The microscopic examination of fungi is conventionally carried out by wet mount staining using lactophenol cotton blue, in combination with different modes of sample preparation: the use of mounting needles, the “adhesive tape lift,” and various agar block techniques are well documented in the literature (2, 3). Such techniques require considerable experience, often incur the disruption of the fragile fungal architecture during sampling, and do not allow observation of the early-stage differentiation of the fungus.
Nitrocellulose is a commonly used membrane for the immobilization and probing of nucleic acids and proteins. Used in conjunction with a filtration device, such membranes are also employed for the detection of coliforms in water. An additional though underused property of nitrocellulose of specific relevance to microscopic imaging is that it may be rendered virtually transparent by exposure to oils.
Combining these observations, in this communication we report a new method for in situ microscopic examination of fungal morphology under high-power magnification, using equipment and materials available in most diagnostic laboratories. The method exploits the passive immobilization of spores on a nitrocellulose membrane, which is then placed on an agar surface to facilitate organism growth. After a suitable period of time, the membrane is stained, rendered transparent by treatment with microscope immersion oil, and then microscopically examined.
Aspergillus oryzae (ATTC 12891) was used as a model organism. Standardized conidial suspensions were prepared from malt agar slant cultures (after incubation for 7 days at 25°C) by the addition of 5 ml saline (0.85% [wt/vol]) containing Tween 80 (0.1% [vol/vol]). The cultures were briefly vortexed and the conidial suspensions recovered by aspiration with a Pasteur pipette. Conidium concentration and the absence of hyphal elements were assessed using a Neubauer chamber, and the conidia were subsequently pelleted by centrifugation at 3,000 rpm for 30 min at 4°C. The pellet was then resuspended in saline-Tween 80 and glycerol (20% [vol/vol]) to yield 1 × 106 conidia ml−1 and stored at −20°C. All conidium dilutions used in the experiments described were performed in sterile phosphate-buffered saline (PBS [pH 7.2]; Oxoid Dulbecco A).
Nitrocellulose squares (approximately 4 by 4 mm) were aseptically cut from a proprietary membrane (Millipore HAWG 047 SO; 0.45 μm) by use of a sterile scissors. In the standardization protocol, each nitrocellulose square was overlaid evenly onto the surface of a malt agar plate and then inoculated either with 5 μl of a standardized conidial suspension (1 × 106 conidia ml−1) or with a PBS control. The plates were incubated at 25°C and test squares removed after various time intervals for drying, staining, and microscopic visualization. In the case of examining conidia from a preexisting fungal colony on an agar plate, the nitrocellulose membrane was aseptically wetted with PBS-Tween 80 (0.1% [vol/vol]) and lightly touched to the growth with a sterile forceps. The membrane was then placed onto the surface of a malt agar plate, incubated at 25°C for germination, and then removed for drying and processing for microscopic examination.
Membranes were dried in sterile petri dishes (3 h, 25°C air incubator) and stained with lactophenol cotton blue (BBL Diagnostic Systems). The membranes were rinsed in PBS-Tween 80, redried at 25°C (3 h), and placed on microscope slides; a minimum volume of immersion oil was added (Olympus AX 9602), and images were captured at various magnifications (Canon Powershot S50 camera attached to a Leica DMLS2 light microscope).
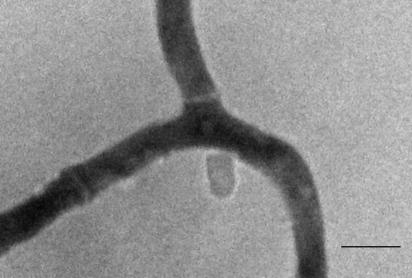
Standardization experiments using a lactophenol cotton blue-stained preparation established the basic imaging parameters; a low level of background interference or artifact due to the structure of the nitrocellulose was encountered. No conidium germination occurred on the nitrocellulose in the absence of a nutrient medium, while image quality did not noticeably vary according to the orientation of the membrane on the microscope slide. Using a standardized conidium suspension, the kinetics of early fungal development could be measured easily and reproducibly. Following conidium swelling, germ tube emergence occurred at 9 hours and hyphal branching at 16 h; there was no difference in the performance of the method when a diluent-moistened nitrocellulose membrane was used to sample fungal growth from an agar plate. The utilization of immersion oil did not adversely affect the imaging of the specimen at low-power magnification (×40, ×100, and ×400). Conversely, it was possible to use the oil immersion lens directly on the sample, thereby affording the imaging of cellular details; an example showing septation is provided in Fig. 1.
FIG. 1.
Septation in A. oryzae visualized on nitrocellulose. Magnification, ×1,000. Bar = 5 μm.
Additional advantages of this system include the ability to modify the nutrient environment by either simple membrane transfer or immersion of the membrane in a liquid medium (thereby countering possible mass transfer limitations). It is well suited to investigating diffusion-mediated processes, such as antimicrobial susceptibility testing, where early-stage differentiators (rate of germination and hyphal extension) may be of utility as indicators of interspecies variability (1).
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Araujo, R., and A. Gonçalves Rodrigues. 2004. Variability of germinative potential among pathogenic species of Aspergillus. J. Clin. Microbiol. 42:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris, J. L. 2000. Safe, low-distortion tape touch method for fungal slide mounts. J. Clin. Microbiol. 38:4683-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Tudela, J. L., and P. Aviles. 1991. Improved adhesive method for microscopic examination of fungi in culture. J. Clin. Microbiol. 29:2604-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]