Abstract
A total of 495 temporally and geographically matched Listeria monocytogenes isolates from human clinical cases, foods, ruminant farms, and urban and natural environments were used to investigate L. monocytogenes pulsed-field gel electrophoresis (PFGE) type diversity. Two-enzyme (AscI and ApaI) PFGE discriminated 310 PFGE types and exhibited higher overall discriminatory power (Simpson's index of discrimination [D] = 0.995) than either EcoRI ribotyping (D = 0.950) or AscI or ApaI single-enzyme PFGE (D = 0.992 for both). Seven PFGE types showed significant associations with specific sources, including one and four PFGE types, respectively, associated with human clinical cases and foods. Spatial analysis of 13 PFGE types occurring >5 times showed that two PFGE types were specific to a single processing facility each, where they appear to have persisted over time. Nine PFGE types were geographically widespread and occurred among isolates from multiple sources. For example, a PFGE type that matched isolates from listeriosis outbreaks in Los Angeles and Switzerland occurred among isolates from farms (n = 7), human clinical cases (n = 4), environmental sources (n = 3), and foods (n = 1). Our data indicate that (i) PFGE is highly discriminatory for the subtyping of L. monocytogenes, (ii) some L. monocytogenes PFGE types are associated with specific sources, and (iii) some L. monocytogenes PFGE types are widely distributed and appear to be stable and pandemic. Large PFGE type databases representing isolates from different sources are thus needed to appropriately interpret subtype data in epidemiological investigations and to identify common as well as source-specific PFGE types.
Listeria monocytogenes is a gram-positive, facultative intracellular pathogen capable of causing severe invasive disease in animals as well as in humans. Invasive infections in humans usually occur in immunocompromised individuals, the elderly, and pregnant women and their neonates (55). The incubation period of human listeriosis ranges from 7 to 60 days. In 1999, the U.S. Centers for Disease Control and Prevention (CDC) estimated that approximately 2,500 human listeriosis cases, including 500 fatal cases, occurred annually in the United States (35). Although the incidence of listeriosis has since declined (15), large multistate outbreaks continue to occur (13, 14). Human listeriosis is almost exclusively a food-borne disease, and L. monocytogenes has been isolated from a variety of raw and ready-to-eat (RTE) products (1, 40, 55), as well as a number of food and nonfood environments (1, 39, 47). Since time and temperature combinations used in food processing and cooking effectively inactivate L. monocytogenes, most food-borne listeriosis cases are believed to be caused by RTE products that are contaminated after processing and allow subsequent growth of the organism (54, 55).
Human listeriosis outbreaks are often difficult to detect, since cases associated with a single outbreak may be geographically dispersed (as illustrated by an outbreak involving patients in as many as 24 U.S. states) (21, 34) and may occur over long periods of time (as illustrated by an outbreak that occurred over more than 5 years) (3, 9). The use of molecular subtyping methods to link human cases that occur over time and space is thus often critical for the initial detection of human listeriosis outbreaks (2, 43, 51, 53). Molecular subtyping methods such as ribotyping (8) and pulsed-field gel electrophoresis (PFGE) (22) have been shown to exhibit much higher discriminatory power than serotyping, which differentiates only 13 L. monocytogenes serotypes (17). PFGE is generally recognized as the most discriminatory subtyping method for L. monocytogenes (22), and widespread and consistent use of PFGE for the routine subtyping of human L. monocytogenes isolates has been shown to facilitate improved detection and control of human listeriosis outbreaks. In particular, the exchange of bacterial PFGE patterns through PulseNet, a national network of public health and food regulatory agency laboratories coordinated by the U.S. CDC (51; http://www.cdc.gov/pulsenet), has shown considerable success (12-14, 20, 21) in identifying and curtailing food-borne listeriosis outbreaks. The importance of PFGE for human food-borne disease surveillance is likely to increase as PulseNet is being expanded internationally (52; http://www.cdc.gov/pulsenet).
In addition to their importance for outbreak detection, molecular subtyping and characterization methods have also provided a better understanding of the population genetics of L. monocytogenes. Specifically, a number of subtyping methods have shown that L. monocytogenes strains can be grouped into at least three distinct genetic lineages (termed genetic lineages I, II, and III) (44, 45, 59). While most subtyping studies of L. monocytogenes have defined two lineages (sometimes also referred to as divisions; see reference 7) with similar serotype groupings, the nomenclature of these lineages is not always consistent. Serotypes 1/2b, 4b, and 3b consistently group into one lineage (37), which has been designated lineage I by us and others (e.g., references 57, 58, and 59) while also being referred to as division II by some investigators (e.g., reference 7). Serotypes 1/2a, 1/2c, and 3a group into another lineage (37), designated lineage II by us and others (e.g., references 57, 58, and 59) while also being referred to as division I (e.g., reference 7). While a third lineage (termed lineage III) has been described as including serotypes 4a and 4c, as well as some serotype 4b strains (37, 57, 59), a recent study indicates the existence of multiple lineages within L. monocytogenes that include isolates with these serotypes (45). Different studies (23, 41) have shown that lineage I strains are generally overrepresented among human isolates, while lineage II strains appear to be overrepresented among food isolates (41). Lineage III strains are rare but have been shown to be overrepresented among isolates from animal listeriosis cases (29). Molecular subtyping studies have been able to identify further specific L. monocytogenes subtypes and clonal groups that appear to be associated with human listeriosis outbreaks and are common among isolates from human listeriosis cases (23, 29, 30). Kathariou (30) specifically designated three L. monocytogenes epidemic clones, (i) epidemic clone I (EC I), linked to listeriosis outbreaks in Nova Scotia (1981), Massachusetts (1983), Los Angeles (1985), Switzerland (1983 to 1987), Denmark (1985 to 1987), and France (1992); (ii) EC II, linked to two outbreaks in the United States in 1998 and 1999 and 2002; and (iii) EC III, a serotype 1/2a strain linked to a single outbreak in the United States in 2000. Increasing evidence indicates that many human disease-associated subtypes, including those representing human epidemic clones, are not only found in foods and food-processing environments but may also be present in urban and natural environments as well as in farm environments (6, 36, 39, 47). Molecular subtyping data have also shown that L. monocytogenes can persist in processing environments for considerable time periods (up to more than 10 years) (30, 43) and that human listeriosis outbreaks can be traced back to persistent contamination by the outbreak subtype in the source plant. Subtype data for L. monocytogenes isolates from foods and food-processing plants can thus sometimes help detect potential outbreak sources (as shown in a listeriosis outbreak in Finland linked to contaminated butter) (33), even though traditional epidemiological methods are still critical in linking a human outbreak to a specific source (58). Considering the broad distribution of L. monocytogenes, including human disease-associated strains, in different environments, it is thus critical to develop a better understanding of the L. monocytogenes PFGE type diversity across populations from different sources in order to further increase the utility of standard PFGE typing for the identification of human listeriosis outbreaks and their sources.
In this study, we used PFGE to characterize 495 temporally and geographically matched L. monocytogenes isolates in an effort to better understand L. monocytogenes PFGE type diversity and the ecology of different L. monocytogenes PFGE types across populations from different sources, including human clinical cases, farm animals and farm environments, and foods, as well as urban and natural environments. Specifically, the goals of our study were to (i) characterize PFGE type diversity among L. monocytogenes isolates from different sources (i.e., human clinical cases, foods, ruminants and ruminant farm environments, and urban and natural environments); (ii) identify PFGE types associated with specific sources; and (iii) characterize the distribution, ecology, and diversity of human disease-associated L. monocytogenes PFGE types with a specific focus on PFGE types representing previously identified epidemic clones.
MATERIALS AND METHODS
L. monocytogenes isolates.
A total of 495 geographically and temporally matched L. monocytogenes isolates collected between 2001 and 2003 were included in this study (Table ST1 in the supplemental material). While the majority of isolates (n = 492) were obtained from sources in New York State, two ruminant farm isolates were from one farm in a directly adjacent state (Pennsylvania) and one human isolate was obtained from a resident of Pennsylvania who presented in New York State. Isolates were obtained from human clinical cases (n = 120), foods (n = 74), ruminant fecal samples and ruminant farms (n = 221), and urban and natural environments (n = 80). For most isolates, isolation and initial molecular characterization using EcoRI automated ribotyping with the RiboPrinter microbial characterization system (DuPont Qualicon, Wilmington, DE) have been reported previously (39, 40, 47-49) (Table ST1 in the supplemental material). EcoRI ribotypes have also been used previously to assign isolates to genetic lineages I, II, and III (59).
PFGE.
PFGE of all isolates was performed using the standard CDC PulseNet protocol (22). Briefly, isolates were grown on brain heart infusion (Difco/BD, Sparks, MD) agar plates at 37°C for 18 h. Bacterial cultures were embedded in 1% agarose plugs (SeaKem Gold agarose; Cambrex, Rockland, ME), lysed, washed, and digested separately with the restriction enzymes AscI and ApaI for at least 5 h at 37°C and 30°C, respectively. Size separation of restricted DNA fragments was performed for 20 to 22 h in 1% agarose gels by using a contour-clamped homogenous electrical field Mapper XA (Bio-Rad Laboratories, Hercules, CA); voltage was set at 6 V/cm with switch times of 4 s to 40.01 s. XbaI-digested Salmonella enterica serotype Braenderup (CDCH9812) DNA was used as a reference size standard (27). Pattern images were captured with a Bio-Rad Gel Doc and the Multi Analyst software version 1.1 (Bio-Rad Laboratories). PFGE patterns were then analyzed and compared using the BioNumerics version 3.5 software (Applied Maths, Saint-Matins-Latem, Belgium). Similarity clustering analyses were performed with BioNumerics by using the unweighted pair group-matching algorithm and the Dice correlation coefficient with a tolerance of 1.5% (22).
Data analyses.
Associations between general source categories (i.e., human, food, farm, and environment) and PFGE types were evaluated using the chi-square test of independence. For these categorical analyses, PFGE types with fewer than five occurrences were pooled into one category termed “rare.” Fisher's exact test was used for analyses if the expected frequency in any cell was less than two or if more than 20% of the expected frequencies were less than five. P values for exact tests for large contingency tables (e.g., 14 by 4) were determined using Monte Carlo simulation. All statistical analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC). Due to the large number of observations investigated, it is possible that the probability of type I error was inflated; however, as previously described (23, 46), rather than generally lowering the significance threshold by adjusting for multiple comparisons, we have provided observed P values for statistical tests to allow readers to evaluate levels of significance according to their own criteria. Specifically, statistical significance is reported at three levels: P of <0.05, <0.005, and <0.0005. In addition, P values with Bonferroni's correction are reported. Overall, a total of 17 and 43 chi-square tests were conducted using lineage and PFGE distribution data, respectively. The Bonferroni's correction-adjusted P value cutoff should thus be lowered to 0.0029 and 0.0011 for lineage and PFGE distribution data, respectively. Thus, P values of <0.0005 should be considered to indicate significance even after Bonferroni's correction.
Simpson's index of discrimination (D) with 95% confidence intervals was calculated as previously described (25, 26). Two subtyping methods were considered significantly different in their discriminatory powers if 95% confidence intervals for D values for both methods did not overlap.
Spatial analysis.
A New York State map was used to visualize the geographical origins of all isolates classified into a given PFGE type that occurred at least five times among the 495 isolates characterized. While information on the county of origin (e.g., the locations of source farms, food establishments sampled, or patient residences) was available for the majority of isolates, geographical origins were plotted using five regions within New York State (western, central, northern, eastern, and metro) (38) to ensure the confidentiality of source locations.
Isolate and data curation.
All available isolate data, including PFGE and ribotype images, are publicly available in the PathogenTracker database (http://www.pathogentracker.net). Isolates are stored in brain heart infusion with 15% glycerol at −80°C.
RESULTS
PFGE diversity and discriminatory ability.
A total of 266 ApaI and 244 AscI PFGE types were differentiated among the 495 L. monocytogenes isolates characterized. Combined analyses of AscI and ApaI PFGE data yielded a total of 310 PFGE types. By comparison, EcoRI ribotyping yielded only 74 ribotypes among the 495 isolates. Calculation of D values further confirmed that two-enzyme PFGE (D = 0.995) is significantly more discriminatory than either AscI or ApaI single-enzyme PFGE (D = 0.992 for each) or EcoRI ribotyping (D = 0.950). AscI or ApaI single-enzyme PFGE was also significantly more discriminatory than EcoRI ribotyping.
Two-enzyme PFGE identified 13 PFGE types occurring at least five times; these 13 PFGE types accounted for 22.8% of the isolates characterized. A total of 235 PFGE types occurred only once and accounted for 47.5% of the isolates. Automated ribotyping identified 25 ribotypes that occurred at least five times, corresponding to 83.5% of the isolates characterized, while the 27 ribotypes that occurred only once corresponded to 5.4% of the isolates. A total of 11 PFGE types included two EcoRI ribotypes. For 47 of the 51 ribotypes that occurred more than once, PFGE was able to further discriminate isolates with the same ribotypes into two or more (up to 41) PFGE types; all ribotypes that occurred ≥5 times were differentiated into at least two PFGE types (Table 1). In particular, two-enzyme PFGE revealed considerable diversity among the four ribotypes corresponding to L. monocytogenes EC I through EC III. Ribotypes DUP-1038B (40 isolates) and DUP-1042B (48 isolates), both corresponding to EC I, could be differentiated into 18 and 34 PFGE types, respectively. Ribotypes DUP-1044A (EC II; 32 isolates) and DUP-1053A (EC III; 6 isolates) could be discriminated into eight and four PFGE types, respectively.
TABLE 1.
PFGE subtype discrimination among L. monocytogenes ribotypes
| Lineage | Ribotype(s) | No. of isolates | No. of PFGE types |
|---|---|---|---|
| I | 116-239-S-2 | 7 | 3 |
| DUP-1038B | 40 | 18 | |
| DUP-1042A | 6 | 6 | |
| DUP-1042B | 48 | 34 | |
| DUP-1042C | 7 | 5 | |
| DUP-1043A | 6 | 5 | |
| DUP-1044A | 32 | 8 | |
| DUP-1044B | 18 | 11 | |
| DUP-1052A | 21 | 16 | |
| Ribotypes with <5 occurrences | 25 | 23 | |
| Total for lineage I | 210 | 129 | |
| II | DUP-1023A | 5 | 5 |
| DUP-1030A | 8 | 8 | |
| DUP-1030B | 13 | 7 | |
| DUP-1039A | 13 | 11 | |
| DUP-1039B | 6 | 2 | |
| DUP-1039C | 66 | 41 | |
| DUP-1039D | 10 | 2 | |
| DUP-1039E | 27 | 16 | |
| DUP-1045A | 16 | 9 | |
| DUP-1045B | 19 | 13 | |
| DUP-1045D | 9 | 7 | |
| DUP-1053A | 6 | 4 | |
| DUP-1054A | 6 | 5 | |
| DUP-1062A | 11 | 3 | |
| DUP-1062E | 7 | 4 | |
| Ribotypes with <5 occurrences | 48 | 43 | |
| Total for lineage II | 270 | 180 | |
| III | DUP-18606 | 5 | 2 |
| Ribotypes with <5 occurrences | 4 | 4 | |
| Total for lineage III | 9 | 6 | |
| Unknown | Ribotypes with <5 occurrences | 6 | 6 |
| Total for all lineages | 495 | NAa |
NA, not applicable. Eleven PFGE types were further discriminated by ribotyping; the total for this column should thus be 321 even though only 310 PFGE types were differentiated among the 495 isolates
PFGE type distributions among L. monocytogenes isolates from human clinical cases, farms, foods, and urban and natural environments.
Categorical analysis of PFGE type distributions by using an overall 14-by-4 table (13 PFGE types plus one category for rare types and four sources) showed that PFGE types were not independently distributed across the four sources (P < 0.005; Monte Carlo estimation of exact test). Subsequent categorical analyses using two-by-four tables (PFGE type A versus not PFGE type A and four sources) revealed that seven PFGE types with at least five occurrences were not independently distributed across the four sources, with P of <0.05. For these seven PFGE types, further categorical analyses were conducted using two-by-two tables to analyze the association between specific PFGE types and individual sources (PFGE type A versus not PFGE type A and source Y versus not source Y); these analyses revealed a number of highly significant associations between specific PFGE types and specific sources, including a number of exclusive associations (Table 2). PFGE types 50 and 52 were associated exclusively with foods (P, <0.0005 for both). PFGE type 22 was significantly overrepresented (P < 0.0005) among human isolates and significantly underrepresented (P < 0.0005) among farm isolates; this PFGE type was not only the most commonly observed human PFGE type (accounting for 13.3% of human isolates) but was also identified as the PFGE type responsible for a multistate listeriosis outbreak in the northeastern United States in 2002 associated with sliced turkey meat (14). A total of 11 human isolates with this PFGE type were obtained during the outbreak period (July through September 2002). Statistical analyses were thus repeated using only one human isolate from the outbreak period to avoid the overrepresentation of PFGE type 22 among human isolates. In these more conservative analyses, PFGE type 22 was still significantly overrepresented among human isolates, although at a lower level of significance (P < 0.05).
TABLE 2.
Distribution of L. monocytogenes lineages and PFGE types among isolates from human clinical cases, foods, ruminant farms, and urban and pristine environments
| Lineagea | PFGE type(s)b | No. of L. monocytogenes isolates from:c
|
Total no. of isolates | |||
|---|---|---|---|---|---|---|
| Humansd | Foodse | Farmsf | Environmentg | |||
| I | Types with <5 occurrences | 53 | 21 | 58 | 22 | 154 |
| 7 | 4 | 1 | 7 | 3 | 15 | |
| 22*** | 16 (+)*** | 4 | 0 (−)*** | 0 | 20 | |
| 38*** | 1 | 0 | 0 (−)* | 6 (+)*** | 7 | |
| 121 | 1 | 0 | 6 | 2 | 9 | |
| 122 | 3 | 0 | 2 | 0 | 5 | |
| Total for lineage I | 78 (+)*** | 26 | 73 (−)*** | 33 | 210 | |
| II | Types with <5 occurrences | 32 | 20 | 119 | 42 | 213 |
| 2* | 3 | 2 | 0 | 0 | 5 | |
| 50*** | 0 | 5 (+)*** | 0 | 0 | 5 | |
| 52*** | 0 | 6 (+)*** | 0 (−)* | 0 | 6 | |
| 189 | 0 | 0 | 8 (+) | 1 | 9 | |
| 240* | 1 | 5 (+)* | 3 | 0 | 9 | |
| 300 | 0 | 0 | 7 (+) | 0 | 7 | |
| 315 | 0 | 0 | 7 (+) | 0 | 7 | |
| 336*** | 2 | 7 (+)*** | 0 (−)* | 0 | 9 | |
| Total for lineage II | 38 (−)*** | 45 | 144 (+)*** | 43 | 270 | |
| III | Types with <5 occurrences | 3 | 0 | 4 | 2 | 9 |
| Unknown | Types with <5 occurrences | 1 | 3 | 0 | 2 | 6 |
| Total for all lineages | 120 | 74 | 221 | 80 | 495 | |
Ribotypes were used to assign isolates to one of the three previously described lineages (59).
PFGE types that were not randomly distributed among sources as determined by categorical analyses are labeled with * (indicating P of ≤0.05), ** (indicating P of ≤0.005), or *** (indicating P of ≤0.0005); all P values of <0.0005 indicate significance even after Bonferroni's correction for multiple comparisons. Individual PFGE types with fewer than five occurrences were grouped together for analysis.
PFGE type and lineage prevalences that were significantly higher (+) or lower (−) for a specific source than expected based on a random distribution as determined by categorical analyses are labeled with * (indicating P of ≤0.05), ** (indicating P of ≤0.005), or *** (indicating P of ≤0.0005); all P values of <0.0005 indicate significance even after Bonferroni's correction for multiple comparisons.
Data are for 120 L. monocytogenes isolates from human clinical cases in New York State between 2001 and 2003 (48, 49).
Data are for 74 L. monocytogenes isolates from foods collected in New York State between 2001 and 2003 (48).
Data are for 221 L. monocytogenes isolates from fecal, feedstuff, soil, and water samples collected from New York State ruminant farms from 2001 to 2003 (39).
Data are for 80 L. monocytogenes isolates collected from urban and natural environments in New York State from 2001 to 2002 (47).
A categorical analysis of lineage distribution using an overall three-by-four table showed that lineages were not independently distributed across isolate sources (P < 0.0005). Six isolates could not be grouped into one of the three genetic lineages as they represented ribotypes not yet assigned to lineages; these isolates were excluded from lineage-based analyses. Individual two-by-two tables for each lineage versus specific sources confirmed that lineage I isolates were significantly overrepresented among human isolates (P < 0.0005) and significantly underrepresented among farm isolates (P < 0.0005) whereas lineage II isolates were significantly underrepresented among human isolates (P < 0.0005) and overrepresented among farm isolates (P < 0.0005) (Table 2). The nine isolates representing lineage III were independently distributed (P = 0.6069).
Spatial distributions of L. monocytogenes isolates from human cases, foods, farms, and natural and urban environments.
Maps of the geographical distribution of the isolate sources corresponding to the 13 PFGE types that occurred at least five times (Fig. 1; Fig. S1 [http://www.foodscience.cornell.edu/cals/foodsci/research/labs/wiedmann/links/upload/efuSupplFigure-s11.pdf]) showed that the spatial distribution of these PFGE types included four general patterns: (i) present only in isolates from a single processing facility (PFGE types 50 and 52); (ii) widely distributed and occurring in isolates from human cases as well as other sources, such as farms and natural and urban environments (PFGE types 7, 38, 121, 122, and 240); (iii) widely distributed among isolates from foods and humans (PFGE types 2, 22, and 336); and (iv) widely distributed among isolates from farms (PFGE types 189, 300, and 315).
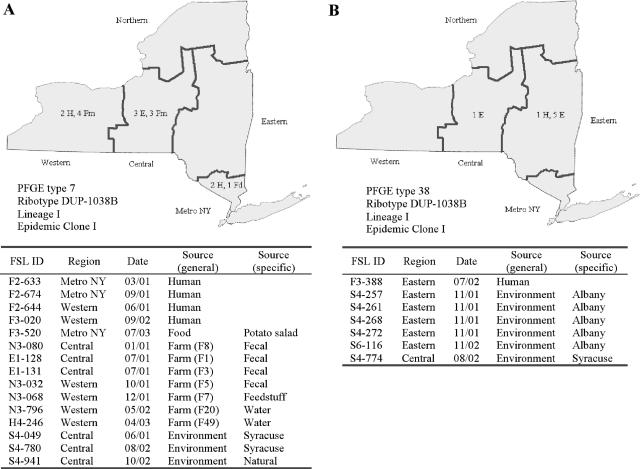
FIG. 1.
Geospatial and source distributions of selected PFGE types. Distributions of PFGE type 7 (A) and PFGE type 38 (B) are shown here; distributions of all other PFGE types that occurred ≥5 times are shown in Fig. S1 (http://www.foodscience.cornell.edu/cals/foodsci/research/labs/wiedmann/links/upload/efuSupplFigure-s11.pdf). Sources of isolates were plotted on New York State maps. Source abbreviations are as follows: H, human; Fd, food; Fm, farm; E, environment. Dates of isolation are given as month/year. While information on the county of origin (e.g., the location of the farm or food establishment sampled or the patient residence) was available for most isolates, geographical origins were plotted using five regions within New York State (western, central, northern, eastern, and metro) (see reference 38) to ensure the confidentiality of source locations. FSL ID, Food Safety Laboratory identification number.
Processor-specific PFGE type 50 (ribotype DUP-1039C) corresponded to isolates collected over a 14-day time period from five products originating from a specific processing facility (Fig. S1A at the URL mentioned above), while PFGE type 52 (ribotype DUP-1039C) occurred three times among samples collected in May of 2001 and then again three times among samples collected in July of 2003 from the same facility (Fig. S1B at the URL mentioned above). These associations were also supported by the overall chi-square analyses, which showed that PFGE types 50 and 52 were significantly associated with foods.
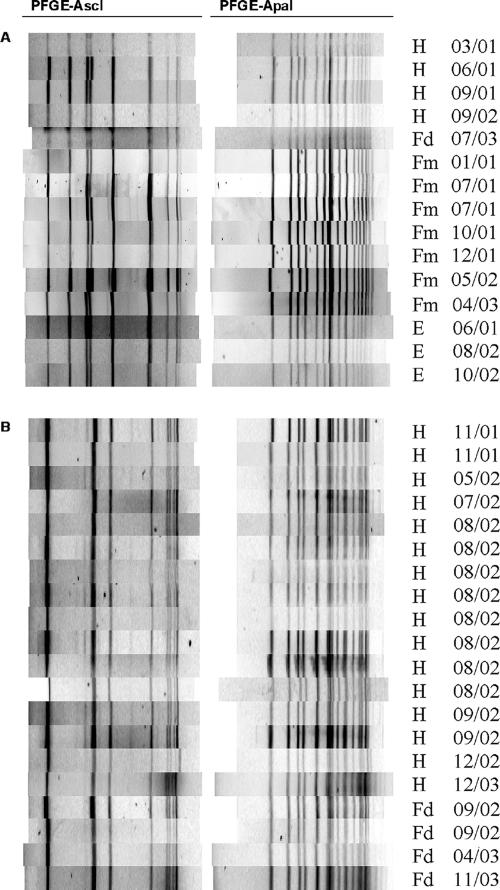
Among the widely distributed PFGE types corresponding to isolates from human cases as well as other sources, PFGE type 7 (ribotype DUP-1038B; EC I) represented not only the second most common PFGE type (15 isolates) but also the only PFGE type occurring in isolates from all sources, including seven different ruminant farms throughout New York State (Fig. 1A). This PFGE type also occurred in isolates from all three study years (2001 through 2003) (Fig. 1A). Comparison with PFGE patterns of human listeriosis outbreak-associated strains (19) revealed that PFGE type 7 matched the PFGE patterns of human and food isolates linked to the 1985 listeriosis outbreak in Los Angeles (10, 31) and the 1983 to 1987 listeriosis outbreak in Switzerland (3, 9) (Fig. 2A). PFGE type 121 (ribotype DUP-1042B; EC I) was temporally and spatially widely distributed and corresponded to isolates from a human case and six different farms, as well as environmental samples collected in two cities in New York State from 2001 through 2003 (Fig. S1C at the URL mentioned above). PFGE type 240 (ribotype DUP-1039D) occurred among isolates from a single human case and three farms in eastern and western New York State, as well as five times in samples from a single processing facility (collected over a 3[1/2]-week period) (Fig. S1E at the URL mentioned above). PFGE type 38 (DUP-1038B; EC I) corresponded to one isolate from a human case as well as six isolates from urban environments, including five isolates obtained in Albany, NY, in 2001 and 2002 (Fig. 1B). PFGE type 122 (ribotype DUP-1044B; ECII) occurred among isolates from three human cases as well as isolates from soil samples from two different farms in New York State (Fig. S1D at the URL mentioned above).
FIG. 2.
PFGE patterns for selected commonly occurring PFGE types. PFGE patterns for isolates exhibiting PFGE type 7 (A) and PFGE type 22 (B) are shown. Source abbreviations are as follows: H, human; Fd, food; Fm, farm; E, environment. Dates of isolation are given as month/year.
Among the three common and widely distributed PFGE types that corresponded only to isolates from human clinical cases and foods, PFGE type 22 (ribotype DUP-1044A; EC II) corresponded to isolates from 16 human listeriosis cases as well as four food items, including two turkey breast samples collected at two different retail operations and two white cheese samples collected from the same processing facility (Fig. S1G at the URL mentioned above). As described above, this PFGE type is identical to the two-enzyme PFGE type of human and food isolates linked to the listeriosis outbreak that occurred in the northeastern United States in 2002 (14, 19) (Fig. 2B). PFGE type 336 (ribotype DUP-1062A) corresponded to isolates from two human clinical cases as well as different foods collected in six different retail and processing facilities throughout New York State (Fig. S1H at the URL mentioned above). PFGE type 2 (ribotype DUP-1039B) corresponded to isolates from three human clinical cases as well as foods collected in two different retail and processing facilities (Fig. S1F at the URL mentioned above).
PFGE types widely distributed among isolates from farms included PFGE types 189 (ribotype DUP-1039C), 300 (ribotype DUP-1030B), and 315 (ribotype DUP-1045A). PFGE type 189 corresponded to isolates from samples collected on eight different farms (located in three regions) as well as one sample from an urban environment (Fig. S1I at the URL mentioned above). PFGE type 300 corresponded to isolates from five farms located across three regions of New York State (Fig. S1J at the URL mentioned above), while PFGE type 315 corresponded to isolates from seven farms located across three regions (Fig. S1K at the URL mentioned above).
DISCUSSION
While some previous studies (e.g., references 24, 32, and 42) have used PFGE to characterize L. monocytogenes isolates from different sources (usually human and food isolates, as well as sometimes isolates from farm animals), characterization of isolates from diverse sources is necessary to understand the subtype diversity among L. monocytogenes strains; these data can be critical in interpreting subtype data as part of outbreak investigations and as part of surveillance efforts. We thus used PFGE to characterize temporally and geographically matched L. monocytogenes isolates from human cases, foods, farms, and natural and urban environments to establish a more detailed understanding of L. monocytogenes PFGE type diversity, distribution, and ecology. Our data indicate that (i) PFGE is more discriminatory for the subtyping of L. monocytogenes than ribotyping; (ii) some L. monocytogenes PFGE types are associated with specific sources; and (iii) some L. monocytogenes PFGE types are widely distributed, including PFGE types corresponding to select epidemic clones that appear to be stable and pandemic.
PFGE is more discriminatory for the subtyping of L. monocytogenes than ribotyping.
Rapid, discriminatory, and standardized molecular subtyping methods are critical for effective food-borne disease surveillance and outbreak investigations. While automated ribotyping provides superior standardization and speed compared to many other molecular subtyping methods for bacterial isolates (58), not surprisingly and consistent with the findings of a number of previous studies (1, 5, 24, 32, 56), two-enzyme PFGE using the standard PulseNet protocol provides significantly higher subtype discrimination than EcoRI ribotyping. While automated ribotyping is thus suitable for population-based studies or subtype characterization of large numbers of isolates (1), the discriminatory power of PFGE clearly is critical for human disease outbreak investigations. Interestingly, while the previous observation (7) that ApaI PFGE may be more discriminatory than AscI PFGE was confirmed by the fact that more ApaI than AscI PFGE types among the isolates characterized here were differentiated, discriminatory powers as determined by Simpson's index of discrimination did not differ significantly between ApaI and AscI PFGE methods. Combined ApaI and AscI PFGE showed significantly higher discriminatory power than single-enzyme PFGE, supporting the importance of PFGE typing with at least two enzymes for L. monocytogenes disease outbreak surveillance and investigations.
Some L. monocytogenes PFGE types are associated with specific sources.
While L. monocytogenes PFGE types present in various sources in New York State show considerable overall diversity, we have identified specific PFGE types that are associated with specific sources. Overall, a total of three specific PFGE types occurred in multiple samples collected at different times from the same processing facilities, consistent with previous observations that L. monocytogenes can persist in processing plants over considerable time periods, from months to more than a decade (4, 19, 43, 50). Interestingly, two PFGE types (50 and 52) were found only in samples from a given processing plant. While it is possible that these PFGE types may be found in other sources if more isolates are characterized, these results illustrate the potential power of large PFGE databases containing a comprehensive selection of food-associated isolates for linking listeriosis cases to potential food sources. For example, matching the PFGE patterns of a number of L. monocytogenes isolates from human listeriosis cases in Finland in 1998 and 1999 and those of an isolate obtained from butter produced in a specific plant in 1997 provided important source tracking information for this outbreak investigation (33). Similarly, matching PFGE patterns of an isolate from a hot dog produced in a specific processing plant in 1989 and those of isolates from a number of patients in a 2000 listeriosis outbreak ultimately helped link this outbreak to RTE deli products produced in the same physical facility that produced the hot dog that yielded the 1989 isolate (11, 13, 43). While PFGE databases may yield apparently food-processing plant-specific L. monocytogenes PFGE patterns, which can help detect outbreak sources, it is critical to understand that PFGE matches alone and in the absence of strong epidemiological linkages cannot be used to implicate a specific food facility as an outbreak source.
In addition to the possibly processing facility-specific PFGE types discussed above, we identified other PFGE types that, although prevalent in foods, were infrequently associated with human cases. In particular, PFGE type 336 (DUP-1062A) corresponded to seven isolates from different widely distributed RTE foods and only two human cases. Interestingly, isolates with this PFGE type represent ribotype DUP-1062A, a clonal group that was previously identified (40) as being characterized by attenuated invasiveness for human intestinal epithelial cells due to a premature stop codon in inlA, an L. monocytogenes gene critical for the invasion of human intestinal epithelial cells. This association between ribotype DUP-1062A and premature stop codons in inlA had been confirmed using 62 isolates with this ribotype (40). PFGE, as well as ribotyping and other subtyping methods (16, 23), thus may also sometimes help to define L. monocytogenes subtypes that are associated with specific sources, including some subtypes for which phenotypic and genetic data provide explanations as to the cause of source associations observed for these specific subtypes.
Some L. monocytogenes PFGE types are widely distributed, including PFGE types corresponding to select epidemic clones that appear to be stable and pandemic.
In addition to PFGE types that appear to be associated with specific sources, we have found a number of PFGE types that appear to be widely distributed and can be detected among isolates from a variety of different sources. Some PFGE types (189, 300, and 315) were widespread among the isolates from the environment and farms but were not found in isolates from humans. Other PFGE types (38, 121, 240, and 336) were widespread and found in multiple sources but were rarely associated with human cases. For example, PFGE type 38, a PFGE type corresponding to isolates with ribotype DUP-1038B (which is grouped into EC I), was assigned to a single human isolate as well as to six isolates from urban environments, including five isolates obtained at two different times over 1 year in a single city, further supporting the persistence of this specific subtype in this city and consistent with initial ribotyping data (47). Similarly, PFGE type 121 (ribotype DUP-1042B; EC I) was widely distributed among isolates from farms and the environment and corresponded to only a single human isolate. Similar to our findings, results from other groups have also previously shown that identical L. monocytogenes PFGE types can sometimes be found among isolates from different foods, food animals, or environments, as well as from humans, without an apparent linkage between nonhuman sources and human cases of disease that yielded identical L. monocytogenes subtypes (6, 24, 42).
Interestingly, we also identified two PFGE types that were previously linked to human listeriosis outbreaks as being widely distributed. PFGE type 7, which is identical to the PFGE type linked to human listeriosis outbreaks in Los Angeles (1985) (10) and Switzerland (1983 to 1987) (9) and thus represents EC I (30), was found among isolates from humans, foods, farms, and farm animals, as well as isolates from environmental sources in the study reported here. We thus hypothesize that this PFGE type represents a stable pandemic clone, which appears to be able to survive successfully in different environments as well as grow in foods and cause human disease. Similarly, PFGE type 22, which is identical to the two-enzyme PFGE type of the strain linked to a multistate listeriosis outbreak in the northeastern United States in 2002 (14), was found among human and food isolates that spanned a three year period and included isolates from foods not associated with the outbreak (i.e., soft cheese), possibly indicating that this outbreak-associated PFGE type is also more widely distributed than previously assumed (14). Overall, our data indicate that some PFGE types are stable and widely distributed. These findings are important for the application of PFGE in food-borne disease surveillance and outbreak detection as they clearly support the fact that alone, PFGE subtype matches between food or environmental isolates and human clinical isolates do not necessarily imply a causal relationship. The more common a PFGE pattern linked to an outbreak is, the more critical strong epidemiological evidence is for linking an outbreak to a specific food source; sometimes the use of additional subtyping methods to differentiate subtypes within highly stable two-enzyme PFGE types may also be needed to further characterize isolates linked to a given outbreak with the necessary level of confidence.
Conclusion.
While the characterization of L. monocytogenes by PFGE typing or other subtyping methods can aid in the identification of listeriosis outbreaks and their food sources (12-14, 20, 21), the data reported here show that large subtype databases representing isolates from different sources may help in the interpretation of subtype data in epidemiological investigations and may facilitate the identification of common, as well as source-specific, subtypes. Due to the ever-increasing complexity and broadening distribution patterns of the food system (43, 51), databases that include subtype patterns from sources around the globe will be particularly useful. Efforts to expand the current U.S. PulseNet database internationally (52), as well as efforts to include PFGE patterns for isolates obtained from foods (e.g., food isolates obtained by the U.S. Department of Agriculture's Food Safety Inspection Service and the Food and Drug Administration) (30, 51), are thus critical to further improve the value of PulseNet and PFGE typing. While the addition of PFGE patterns for animal isolates (e.g., through the proposed VetNet system) (18, 28) is likely to enhance the value of the PulseNet databases even further, even more comprehensive databases that include isolates from different environmental sources may be needed to fully understand food-borne pathogen and L. monocytogenes diversity and to provide data that can be useful in providing context to epidemiological investigations. The large number of PFGE types found among L. monocytogenes isolates specifically indicates that PFGE databases may need to contain information on thousands of isolates from broad geographical and source ranges.
Supplementary Material
Acknowledgments
This work was supported by USDA special research grants 2002-34459-11758, 2003-34459-12999, and 2004-34459-14296.
We thank P. McGann, M. Garner, and K. Nightingale for helpful discussions and comments on manuscript drafts.
Footnotes
Published ahead of print on 3 January 2007.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Aarnisalo, K., T. Autio, A. M. Sjoberg, J. Lunden, H. Korkeala, and M. L. Suihko. 2003. Typing of Listeria monocytogenes isolates originating from the food processing industry with automated ribotyping and pulsed-field gel electrophoresis. J. Food Prot. 66:249-255. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., M. L. Cohen, and D. L. Swerdlow. 1997. Emerging foodborne diseases. Emerg. Infect. Dis. 3:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BC Centre for Disease Control. 2 July 2002, revision date. Food poisoning outbreak: Listeria monocytogenes, soft ripened cheese, Switzerland. BC Centre for Disease Control, Vancouver, British Columbia, Canada. http://www.bccdc.org/downloads/pdf/fps/reports/Outbreak_Listeria_Mold_Ripened_Cheese.pdf.
- 4.Berrang, M. E., R. J. Meinersmann, J. F. Frank, D. P. Smith, and L. L. Genzlinger. 2005. Distribution of Listeria monocytogenes subtypes within a poultry further processing plant. J. Food Prot. 68:980-985. [DOI] [PubMed] [Google Scholar]
- 5.Borucki, M. K., S. H. Kim, D. R. Call, S. C. Smole, and F. Pagotto. 2004. Selective discrimination of Listeria monocytogenes epidemic strains by a mixed-genome DNA microarray compared to discrimination by pulsed-field gel electrophoresis, ribotyping, and multilocus sequence typing. J. Clin. Microbiol. 42:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borucki, M. K., J. Reynolds, C. C. Gay, K. L. McElwain, S. H. Kim, D. P. Knowles, and J. Hu. 2004. Dairy farm reservoir of Listeria monocytogenes sporadic and epidemic strains. J. Food Prot. 67:2496-2499. [DOI] [PubMed] [Google Scholar]
- 7.Brosch, R., M. Brett, B. Catimel, J. B. Luchansky, B. Ojeniyi, and J. Rocourt. 1996. Genomic fingerprinting of 80 strains from the WHO multicenter international typing study of Listeria monocytogenes via pulsed-field gel electrophoresis (PFGE). Int. J. Food Microbiol. 32:343-355. [DOI] [PubMed] [Google Scholar]
- 8.Bruce, J. L. 1996. Automated system rapidly identifies and characterizes microorganisms in food. Food Technol. 50:77-81. [Google Scholar]
- 9.Bula, C. J., J. Bille, and M. P. Glauser. 1995. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clin. Infect. Dis. 20:66-72. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. 1985. Listeriosis outbreak associated with Mexican-style cheese—California. Morb. Mortal. Wkly. Rep. 34:357-359. [PubMed] [Google Scholar]
- 11.Centers for Disease Control. 1989. Listeriosis associated with consumption of turkey franks. Morb. Mortal. Wkly. Rep. 38:267-268. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1999. Update: multistate outbreak of listeriosis—United States, 1998-1999. Morb. Mortal. Wkly. Rep. 47:1117-1118. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 2000. Multistate outbreak of listeriosis—United States, 2000. Morb. Mortal. Wkly. Rep. 49:1129-1130. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 2002. Outbreak of listeriosis—northeastern United States, 2002. Morb. Mortal. Wkly. Rep. 51:950-951. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2003. Preliminary FoodNet data on the incidence of foodborne illnesses—selected sites, United States, 2002. Morb. Mortal. Wkly. Rep. 52:340-343. [PubMed] [Google Scholar]
- 16.Chou, C. H., J. L. Solva, and C. Wang. 2006. Prevalence and typing of Listeria monocytogenes in raw catfish fillets. J. Food Prot. 69:815-819. [DOI] [PubMed] [Google Scholar]
- 17.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorka-Cray, P. J. 29 October 2006, accession date. NARMS update. Agricultural Research Service, U.S. Department of Agriculture, Washington, DC. http://www.aphl.org/conferences/pulsenet_update_meeting_2005/files/2005/Wednesday/Cray_USDA_VetNet.pdf.
- 19.Fugett, E. B., E. Fortes, C. Nnoka, and M. Wiedmann. 2006. The International Life Science Institute North America Listeria monocytogenes strain collection: development of standard L. monocytogenes strain sets for research and validation studies. J. Food Prot. 69:2929-2938. [DOI] [PubMed]
- 20.Gottlieb, S. L., E. C. Newbern, P. M. Griffin, L. M. Graves, R. M. Hoekstra, N. L. Baker, S. B. Hunter, K. G. Holt, F. Ramsey, M. Head, P. Levine, G. Johnson, D. Schoonmaker-Bopp, V. Reddy, L. Kornstein, M. Gerwel, J. Nsubuga, L. Edwards, S. Stonecipher, S. Hurd, D. Austin, M. A. Jefferson, S. D. Young, K. Hise, E. D. Chernak, and J. Sobel. 2006. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin. Infect. Dis. 42:29-36. [DOI] [PubMed] [Google Scholar]
- 21.Graves, L. M., S. B. Hunter, A. R. Ong, D. Schoonmaker-Bopp, K. Hise, L. Kornstein, W. E. DeWitt, P. S. Hayes, E. Dunne, P. Mead, and B. Swaminathan. 2005. Microbiological aspects of the investigation that traced the 1998 outbreak of listeriosis in the United States to contaminated hot dogs and establishment of molecular subtyping-based surveillance for Listeria monocytogenes in the PulseNet network. J. Clin. Microbiol. 43:2350-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graves, L. M., and B. Swaminathan. 2001. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 65:55-62. [DOI] [PubMed] [Google Scholar]
- 23.Gray, M. J., R. N. Zadoks, E. D. Fortes, B. Dogan, S. Cai, Y. Chen, V. N. Scott, D. E. Gombas, K. J. Boor, and M. Wiedmann. 2004. Listeria monocytogenes isolates from foods and humans form distinct but overlapping populations. Appl. Environ. Microbiol. 70:5833-5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grif, K., I. Heller, M. Wagner, M. Dierich, and R. Wurzner. 2006. A comparison of Listeria monocytogenes serovar 4b isolates of clinical and food origin in Austria by automated ribotyping and pulsed-field gel electrophoresis. Foodborne Pathog. Dis. 3:138-141. [DOI] [PubMed] [Google Scholar]
- 25.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Van Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson, C., J. Frye, M. Englen, J. Bailey, R. Meinersmann, M. Berrang, and C. Paula. 21 March 2006, revision date. NARMS, VetNet and CAHFSE. Agricultural Research Service, U.S. Department of Agriculture, Washington, DC. http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=191019.
- 29.Jeffers, G. T., J. L. Bruce, P. L. McDonough, J. Scarlett, K. J. Boor, and M. Wiedmann. 2001. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology 147:1095-1104. [DOI] [PubMed] [Google Scholar]
- 30.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 31.Linnan, M. J., L. Mascola, X. D. Lou, V. Goulet, S. May, C. Salminen, D. W. Hird, M. L. Yonekura, P. Hayes, and R. Weaver. 1988. Epidemic listeriosis associated with Mexican-style cheese. N. Engl. J. Med. 319:823-828. [DOI] [PubMed] [Google Scholar]
- 32.Lukinmaa, S., K. Aarnisalo, M. L. Suihko, and A. Siitonen. 2003. Diversity of Listeria monocytogenes isolates of human and food origin studied by serotyping, automated ribotyping and pulsed field gel electrophoresis. Clin. Microbiol. Infect. 10:562-568. [DOI] [PubMed] [Google Scholar]
- 33.Lyytikainen, O., T. Autio, R. Maijala, P. Ruutu, T. Honkanen-Buzalski, M. Miettinen, M. Hatakka, J. Mikkola, V. J. Antilla, T. Johansson, L. Rantala, T. Aalto, H. Korkeala, and A. Siitonen. 2000. An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J. Infect. Dis. 181:1838-1841. [DOI] [PubMed] [Google Scholar]
- 34.Mead, P. S., E. F. Dunne, L. Graves, M. Wiedmann, M. Patrick, S. Hunter, E. Salehi, F. Mostashari, A. Craig, P. Mshar, T. Bannerman, B. D. Sauders, P. Hayes, W. DeWitt, P. Sparling, P. Griffin, D. Morse, L. Slutsker, and B. Swaminathan. 2005. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol. Infect. 1:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mereghetti, L., P. Lanotte, V. Savoye-Marczuk, N. Marquet-Van Der Mee, A. Audurier, and R. Quentin. 2002. Combined ribotyping and random multiprimer DNA analysis to probe the population structure of Listeria monocytogenes. Appl. Environ. Microbiol. 68:2849-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadon, C. A., D. L. Woodward, C. Young, F. G. Rodgers, and M. Wiedmann. 2001. Correlations between molecular subtyping and serotyping of Listeria monocytogenes. J. Clin. Microbiol. 39:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.New York State Department of Agriculture and Markets. 29 October 2006, accession date. New York State dairy statistics 2004 annual summary. New York State Department of Agriculture and Markets, Albany, NY. http://www.agmkt.state.ny.us/DI/NYSAnnStat2004.pdf.
- 39.Nightingale, K. K., Y. H. Schukken, C. R. Nightingale, E. D. Fortes, A. J. Ho, Z. Her, Y. T. Grohn, P. L. McDonough, and M. Wiedmann. 2004. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 70:4458-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nightingale, K. K., K. Windham, K. E. Martin, M. Yeung, and M. Wiedmann. 2005. Select Listeria monocytogenes subtypes commonly found in foods carry distinct nonsense mutations in inlA, leading to expression of truncated and secreted internalin A, and are associated with a reduced invasion phenotype for human intestinal epithelial cells. Appl. Environ. Microbiol. 71:8764-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton, D. M., J. M. Scarlett, K. Horton, D. Sue, J. Thimothe, K. J. Boor, and M. Wiedmann. 2001. Characterization and pathogenic potential of Listeria monocytogenes isolates from the smoked fish industry. Appl. Environ. Microbiol. 67:646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okwumabua, O., M. O'Connor, E. Shull, K. Strelow, M. Hamacher, T. Kurzynski, and D. Warshauer. 2005. Characterization of Listeria monocytogenes isolates from food animal clinical cases: PFGE pattern similarity to strains from human listeriosis cases. FEMS Microbiol. Lett. 249:275-281. [DOI] [PubMed] [Google Scholar]
- 43.Olsen, S. J., M. Patrick, S. B. Hunter, V. Reddy, L. Kornstein, W. R. MacKenzie, K. Lane, S. Bidol, G. A. Stoltman, D. M. Frye, I. Lee, S. Hurd, T. F. Jones, T. N. LaPorte, W. Dewitt, L. Graves, M. Wiedmann, D. J. Schoonmaker-Bopp, A. J. Huang, C. Vincent, A. Bugenhagen, J. Corby, E. R. Carloni, M. E. Holcomb, R. F. Woron, S. M. Zansky, G. Dowdle, F. Smith, S. Ahrabi-Fard, A. R. Ong, N. Tucker, N. A. Hynes, and P. Mead. 2005. Multistate outbreak of Listeria monocytogenes infection linked to delicatessen turkey meat. Clin. Infect. Dis. 40:962-967. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen, O. F., P. Skouboe, L. Dons, L. Rossen, and J. E. Olsen. 1995. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology 141:2053-2061. [DOI] [PubMed] [Google Scholar]
- 45.Roberts, A., K. Nightingale, G. Jeffers, E. Fortes, J. M. Kongo, and M. Wiedmann. 2006. Genetic and phenotypic characterization of Listeria monocytogenes lineage III. Microbiology 152:685-693. [DOI] [PubMed] [Google Scholar]
- 46.Rothman, K. J., and S. Greenland. 1998. Modern epidemiology, p. 183-199. Lippincott-Raven, Philadelphia, PA.
- 47.Sauders, B. D., M. Z. Durak, E. Fortes, K. Windham, Y. Schukken, A. J. Lembo, Jr., B. Akey, K. K. Nightingale, and M. Wiedmann. 2006. Molecular characterization of Listeria monocytogenes from natural and urban environments. J. Food Prot. 69:93-105. [DOI] [PubMed] [Google Scholar]
- 48.Sauders, B. D., K. Mangione, C. Vincent, J. Schermerhorn, C. M. Farchione, N. B. Dumas, D. Bopp, L. Kornstein, E. D. Fortes, K. Windham, and M. Wiedmann. 2004. Distribution of Listeria monocytogenes molecular subtypes among human and food isolates from New York State shows persistence of human disease-associated Listeria monocytogenes strains in retail environments. J. Food Prot. 67:1417-1428. [DOI] [PubMed] [Google Scholar]
- 49.Sauders, B. D., Y. Schukken, L. Kornstein, V. Reddy, T. Bannerman, E. Salehi, N. Dumas, B. J. Anderson, J. P. Massey, and M. Wiedmann. 2006. Molecular epidemiology and cluster analysis of human listeriosis cases in three U.S. states. J. Food Prot. 69:1680-1689. [DOI] [PubMed] [Google Scholar]
- 50.Senczek, D., R. Stephan, and F. Untermann. 2000. Pulsed-field gel electrophoresis (PFGE) typing of Listeria strains isolated from a meat processing plant over a 2-year period. Int. J. Food Microbiol. 62:155-159. [DOI] [PubMed] [Google Scholar]
- 51.Swaminathan, B., T. J. Barrett, S. B. Hunter, and R. V. Tauxe. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swaminathan, B., P. Gerner-Smidt, L. K. Ng, S. Lukinmaa, K. M. Kam, S. Rolando, E. P. Gutierrez, and N. Binsztein. 2006. Building PulseNet international: an interconnected system of laboratory networks to facilitate timely public health recognition and response to foodborne disease outbreaks and emerging foodborne diseases. Foodborne Pathog. Dis. 3:36-50. [DOI] [PubMed] [Google Scholar]
- 53.Tauxe, R. V. 1997. Emerging foodborne diseases: an evolving public health challenge. Emerg. Infect. Dis. 3:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tompkin, R. B. 2002. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 65:709-725. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Food and Drug Administration's Center for Food Safety and Applied Nutrition, U.S. Department of Agriculture's Food Safety and Inspection Service, and the Centers for Disease Control and Prevention. 10 October 2005, revision date. Quantitative assessment of relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. U. S. Food and Drug Administration's Center for Food Safety and Applied Nutrition, Washington, DC. http://www.foodsafety.gov/∼dms/lmr2-toc.html.
- 56.Vogel, B. F., V. Fussing, B. Ojeniyi, L. Gram, and P. Ahrens. 2004. High-resolution genotyping of Listeria monocytogenes by fluorescent amplified fragment polymorphism analysis compared to pulsed-field gel electrophoresis, random amplified polymorphic DNA analysis, ribotyping, and PCR-restriction fragment length polymorphism analysis. J. Food Prot. 67:1656-1665. [DOI] [PubMed] [Google Scholar]
- 57.Ward, T. J., L. Gorski, M. K. Borucki, R. E. Mandrell, J. Hutchins, and K. Pupedis. 2004. Intraspecific phylogeny and lineage group identification based on the prfA virulence gene cluster of Listeria monocytogenes. J. Bacteriol. 186:4994-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiedmann, M. 2002. Molecular subtyping methods for Listeria monocytogenes. J. AOAC Int. 85:524-531. [PubMed] [Google Scholar]
- 59.Wiedmann, M., J. L. Bruce, C. Keating, A. E. Johnson, P. L. McDonough, and C. A. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.