Abstract
Isolation of Mycoplasma genitalium from clinical specimens remains difficult. We describe an improvement of the Vero cell coculture method in which the growth of M. genitalium was monitored by quantitative real-time PCR. Four new M. genitalium strains were isolated from six first-void urine specimens of male Japanese patients with urethritis. In two of them, only M. genitalium was detected: one also contained Ureaplasma urealyticum, and one contained Chlamydia trachomatis, Neisseria gonorrhoeae, U. urealyticum, and Ureaplasma parvum. In the specimens yielding isolates of M. genitalium, growth was documented by quantitative PCR after two to five passages in Vero cells. The complete isolation procedure from the initial inoculation to completion of single-colony cloning took about 1 year. Isolation of M. genitalium from urine specimens proved to be more difficult than from swab specimens. Due to the cytotoxic effect of urine, a procedure involving washing of the urinary sediment was introduced. Furthermore, prolonged storage of the urine specimens before culture was shown to be detrimental to the success of isolation, as shown by the lack of success in attempts to isolate M. genitalium from mailed urine specimens as well as by simulation experiments. High concentrations of penicillin G and amphotericin B were surprisingly inhibitory to the growth of wild-type M. genitalium strains, but penicillin G at 200 IU/ml and polymyxin B at 500 μg/ml could be used as selective antibiotics to avoid bacterial overgrowth in the Vero cell cultures.
Mycoplasma genitalium is one of the pathogens of male urethritis and is sexually transmissible. Two strains of M. genitalium were originally isolated from urethral swabs of male patients with nongonococcal urethritis by direct inoculation of the swabs into SP4 mycoplasma medium (14). However, other investigators failed to isolate new strains from urogenital tract specimens for about 10 years after the first report. In 1996, Jensen et al. recovered five new M. genitalium strains by using Vero cells for initial cocultivation of M. genitalium (8). However, isolation of M. genitalium from clinical specimens remained difficult and time-consuming.
In the recent decade, detection of M. genitalium by PCR in first-void urine (FVU) specimens has been shown to be comparable to PCR on urethral swabs and is easier to collect and painless for patients (2, 6, 9). However, M. genitalium has not previously been isolated in axenic culture from FVU. In this study, we improved the culture method and demonstrated its ability to isolate M. genitalium from FVU.
(Part of this work was presented at the 15th Meeting of the Japanese Society for Sexually Transmitted Diseases, 4 to 5 December 2004, Tokyo, Japan.)
MATERIALS AND METHODS
Urine specimens.
FVU specimens were collected from 109 Japanese male patients with urethritis. Approximately 30 ml of the urine was centrifuged at 10,000 × g for 30 min. The pellet was resuspended in 1 ml of the same urine and was divided into 0.25-ml aliquots which were stored at −80°C. These frozen specimens were transported on dry ice, and all procedures below were performed at the Statens Serum Institut, Copenhagen, Denmark. M. genitalium, Chlamydia trachomatis, Neisseria gonorrhoeae, Ureaplasma urealyticum, and Ureaplasma parvum were detected by PCR performed on one of the aliquots of the urine specimens (6). The M. genitalium DNA load was determined by a TaqMan 5′ nuclease real-time PCR as previous described (7). Urine specimens containing more than 40,000 genome equivalents (geq) of M. genitalium per ml were selected for the attempts to isolate M. genitalium. Of the 109 urine specimens collected, 6 contained sufficiently high titers for the isolation attempts. The average number of geq of M. genitalium in these specimens was 692,100/ml.
Culture of Vero cells.
Vero cells were maintained in Eagle's minimal essential medium (MEM) supplemented with 2% Ultroser G serum substitute (Ciphergen, Cergy-Saint-Christophe, France) and 3% sodium bicarbonate, without any antibiotics at 37°C. Vero cells were propagated in flat-bottom culture tubes (Nunclon Delta flat-bottom screw-cap tubes; NUNC, Roskilde, Denmark) or 15-ml plastic culture flasks (NUNC, Roskilde, Denmark) seeded with 2.5 × 104 cells per ml.
Initial cultivation of M. genitalium from urine specimens in Vero cell cultures.
Initial attempts to cultivate M. genitalium were performed by a modification of the method described by Jensen et al. (8). Initially, 0.2 ml of urine was inoculated directly into 2 ml of Vero cells, but since it was found that most of the urine specimens were cytotoxic, a washing procedure was introduced. In brief, the stored urine specimen was thawed and 0.2 ml was diluted in 1 ml of Eagle's MEM. The suspension was centrifuged at 30,000 × g for 15 min and was washed three times in 1.9 ml of Eagle's MEM. The pellet was resuspended in 1 ml of Eagle's MEM with 2% Ultroser G and inoculated into 2 ml of a freshly prepared Vero cell suspension at 2.5 × 104 cells per ml in Eagle's MEM supplemented with 2% Ultroser G, 200 IU/ml of penicillin G (LeoPharma, Ballerup, Denmark), and 500 μg/ml of polymyxin B (Sigma-Aldrich Denmark, Vallensbaek Strand, Denmark) in flat-bottom culture tubes and incubated at 37°C with 5% CO2. The cells were observed daily for the first week and then at 2- to 3-day intervals. If cells detached, fresh Vero cells were added (about 2.5 × 104 cells). The medium was not changed during the first 14 days even if it was acidified. Each week, 100 μl of the supernatant was assayed for growth using the quantitative PCR (qPCR) (7).
Subculture of M. genitalium strains and adaptation to axenic culture.
Attaching Vero cells were carefully scraped off 14 days after the initial inoculation, and 1 ml of the culture medium containing Vero cells was added to a new tube containing 2 ml of a fresh Vero cell suspension. At the same time, 0.25 ml and 0.1 ml of the cell-containing medium was inoculated into 2 ml of Friis' broth (FB) medium and onto Friis' agar (8), respectively. In addition, 0.1 ml was assayed for growth in the qPCR (4). The remaining cell-containing medium was stored at −80°C.
When growth was confirmed after some subcultures and reached >10,000 geq/5 μl of pretreated specimen, 1 ml of the culture medium containing Vero cells was inoculated into 15 ml of a Vero cell suspension in a culture flask. M. genitalium cells in culture flasks were also subcultured every 14 days as described above. Subcultures of M. genitalium in flasks continued until growth of M. genitalium in FB medium or on Friis' agar was confirmed. If, for some reason, the growth of M. genitalium was stopped, cell-containing medium stored as a backup at −80°C was thawed and used to reinitiate culturing in flat-bottomed culture tubes.
The FB medium was incubated at 37°C in tightly closed glass tubes. When the color of the FB medium changed from orange to yellow, FB medium was filtered through a filter with a pore size of 0.45 μm and 0.1 ml was inoculated onto Friis's agar and incubated at 37°C in 5% CO2 and 95% N2. When colonies of M. genitalium were observed on Friis's agar, single and well-separated colonies were picked and each was inoculated into a separate tube with 2 ml of FB medium. This procedure was repeated three times, and the final cloned strain of M. genitalium was grown and stored at −80°C.
Simulation experiments for determination of the effect of prolonged storage of M. genitalium in urine specimens on isolation.
Since initial attempts to isolate M. genitalium from mailed urine specimens were unsuccessful, simulation experiments were conducted in order to demonstrate the preservation of viability of M. genitalium under different transport conditions. For the experiments, M. genitalium strain R6G from a urethral swab specimen from a Danish male patient was used. This strain was adapted to growth in Vero cell culture during the present study but was unable to be propagated in axenic culture media. Fresh urine free of M. genitalium from a healthy man was passed through a filter with a pore size of 0.45 μm. Four plastic tissue culture flasks were prepared: two were filled with 30 ml of the filtered urine, and the other two were filled with 30 ml of SP4 mycoplasma medium without any antibiotics. Each flask was inoculated with 0.5 ml of cultured R6G with Vero cells (a total of 1.6 × 108 geq). One of each flask containing urine or SP4 mycoplasma medium was incubated at room temperature, and the other set was incubated at 4°C. At 1, 2, 4, 7, 14, and 21 days after the incubation, 1.9 ml of urine or SP4 mycoplasma medium was withdrawn from each flask and was frozen at −80°C. M. genitalium DNA loads from each specimen were determined by qPCR in order to monitor the temporal change of the M. genitalium DNA load in each flask.
In order to determine the infectivity of M. genitalium R6G after storage, a specimen from each time point and incubation condition was subjected to culture essentially using the procedure described above. In brief, the washed specimen was added to 2.0 ml of fresh Vero cell suspension (about 3.8 × 104 cells) in a well of a 24-well tissue culture plate (Multiwell; Becton Dickinson, France) and incubated at 37°C in 5% CO2. At 7, 14, and 21 days after inoculation, 0.1 ml of the supernatant was harvested from each well, the M. genitalium DNA loads were determined by qPCR, and growth curves of M. genitalium were established.
Effects of selective antibiotics on the growth of M. genitalium in Vero cell culture.
In order to determine the best selective antibiotic for primary isolation in the Vero cell culture procedure, the antibiotic susceptibility of 11 primary isolates of M. genitalium as well as the G37T strain and an early passage of the M30 urogenital strain (listed in Table 2) were tested as described previously (4). The antibiotics tested were penicillin G, ampicillin, polymyxin B, and amphotericin B. The M. genitalium strains were inoculated into 2.4 ml of Vero cell suspension with a range of concentrations of the antibiotics in wells of flat-bottom tissue culture plates at 37°C in 5% CO2. At 21 days of incubation, 0.1 ml of the supernatant was harvested from each well for calculation of M. genitalium DNA loads by qPCR and the inhibitory rates of antibiotics were calculated by the following formula: inhibition rate (%) = [(average of DNA loads in control wells − DNA load in test well)/(average of DNA loads in control wells)] × 100.
TABLE 2.
Inhibition rates of selective antibiotics at different concentrations on a selection of M. genitalium strainsa
| Strain | % Inhibition at antibiotic concnb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin (μg/ml)
|
Penicillin G (IU/ml)
|
Amphotericin B (μg/ml)
|
Polymyxin B (μg/ml)
|
|||||||||
| 500 | 250 | 125 | 500 | 250 | 125 | 25 | 12.5 | 6.3 | 500 | 250 | 125 | |
| G37T | 63.2 | 6.2 | 9.7 | 0 | 20.0 | 4.5 | 44.6 | 0 | 0 | 29.1 | 2.7 | NTc |
| M30 | 67.1 | 23.0 | 41.7 | 44.2 | 25.5 | 12.5 | 95.2 | 13.4 | 17.1 | 79.9 | 53.6 | NT |
| M2282 | 51.8 | 3.18 | NT | 27.6 | 0 | NT | 0 | 0 | NT | 0 | 0 | NT |
| M2300 | 64.9 | 33.6 | 32.7 | 33.6 | 12.4 | 18.0 | 65.3 | 10.0 | 8.8 | 31.0 | 19.2 | NT |
| M2321 | 33.1 | 21.8 | 0 | 3.3 | 0 | 0 | 6.0 | 7.8 | 23.3 | 55.0 | 67.0 | NT |
| M2341 | 94.6 | 31.6 | 68.4 | 42.0 | 59.9 | 21.8 | 39.5 | 47.4 | 10.1 | 62.3 | 44.3 | NT |
| M6090 | 76.1 | 0 | 0 | 0 | 0 | 0 | 95.5 | 0 | 0 | 0 | 0 | NT |
| M6151 | 69.3 | 61.6 | 26.8 | 69.6 | 48.4 | 0 | 100 | 0 | 0 | 29.5 | 0 | NT |
| R5G (M6280) | 96.7 | 25.8 | 0 | 42.3 | 46.7 | 35.9 | 100 | 67.3 | 63.9 | 70.2 | 58.3 | NT |
| R6G (M6281) | 99.7 | 92.1 | 0 | 99.5 | 91.8 | 0 | 99.9 | 62.4 | 35.4 | 19.5 | 24.0 | 0 |
| R65G (M6285) | 99.9 | 79.6 | 37.8 | 99.1 | 7.1 | 16.4 | 100 | 72.5 | 0 | 83.0 | 0 | NT |
| R66G (M6328) | 99.9 | 96.9 | 94.3 | 99.9 | 56.9 | 28.3 | 99.9 | 99.9 | 76.9 | 98.2 | 0 | NT |
| R67G (M6286) | 99.4 | 95.8 | 73.2 | 94.9 | 0 | 0 | 99.5 | 71.0 | 0 | 0 | 0 | NT |
M. genitalium strains were grown in Vero cell suspensions with selective antibiotics at different concentrations in wells of flat-bottom tissue culture plates at 37°C in 5% CO2. The M. genitalium DNA load in the supernatant was determined by quantitative real-time PCR 21 days after the inoculation of the M. genitalium strains.
The inhibition rate was calculated by the following formula: inhibition rate (%) = [(average of DNA loads in control wells − DNA loads in test well)/(average of DNA loads in control wells)] × 100.
NT, not tested.
RESULTS
Isolation of M. genitalium strains from urine specimens.
Of the six urine specimens containing >40,000 geq/ml, four yielded growth of new M. genitalium strains in the Vero cell culture system (Table 1). In two of them, only M. genitalium was detected: one also contained U. urealyticum, and one contained C. trachomatis, N. gonorrhoeae, U. urealyticum, and U. parvum. These other pathogens could not be detected from the cloned isolates by PCR. In the specimens yielding isolates of M. genitalium, growth was documented by qPCR after two to five passages in Vero cells. The complete isolation procedure from the initial inoculation to completion of the cloning took about 1 year.
TABLE 1.
First-void urine specimens cultured for M. genitalium
| Specimen name | Detection of microorganism from urine
|
DNA load of M. genitalium (geq/ml)
|
First day of confirmed M. genitalium growth
|
Final name | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. genitalium | C. trachomatis | N. gonorrhoeae | U. urealyticum | U. parvum | Urine | Washed inoculum | Vero cells | FB medium | Friis' agar | ||
| R53G | + | − | − | + | − | 1,742,520 | 68,760 | 70 | 161 | 189 | M6287 |
| R69G | + | + | + | + | + | 62,520 | 9,160 | 60 | 76 | 80 | M6284 |
| R70G | + | − | − | − | − | 429,400 | 12,1800 | 74 | 80 | 73 | M6282 |
| R71G | + | + | − | − | + | 180,200 | 41,560 | ||||
| R72G | + | − | + | − | − | 1,232,760 | 3,800 | ||||
| R74G | + | − | − | − | − | 506,120 | 51,280 | 35 | 73 | 80 | M6283 |
Effect of storage on DNA loads of M. genitalium in urine and SP4 medium.
The M. genitalium DNA load in urine and SP4 mycoplasma medium after up to 21 days of incubation at room temperature or at 4°C after the inoculation was compared to that of the specimens collected just after the inoculation of R6G. No significant change in the DNA load could be shown even after extended storage at room temperature, indicating that transport of specimens at ambient temperature should be possible.
Effect of storage on growth of M. genitalium in urine and SP4 medium.
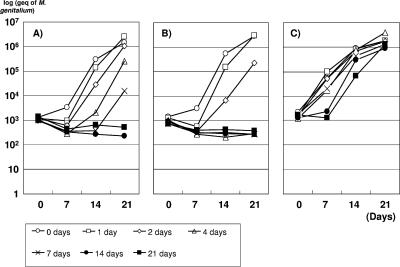
As shown in Fig. 1, the growth curves of R6G obtained after storage in urine or SP4 medium at room temperature or at 4°C varied significantly. SP4 mycoplasma medium clearly preserved viability better than urine as M. genitalium could be grown even after storage at room temperature for up to 21 days, although storage for more than 7 days resulted in an initial lag phase (Fig. 1C). In contrast, M. genitalium R6G stored in urine rapidly lost its viability, in particular when the urine was kept at room temperature, where viability was completely lost after storage for >2 days (Fig. 1B). At 4°C, viability was preserved for 7 days, although a significant initial lag phase was seen after storage for >2 days (Fig. 1A).
FIG. 1.
M. genitalium strain R6G grown in Vero cell culture from a urethral swab specimen from a Danish man was added to urine or SP4 mycoplasma medium kept at room temperature or at 4°C. At 1, 2, 4, 7, 14, and 21 days of incubation, cells from 1.9 ml of urine or SP4 mycoplasma medium were harvested and frozen at −80°C. Each specimen was inoculated into Vero cell cultures, and growth was monitored by quantitative PCR. (A and B) R6G in urine at 4°C (A) and room temperature (B). (C) R6G in SP4 medium at room temperature.
Effects of selective antibiotics on the growth of M. genitalium in Vero cell culture.
Thirteen M. genitalium strains were grown in Vero cell culture with selective antibiotics at different concentrations. Ampicillin, penicillin G, amphotericin B, and polymyxin B all inhibited the growth of any M. genitalium strains to some degree at high concentrations (Table 2). Amphotericin B was the strongest inhibitor of M. genitalium, as 25 μg/ml inhibited the growth of eight strains by more than 90%. Surprisingly, M2282 was not significantly influenced by amphotericin B. Polymyxin B at 500 μg/ml inhibited 1 strain more than 90%, but apparently, 250 μg/ml could be used without detrimental effects. The β-lactam antibiotics ampicillin and penicillin G were surprisingly inhibitory at high concentrations. Even at 125 μg/ml, one strain was inhibited by ampicillin, whereas penicillin G could be safely used at 125 IU/ml.
DISCUSSION
In 1996, Jensen et al. reported that four M. genitalium strains were isolated from urethral swabs of men with nongonococcal urethritis (8). Using this procedure, nine strains could grow in Vero cells, but five strains were lost after passages for adaptation to growth in FB medium. Later, one of these strains (M2282) was recovered. In the literature, M. genitalium strains have been isolated from urethral swabs (8, 10, 14), cervical swabs (1), and respiratory tract specimens (13). However, M. genitalium has not been isolated from urine specimens in stable axenic cultures, although propagation in HEp-2 cells was reported by Totten et al. (12).
In this study, modified methods were developed to isolate M. genitalium from FVU specimens. A very essential improvement was the use of qPCR for monitoring the growth of M. genitalium. Besides being useful in the optimization of the culture procedure, we also discovered that qPCR could be applied for drug susceptibility testing (4). This idea was developed out of the surprising observation that some M. genitalium strains were inhibited by ampicillin, penicillin G, amphotericin B, or polymyxin B. In other mycoplasmas including Mycoplasma neurolyticum, Mycoplasma hyopneumoniae, Mycoplasma flocculare, and Mycoplasma dispar, high concentrations of ampicillin and penicillin G were reported to inhibit growth (3). In contrast, it was reported that M. genitalium G37T was insensitive to penicillin G (MIC of >3,000 IU/ml) and ampicillin (MIC of >2,000 IU/ml) (11). Susceptibility testing of M. genitalium as well as other mollicutes has traditionally been performed by broth or agar dilution methods, in which the growth of M. genitalium was monitored by the observation of a color change of the mycoplasma medium or by development of colonies. However, using these methods, a partial inhibition that might be detrimental in the initial phase of axenic growth could be overlooked. Furthermore, the different compositions of the growth media in terms of pH and protein concentration may significantly change the inhibitory effect of the antibiotics. Monitoring inhibition by calculation of DNA loads by qPCR, we could show that the growth of M. genitalium G37T was inhibited 63% by 500 μg/ml of ampicillin, 45% by 25 μg/ml of amphotericin B, and 29% by 500 μg/ml of polymyxin B but not by penicillin G. However, other M. genitalium strains had other patterns of inhibition. The mechanisms of inhibition of M. genitalium are unclear, and in some cases, inhibition might be caused by impurities in the antibiotic preparations. A high concentration of amphotericin B has a cytopathic effect on the Vero cells, which might be one of the mechanisms of inhibition, but since the drug is not completely selective in its binding to ergosterol present in fungi, some binding to the cholesterol in the mycoplasma membrane may also occur, explaining the growth inhibition.
As a sound principle, antibiotics were not used for maintenance of the Vero cells. However, the Vero cells were easily contaminated when inoculated with clinical specimens. Therefore, 200 IU/ml of penicillin G and 500 μg/ml of polymyxin B were added to the medium during the initial cultivation of M. genitalium.
Another essential change from the initial procedure was the increased duration between each subculture of M. genitalium in the Vero cell cultures. Some strains grew slower than expected previously, and M. genitalium growth could not be demonstrated until after 2 weeks of incubation. Vero cells started to detach from the surface of the culture vessels at 10 to 21 days after the passage of M. genitalium. We consequently decided to subculture M. genitalium every 2 weeks, but the Vero cells should be observed at regular intervals, and when the Vero cells start to detach, passage should be carried out or fresh cells should be added, depending on the time after inoculation.
Isolation of M. genitalium from urine specimens proved to be more difficult than from swab specimens. When urine specimens were inoculated directly into the Vero cell culture, the monolayer of Vero cells was destroyed by some contents of urine. Consequently, a washing procedure was introduced. Furthermore, prolonged storage of the urine specimens before culture appeared to be detrimental to the success of isolation, as illustrated both by simulation experiments in which M. genitalium was added to urine (Fig. 1) and by the lack of success in previous attempts to isolate M. genitalium from mailed urine specimens.
It was encouraging that the M. genitalium DNA load in urine specimens was stable for at least 3 weeks. Therefore, urine specimens are useful for diagnosis for M. genitalium by PCR. However, the infectivity on Vero cells was lost by storage in urine for more than 2 days at room air temperature. Refrigeration of the urine increased the duration of infectivity, but for isolation of M. genitalium from urine specimens, we recommend that the urine should centrifuged and the pellet resuspended in mycoplasma broth and stored at −80°C as soon as possible.
Using the Vero cell culture system in which the M. genitalium strains grow to high titers may lead to cross-contamination between the cultures. In order to document the absence of such cross-contamination, we DNA typed the M. genitalium strains directly from the urine specimens and from the cloned isolates using the MgPa 1-3 typing system recently described (5). Although strains M6284 and M6287 had the same DNA type, subsequent supplementary typing using other parts of the mgpB gene clearly documented that the strains were different from each other but identical to the inoculum strains (data not shown).
In conclusion, we successfully developed an improved method allowing us to isolate M. genitalium strains from FVU specimens stored for extended periods at −80°C. Although four out of six strains could be isolated, the procedure is still extremely slow and labor intensive. However, given the ability to assess the antibiotic susceptibility pattern by performing the testing already when the strains are growing in the Vero cells by using the qPCR, new possibilities for establishing a correlation between the in vitro MIC and clinical response have arisen.
Acknowledgments
Berit Larsen, Gitte Riisgaard Christensen, and Lisbeth Egelykke Stolpe provided technical assistance with culturing M. genitalium, and Birthe Dohn supported the quantitative real-time PCR.
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Baseman, J. B., M. Cagle, J. E. Korte, C. Herrera, W. G. Rasmussen, J. G. Baseman, R. Shain, and J. M. Piper. 2004. Diagnostic assessment of Mycoplasma genitalium in culture-positive women. J. Clin. Microbiol. 42:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deguchi, T., T. Yoshida, S. Yokoi, M. Ito, M. Tamaki, H. Ishiko, and S.-I. Maeda. 2002. Longitudinal quantitative detection by real-time PCR of Mycoplasma genitalium in first-pass urine of men with recurrent nongonococcal urethritis. J. Clin. Microbiol. 40:3854-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freundt, E. A. 1983. Culture media for classic mycoplasmas. Methods Mycoplasmol. 1:127-135. [Google Scholar]
- 4.Hamasuna, R., Y. Osada, and J. S. Jensen. 2005. Antibiotic susceptibility testing of Mycoplasma genitalium by TaqMan 5′ nuclease real-time PCR. Antimicrob. Agents Chemother. 49:4993-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjorth, S. V., E. Björnelius, P. Lidbrink, L. Falk, B. Dohn, L. Berthelsen, L. Ma, D. H. Martin, and J. S. Jensen. 2006. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J. Clin. Microbiol. 44:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen, J. S., E. Björnelius, B. Dohn, and P. Lidbrink. 2004. Comparison of first void urine and urogenital swab specimens for detection of Mycoplasma genitalium and Chlamydia trachomatis by polymerase chain reaction in patients attending a sexually transmitted disease clinic. Sex. Transm. Dis. 31:499-507. [DOI] [PubMed] [Google Scholar]
- 7.Jensen, J. S., E. Björnelius, B. Dohn, and P. Lidbrink. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurstrand, M., J. S. Jensen, H. Fredlund, L. Falk, and P. Molling. 2005. Detection of Mycoplasma genitalium in urogenital specimens by real-time PCR and by conventional PCR assay. J. Med. Microbiol. 54:23-29. [DOI] [PubMed] [Google Scholar]
- 10.Luo, D., W. Xu, G. Liang, S. Wang, Z. Wang, Z. Bi, and W. Zhu. 1999. Isolation and identification of Mycoplasma genitalium from high risk populations of sexually transmitted diseases in China. Chin. Med. J. 112:489-492. [PubMed] [Google Scholar]
- 11.Taylor-Robinson, D., J. G. Tully, P. M. Furr, R. M. Cole, D. L. Rose, and N. F. Hanna. 1981. Urogenital mycoplasma infection of man: a review with observations on a recently discovered mycoplasma. Isr. J. Med. Sci. 17:524-530. [PubMed] [Google Scholar]
- 12.Totten, P. A., M. A. Schwartz, K. E. Sjostrom, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183:269-276. [DOI] [PubMed] [Google Scholar]
- 13.Tully, J. G., D. L. Rose, J. B. Baseman, S. F. Dallo, A. L. Lazzell, and C. P. Davis. 1995. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J. Clin. Microbiol. 33:1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]