolate was shown to be nonsusceptible to linezolid.
The β-hemolytic streptococci were very susceptible to the tested agents, although a 20.2% resistance rate for erythromycin was found, and among the ER-CS phenotypes, a 60.0% inducible clindamycin resistance rate was observed. Rare strains showed reproducible nonsusceptible disk zone diameters in response to penicillin, ceftriaxone, levofloxacin, and linezolid. These strains were considered as the natural extreme in the distribution of the wild-type susceptible zone diameters, which were typically only 1 or 2 mm smaller than the CLSI breakpoint zone.
The CLSI documents have comments that describe the cefoxitin disk test as being comparable to the oxacillin disk test for the prediction of mecA-mediated resistance and indicate that the cefoxitin disk results are the more easily interpreted and preferred method (7). Table 3 shows the categorical accuracy of using cefoxitin compared to oxacillin disk tests to predict mecA-mediated resistance for the S. aureus isolates, as performed by the local medical centers in this study. A total of 1,761 S. aureus isolates had disk zone diameter results for both oxacillin and cefoxitin. Using cefoxitin as a surrogate to predict oxacillin susceptibility resulted in absolute categorical agreement for 96.8% of the strains tested with a very-major-error (false-susceptible) rate of 2.3%. Only 15 isolates (0.9%) had oxacillin results that were within the 2-mm intermediate category for oxacillin, and 80.0% of these strains were considered resistant by the cefoxitin disk test. According to the CLSI recommendations, the cefoxitin disk test, oxacillin MIC test, oxacillin-salt agar screening test, or molecular methods (mecA or PBP 2a testing) should be used to report the 15 intermediate isolates as susceptible or resistant to oxacillin.
TABLE 3.
Comparison of categorical accuracies for oxacillin and cefoxitin disk results when 1,761 S. aureus strains were tested in the dalbavancin in vitro study
| Cefoxitin susceptibility category | No. of strains (%) in indicated oxacillin susceptibility category
|
||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| Susceptible | 748 (42.6)a | 3 (0.2) | 1 (<0.1) |
| Resistant | 37 (2.3)b | 12 (0.7) | 957 (54.2)a |
Absolute categorical agreement was 96.8%, and minor errors were at only 0.9%.
Assuming cefoxitin results as presented in reference 7, very major (false-susceptible) errors were noted at 2.3%.
Currently, there is no dalbavancin disk diffusion test or an approved automated system for testing this antimicrobial agent, although commercial (dry-form) microdilution panels have been validated (14). One study demonstrated that the use of currently tested glycopeptides, including vancomycin and teicoplanin, would accurately characterize the susceptibility to dalbavancin (16). A study comparing dalbavancin MICs determined by Etest and reference broth microdilution methods validated the Etest as an accurate procedure with a very high level of intermethod agreement (9). However, dalbavancin MICs obtained with Etest may be falsely elevated due to interpretive errors when reading the ellipse of inhibition. An image of an Etest result testing dalbavancin against a clinical isolate of S. aureus is shown in Fig. 3. This figure provides a visualization of two problematic affects associated with testing large-molecular-weight compounds (dalbavancin and related peptide compounds) having compromised diffusion characteristics in agar media when determining the appropriate MIC. The ellipse created by dalbavancin tends to become distorted, with a narrowing effect at concentrations that are near the true MIC. As demonstrated in the figure, this effect begins at approximately 0.25 μg/ml for this particular strain. According to manufacturer's recommendations, results should not be extrapolated from the curvature towards the strip edge. Also, different intersections on either side of the strip should be read as the higher MIC (incorrect interpretations are indicated in Fig. 3). The MIC for this strain should be read at 0.06 μg/ml, which was verified by reference broth microdilution testing (7).
FIG. 3.
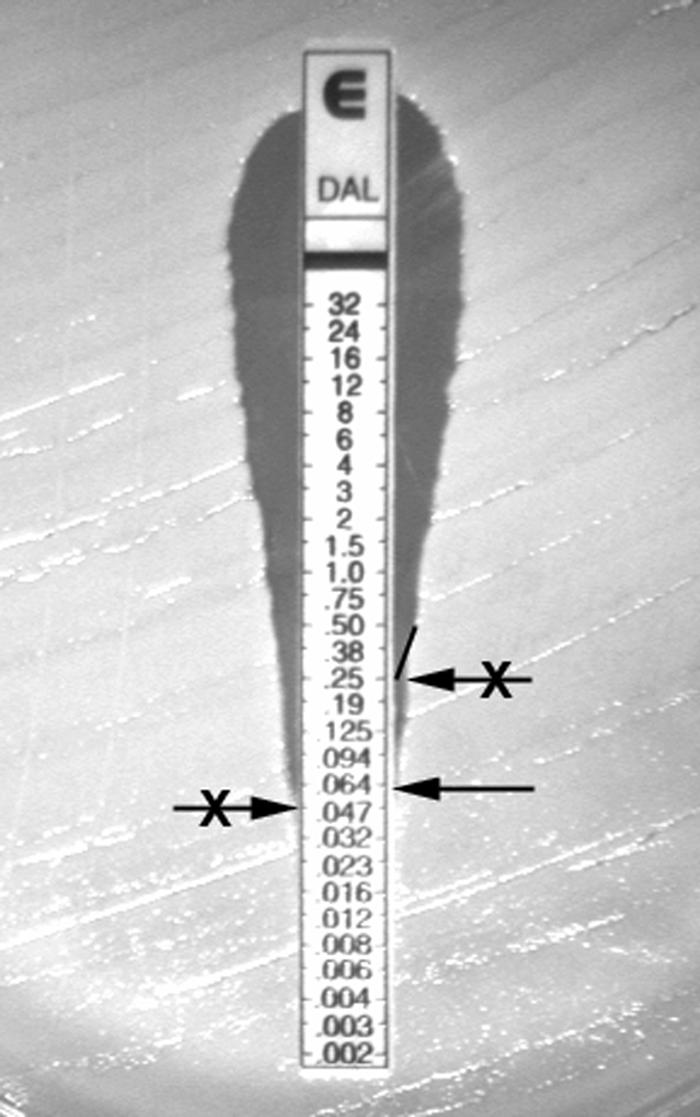
Dalbavancin Etest result tested against a clinical isolate of S. aureus demonstrating a narrow ellipse with the appropriate MIC interpretation (arrow without an X). Incorrect interpretations are arrows with X's.
DISCUSSION
This study generated susceptibility data against a large number of contemporary (2005 and 2006) isolates from numerous hospitals geographically dispersed throughout the United States and provided the local laboratories with a test method with which to determine the potency of a novel lipoglycopeptide, dalbavancin, against gram-positive strains isolated in their community. An approval letter by the FDA has been issued for dalbavancin for the treatment of moderate to severe SSSI; susceptibility breakpoints have also been suggested (used here), and QC ranges have been published by the CLSI (7) based on studies reported by Anderegg et al. (1). Dalbavancin showed excellent in vitro activity when the Etest (AB BIODISK) method was utilized against indicated species of staphylococci (MIC90, 0.125 to 0.19 μg/ml) and β-hemolytic streptococci (MIC90, 0.047 μg/ml), which are common causes of SSSI. However, care must be exercised when determining the MICs obtained with Etest, as these MICs may be falsely elevated due to interpretive errors in reading the narrow inhibitory ellipses produced by dalbavancin (Fig. 3). This antimicrobial agent was demonstrated to be 8- to 16-fold more potent than vancomycin against these pathogens, confirming several earlier studies (15, 17, 23). This study also documented that very high resistance rates for commonly used agents exist and were particularly worrisome in regards to oxacillin-resistant staphylococci and that MLSB resistance was quite high among streptococci. Cefoxitin, used as the preferred surrogate disk test marker for predicting oxacillin susceptibility (6, 7, 26), showed that utilizing the oxacillin disk result produced very major errors in over 2% of the strains and that only a small number of isolates (<1%) would need confirmation tests due to intermediate oxacillin disk test categorization (minor error). An investigation that studied the sensitivities and specificities of the oxacillin and cefoxitin disk test compared to those of the reference standard (mecA testing) showed similar results for S. aureus, with ≥95% sensitivity for both disk tests (26). However, when tested against CoNS, the cefoxitin disk test was significantly more accurate than the oxacillin disk test, with sensitivities of 90.5 and 83.4%, respectively (26).
Surveillance studies that have tested large numbers of isolates from worldwide collections indicate that dalbavancin potency data were similar to the data generated here. These studies tested isolates by use of validated broth microdilution methods, with MIC90 values that ranged from 0.06 to 0.12 μg/ml for staphylococci and 0.016 to 0.03 μg/ml for streptococci (15, 17, 23). Yet another study documented that dalbavancin was also very active against antimicrobial-resistant gram-positive pathogens, demonstrating that dalbavancin susceptibility was not influenced by staphylococcal strains having a decreased susceptibility to vancomycin, linezolid, or quinupristin-dalfopristin (24). Dalbavancin is known to be bactericidal and may provide complete or partial synergy in vitro when used in combination with other antimicrobial classes (13).
The recent favorable findings from phase 2 and 3 clinical trials on the efficacy of dalbavancin against species that cause SSSI (5, 12, 18, 22), the prolonged elimination half-life, which allows weekly dosing, and the acceptable safety profile, along with the activity measurements that have been provided by this U.S. multicenter trial, all combine to make this antimicrobial agent a novel treatment alternative in an era of increasing bacterial resistance.
Acknowledgments
We appreciate the efforts of P. R. Rhomberg for his assistance in the manuscript preparation. We also thank N. D. O'Mara-Morrissey for assistance with manuscript production and M. G. Stilwell for his assistance with the database and website design, and data analysis. We are grateful to the study site participants for their time and efforts in completing this important investigation.
This study was financially supported by grants from Vicuron and Pfizer Inc.
Footnotes
Published ahead of print on 10 January 2007.
REFERENCES
- 1.Anderegg, T. R., D. J. Biedenbach, and R. N. Jones. 2003. Initial quality control evaluations for susceptibility testing of dalbavancin (BI397), an investigational glycopeptide with potent gram-positive activity. J. Clin. Microbiol. 41:2795-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biedenbach, D. J., J. M. Stephen, and R. N. Jones. 2003. Antimicrobial susceptibility profile among β-haemolytic Streptococcus spp. collected in the SENTRY Antimicrobial Surveillance Program-North America, 2001. Diagn. Microbiol. Infect. Dis. 46:291-294. [DOI] [PubMed] [Google Scholar]
- 3.Biedenbach, D. J., M. A. Toleman, T. R. Walsh, and R. N. Jones. 2006. Characterization of fluoroquinolone-resistant β-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn. Microbiol. Infect. Dis. 55:119-127. [DOI] [PubMed] [Google Scholar]
- 4.Bowker, K. E., A. R. Noel, and A. P. Macgowan. 2006. Pharmacodynamics of dalbavancin studied in an in vitro pharmacokinetic system. J. Antimicrob Chemother. 58:802-805. [DOI] [PubMed] [Google Scholar]
- 5.Carmeli, Y., C. Rothermel, D. Sheehan, P. Hogan, and M. Mendelson. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-1211.
- 6.CLSI. 2006. Performance standards for antimicrobial disk susceptibility tests, 9th edition. Approved standard M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.CLSI. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Ergin, A., S. Ercis, and G. Hascelik. 2003. In vitro susceptibility, tolerance and MLS resistance phenotypes of group C and group G streptococci isolated in Turkey between 1995 and 2002. Int. J. Antimicrob. Agents 22:160-163. [DOI] [PubMed] [Google Scholar]
- 9.Fritsche, T. R., R. P. Rennie, B. P. Goldstein, and R. N. Jones. 2006. Comparison of dalbavancin MIC values determined by Etest (AB BIODISK) and reference dilution methods using gram-positive organisms. J. Clin. Microbiol. 44:2988-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein, E. J., D. M. Citron, Y. A. Warren, K. L. Tyrrell, C. V. Merriam, and H. T. Fernandez. 2006. In vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infections. Antimicrob. Agents Chemother. 50:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanslik, T., C. Hartig, C. Jurand, L. Armand-Lefevre, V. Jubault, E. Rouveix, O. Dubourg, J. Prinseau, A. Baglin, and M. H. Nicolas-Chanoine. 2003. Clinical significance of tolerant strains of streptococci in adults with infective endocarditis. Clin. Microbiol. Infect. 9:852-857. [DOI] [PubMed] [Google Scholar]
- 12.Jauregui, L. E., S. Babazadeh, E. Seltzer, L. Goldberg, D. Krievins, M. Frederick, D. Krause, I. Satilovs, Z. Endzinas, J. Breaux, and W. O'Riordan. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 41:1407-1415. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, D. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2006. Evaluation of dalbavancin in combination with nine antimicrobial agents to detect enhanced or antagonistic interactions. Int. J. Antimicrob. Agents 27:557-560. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., J. M. Streit, and T. R. Fritsche. 2004. Validation of commercial dry-form broth microdilution panels and test reproducibility for susceptibility testing of dalbavancin, a new very long-acting glycopeptide. Int. J. Antimicrob. Agents 23:197-199. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. N., T. R. Fritsche, H. S. Sader, and B. P. Goldstein. 2005. Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. J. Chemother. 17:593-600. [DOI] [PubMed] [Google Scholar]
- 16.Jones, R. N., H. S. Sader, T. R. Fritsche, P. A. Hogan, and D. J. Sheehan. 2006. Selection of a surrogate agent (vancomycin or teicoplanin) for initial susceptibility testing of dalbavancin: results from an international antimicrobial surveillance program. J. Clin. Microbiol. 44:2622-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, R. N., M. G. Stilwell, H. S. Sader, T. R. Fritsche, and B. P. Goldstein. 2006. Spectrum and potency of dalbavancin tested against 3,322 Gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn. Microbiol. Infect. Dis. 54:149-153. [DOI] [PubMed] [Google Scholar]
- 18.Lin, S. W., P. L. Carver, and D. D. DePestel. 2006. Dalbavancin: a new option for the treatment of Gram-positive infections. Ann. Pharmacother. 40:449-460. [DOI] [PubMed] [Google Scholar]
- 19.Lopez, S., C. Hackbarth, G. Romano, J. Trias, D. Jabes, and B. P. Goldstein. 2005. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J Antimicrob Chemother. 55(Suppl. 2):ii21-ii24. [DOI] [PubMed] [Google Scholar]
- 20.Moet, G. J., R. N. Jones, D. J. Biedenbach, M. G. Stilwell, and T. R. Fritsche. 2007. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998-2004). Diagn. Microbiol. Infect. Dis. 57:7-13 [DOI] [PubMed] [Google Scholar]
- 21.Pope, S. D., and A. M. Roecker. 2006. Dalbavancin: a novel lipoglycopeptide antibacterial. Pharmacotherapy 26:908-918. [DOI] [PubMed] [Google Scholar]
- 22.Seltzer, E., M. B. Dorr, B. P. Goldstein, M. Perry, J. A. Dowell, and T. Henkel. 2003. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin. Infect. Dis. 37:1298-1303. [DOI] [PubMed] [Google Scholar]
- 23.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis. 48:137-143. [DOI] [PubMed] [Google Scholar]
- 24.Streit, J. M., H. S. Sader, T. R. Fritsche, and R. N. Jones. 2005. Dalbavancin activity against selected populations of antimicrobial-resistant Gram-positive pathogens. Diagn. Microbiol. Infect. Dis. 53:307-310. [DOI] [PubMed] [Google Scholar]
- 25.Zaoutis, T., B. Schneider, L. Steele Moore, and J. D. Klein. 1999. Antibiotic susceptibilities of group C and group G streptococci isolated from patients with invasive infections: evidence of vancomycin tolerance among group G serotypes. J. Clin. Microbiol. 37:3380-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu, L. X., Z. W. Zhang, C. Wang, H. W. Yang, Q. Zhang, and J. Cheng. 2006. Evaluation of the CLSI cefoxitin 30-μg disk-diffusion method for detecting methicillin resistance in staphylococci. Clin. Microbiol. Infect. 12:1039-1042. [DOI] [PubMed] [Google Scholar]