Abstract
Necrotizing fasciitis due to Vibrio vulnificus may result in overwhelming sepsis, leading to death in some patients. Significant risk factors for severe disease include preexisting liver disease. We report a case of Vibrio vulnificus necrotizing fasciitis in a patient with previously undiagnosed chronic hepatitis and cirrhosis.
CASE REPORT
The patient was a 50-year-old Cambodian male with no significant prior medical history who developed bilateral lower-extremity pain and shortness of breath approximately 24 h prior to admission. He also complained of subjective fever and resolved watery diarrhea. As a chef at a local Asian restaurant, he prepared food for the restaurant, including fresh seafood. On presentation to the emergency department (ED), the patient's vital statistics were as follows: temperature, 97.7°F; heart rate, 114 beats/min; blood pressure, 132/90 mm Hg; respiratory rate, 26; and SpO2 (saturation of oxyhemoglobin), 93% on room air. The patient complained primarily of shortness of breath and leg pain and was noted to be developing lower-extremity swelling and erythema bilaterally. The patient was evaluated for deep venous thrombosis and acute pulmonary embolus (PE) with both PE protocol chest computed tomography and bilateral duplex ultrasonography of his lower extremities. In addition, blood cultures were performed to evaluate for acute infection.
Chest computed tomography did not reveal evidence of PE but did demonstrate complete situs inversus and apparent cirrhosis with ascites; ultrasound revealed no venous thrombosis. His medical history included ingestion of approximately one to two beers per day for several years. He smoked one pack of cigarettes per day for more than 10 years and denied traveling outside of Tennessee since moving from Cambodia 17 years previously. The patient's initial laboratory analysis (compared to normal values) revealed creatine kinase, 261 units/liter (30 to 210 units/liter); white blood cell count, 2.3 × 103 cells/μl (3.9 × 103 to 10.3 × 103 cells/μl); platelets, 33,000/μl (135,000 to 370,000/μl); bicarbonate, 14 mmol/liter (23 to 30 mmol/liter), pH 7.34; blood urea nitrogen, 28 mg/dl (5 to 25 mg/dl); creatinine, 1.9 mg/dl (0.7 to 1.6 mg/dl); glucose, 65 mg/dl (70 to 110 mg/dl); international normalized ratio, 1.8; and total bilirubin, 2.8 mg/dl (0.2 to 1.2 mg/dl). The patient had a single episode of watery diarrhea while in the ED (a stool sample was not obtained), and his blood pressure became tenuous, with a systolic blood pressure in the 80s despite the rapid infusion of 3 liters of 0.9% saline. He was admitted to the medical intensive care unit (MICU) for further evaluation and management of presumed septic shock due to severe enteritis.
On his initial examination in the MICU, the patient's temperature was 96.7°F, his heart rate was 115 beats/min, his blood pressure was 96/62 mm Hg on norepinephrine and vasopressin infusions, his respiratory rate was 26, and his SpO2 was 93% on 3 liters per minute by nasal cannula. His sclerae were anicteric, and his liver edge was palpable 3 cm below the left costal margin. He had brawny, pitting edema of the lower extremities to the knees bilaterally. He became progressively less alert, and an additional history, including whether he ingested or simply handled the raw seafood present in the restaurant, was not obtainable. Over the next 4 hours, he developed purpuric, nonblanching skin lesions over the anterior and then posterior surfaces of his legs as well as on his inner thighs bilaterally; he had one such lesion on his left lower abdomen as well.
A repeated laboratory analysis revealed lactic acid, 14.8 mmol/liter; creatine kinase, 11,294 units/liter; bicarbonate, 10 mEq/liter (pH 7.15), with a PaO2 (alveolar partial pressure of O2) of 81 mm Hg on 3 liters per minute by nasal cannula; glucose, 29 mg/dl; and cortisol, 94.3 μg/dl. Broad-spectrum intravenous antibiotics were initiated, with piperacillin-tazobactam at 3.375 g every 6 h and vancomycin at 1 g every 12 h. Over the next 6 hours, the patient's blood pressure was supported with increasing doses of norepinephrine, and the patient was intubated. Meanwhile, the patient's lower-extremity lesions enlarged and became vesiculobullous (Fig. 1A), his distal lower-extremity pulses became weak, and his condition continued to deteriorate as evidenced by multiorgan failure. Surgical consultation was obtained, and the patient underwent through-the-knee amputations and adductor thigh fasciotomies bilaterally. The subsequent development of abdominal compartment syndrome prompted a bedside decompressive celiotomy in the surgical intensive care unit. Given the poor prognosis due to progressive multiorgan failure, aggressive support was withdrawn after consultation with the family. The patient died approximately 32 h after presentation to the ED.
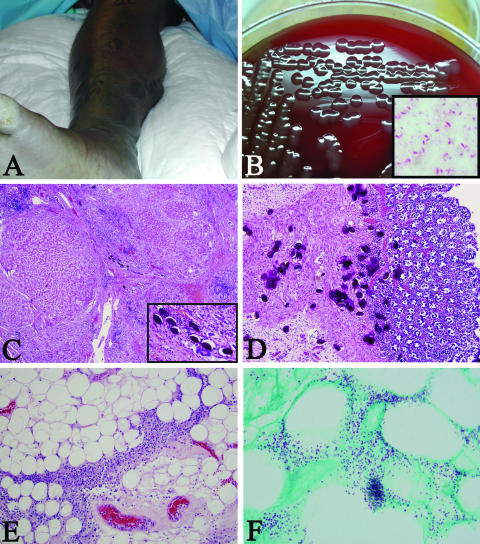
FIG. 1.
(A) Clinical view of the right lower extremity showing extensive bulla formation, erythema, and edema. (B) Isolated colonies on blood agar and a modified Gram stain (inset) of these colonies counterstained with carbolfuchsin, illustrating a slightly curved encapsulated (negative space) bacillus (inset, original magnification ×1,000). (C) Photomicrograph of the liver demonstrating macronodular cirrhosis, chronic hepatitis, and calcified Schistosoma ova (hematoxylin and eosin [H&E] stain; original magnification ×200; inset, original magnification ×400). (D) Photomicrograph of the large intestine showing numerous calcified Schistosoma ova (H&E stain; original magnification ×200). (E) Photomicrograph of the subcutis showing an intense mononuclear inflammatory infiltrate in a septal pattern (H&E stain; original magnification ×200). (F) Photomicrograph of gram-stained tissue illustrating numerous, small, gram-negative bacilli in the subcutaneous adipose tissue (phenol green tissue Gram stain; original magnification ×1,000).
Four premortem blood cultures and a wound culture of the left-leg amputation site were positive for an encapsulated, aerobic, gram-negative bacillus. These bacilli grew as mucoid colonies on blood agar (Fig. 1B) and were negative for lactose fermentation on MacConkey agar. The colonies were spot oxidase and indole test positive, and API 20E testing showed the organism to be positive for glucose fermentation and amygdalin. The organism was also found to be positive for ortho-nitrophenyl-β-d-galactopyranoside, lysine decarboxylase, and ornithine decarboxylase and negative for arginine dihydrolase. The API 20E identity determination was 99.9% for Vibrio vulnificus. Further work-up by conventional biochemical methods and growth characteristics performed at the Tennessee Department of Health Laboratory (TDOHL) confirmed the isolate to be Vibrio vulnificus. At the TDOHL, the organism did not grow in 0% NaCl and was motile. It was positive for indole, citrate, lysine, oxidase, and acetate analyses. It demonstrated acid production from glucose, lactose, salicin, maltose, cellobiose, and glycerol (at 48 h). It was negative for the utilization of malonate, sucrose, mannitol, dulcitol, adonitol, inositol, sorbitol, arabinose, raffinose, rhamnose, xylose, and mucate. The isolate was also negative for methyl red, Voges-Proskauer, arginine, and ornithine tests and did not produce urease or hydrolyze esculin.
PCR amplification of the first 500 base pairs of the isolate's 16S rRNA gene was performed using a MicroSeq 500 16S bacterial sequencing kit according to procedures described previously (30). Bi-directional sequences of the PCR amplification product were determined, and a phylogenetic analysis was performed with a MicroSeq Database Library (Applied Biosystems, Foster City, CA). The phylogenetic analysis (GenBank accession number DQ923054) revealed that the isolate was most closely related to V. vulnificus. Nucleotide sequences were 100% identical to a V. vulnificus prototype, ATCC 27562 (2, 25).
Antibiotic susceptibility testing using the Kirby-Bauer, broth dilution, and E-test methods showed the isolate to be pan-susceptible, with the following MICs: amikacin, 4.0 μg/ml; amoxicillin-clavulanic acid, 0.5 μg/ml; ampicillin, 1.0 μg/ml; ampicillin-sulbactam, 8.0 μg/ml; cefepime, 0.5 μg/ml; cefotaxime, 4.0 μg/ml; ceftazidime, 1.0 μg/ml; cephalothin, 8.0 μg/ml; ciprofloxacin, 0.06 μg/ml; gentamicin, 2.0 μg/ml; imipenem, 0.25 μg/ml; piperacillin, 4.0 μg/ml; sulfamethoxazole-trimethoprim, 0.5 μg/ml; piperacillin-tazobactam, 2.0 μg/ml; and tetracycline, 0.38 μg/ml (6, 7). A stool culture was not ordered or performed.
At autopsy, external examination of the patient revealed sloughing and bullae on the left side, the right thigh, and the right chest. There were no surgical scars noted, but the legs had been amputated through the knees.
Internally, the situs inversus discovered by premortem radiography was found to be complete, with no associated organ dysgenesis. Right (60 ml) and left (250 ml) serosanguinous pleural effusions were seen. There was extensive soft tissue edema, hemorrhage, and abscess formation in the right chest wall. No pulmonary emboli were identified, and the lungs were congested, with a combined weight of 2,350 g. Histologically, there was early acute pneumonia in the left upper lobe and focal hemorrhage and inflammation throughout all lung fields. Postmortem lung and blood bacterial cultures were negative.
In the abdominal cavity, there was 500 ml of serosanguinous fluid, and the wall of the pelvis was notable for soft tissue abscesses, hemorrhage, and necrosis of the psoas muscle. Hepatosplenomegaly was present, and the liver demonstrated macronodular cirrhosis, chronic hepatitis, and calcified Schistosoma sp. ova (Fig. 1C), each measuring approximately 70 μm by 50 μm. Grossly, there was a suggestion of a component of pipestem fibrosis that, upon microscopic examination, was obscured by advanced nodular cirrhosis with chronic hepatitis. Calcified Schistosoma ova were also extensively present in the large bowel (Fig. 1D).
Histologic sections of the surgically amputated lower legs showed subepidermal bullae formation with the presence of an intense mononuclear inflammatory infiltrate that was present in the dermis, subcutaneous adipose tissue, and muscle (Fig. 1E). Phenol green tissue Gram stain showed numerous, small, gram-negative bacilli at the dermal-epidermal junction, the deeper dermis, and the subcutaneous adipose tissue (Fig. 1F).
Necrotizing fasciitis due to Vibrio vulnificus can produce an overwhelming toxic shock-like syndrome that results in rapid deterioration and death (15, 16, 29, 32). It is markedly associated with chronic liver disease such as cirrhosis caused by chronic hepatitis B or C infection. Other high-risk conditions that predispose to severe infection with Vibrio vulnificus include hemochromatosis, current malignancy, AIDS and other immunocompromised states, and achlorhydria (14, 15). As yet, no superantigen has been implicated as a cause of this form of necrotizing fasciitis. The current literature suggests that host factors play a large role in the fulminant nature of the disease in these susceptible patients (4, 14). Exposure to the organism usually occurs through ingestion of shellfish or inoculation via traumatic injury in marine environments. It is likely that our patient either could have ingested raw shellfish or was exposed while preparing seafood at his place of employment.
Vibrio vulnificus is a halophilic, motile, comma-shaped, gram-negative bacillus from the family Vibrionaceae. It is associated with warm coastal waters such as the Gulf of Mexico and is seen during the warmer months, generally from mid-May to mid-September. The organism is isolated in high concentrations from shellfish (especially oysters), and during the summer months, it can be seen in concentrations as high as 103 to 104 CFU per gram of tissue (16, 31). In some epidemiologic studies, greater than 50% of shellfish and 11% of crabs harbor the organism during warmer months (18). Of 422 cases of Vibrio vulnificus infection reported to the CDC over a 9-year period, 43% were primary septicemia, 45% were wound infections, 5% were gastroenteritis, and in 7%, the source could not be determined (26). The overall case fatality rate is 25%, and infection is estimated to result in 90% of all deaths related to the ingestion of seafood (23). Primary septicemia is seen without an apparent source of inoculation and is attributed to the ingestion of contaminated shellfish, with blood-borne dissemination through the gastrointestinal tract, particularly in patients with cirrhosis.
High iron levels appear to be a marked predictor of the severity of infection with Vibrio vulnificus. Animal studies indicate that high levels of iron can lower the 50% lethal dose of Vibrio vulnificus to one cell in mice (14, 16). This appears to be related to iron sequestration from transferrin and hemoglobin and the production of siderophores (14, 17, 31, 33). Cirrhosis is also a significant risk factor, likely related to dysfunction in portal drainage, hepatocellular functions, and impaired iron metabolism (5, 14, 17, 27, 31, 33). Capsular polysaccharide and lipopolysaccharide help to prevent complement-mediated lysis and also induce the characteristically potent systemic inflammatory response syndrome seen in these patients (5, 14, 27). Other important virulence factors for infection with Vibrio vulnificus include pili for attachment and colonization and cytolysins, cytotoxins (RtxA), and metalloproteases that facilitate tissue infiltration and destruction, intravascular dissemination, and hemolysis (5, 14, 27).
As determined serologically, our patient had chronic liver disease secondary to previously undiagnosed chronic hepatitis B infection. Autopsy histology confirmed the chronic hepatitis and also demonstrated established cirrhosis as well as evidence of previous schistosoma infection in the liver and colon. Gross examination of the liver suggested a possible component of pipestem fibrosis that was obscured microscopically by advanced cirrhosis. Pipestem fibrosis is a characteristic finding seen in advanced schistosome-induced liver disease. It might be possible that the patient's schistosomal infection contributed to his liver disease, although to what extent is uncertain. No definitive histologic evidence was present (e.g., no spines were seen) that would indicate the species of schistosome, but based on the patient's history of previous residence in Cambodia and the ovum size (70 μm by 50 μm), the most likely organism would be Schistosoma mekongi.
The histopathology of Vibrio vulnificus is usually revealed as an intense cellulitis involving the skin and subcutaneous tissues in a septal distribution (3). Numerous bacteria are found in the superficial dermis, and bullae that form at the dermal-epidermal interface are devoid of inflammatory cells. The infiltrate is usually neutrophilic, which makes our case unusual in that the infiltrate was composed of mostly mononuclear cells (Fig. 1E) (3). This may be related to the rapidity of the development of overwhelming infection and possibly to a relatively immunosuppressed state secondary to chronic liver disease. Characteristically, there are vessel thrombosis and numerous perivascular accumulations of bacteria, and vascular and perivascular neutrophils are present without fibrinoid necrosis. The inflammation, as in our case, can also extend to involve adjacent muscle, with extensive myositis (3).
Laboratory diagnosis of Vibrio vulnificus infection can be performed by using stool, blood, or lesion cultures. The Gram stain shows a mildly pleomorphic, slightly curved gram-negative bacillus. It grows well in peptone water enrichment broth and can then be subcultured on thiosulfate citrate bile salts sucrose (TCBS) agar, which exhibits green colonies. Direct inoculation of stool specimens on TCBS is routinely performed. The organism grows well on 5% sheep blood and chocolate agars, as well as in broths of blood culture systems, brain-heart infusion broth, and thioglycolate broth (12). On blood agar, the colonies are medium to large, smooth, and opaque and have a greenish color (Fig. 1B). The organism also grows on MacConkey agar but shows variable demonstration of lactose fermentation (12). In our case, the organism appeared to be lactose negative on MacConkey agar but demonstrated the ability to ferment lactose by a traditional biochemical test. A spot oxidase result is positive, as is the case for Pseudomonas spp., and the spot indole test is usually positive (Pseudomonas spp. are indole negative), suggesting Vibrio, Aeromonas, or Plesiomonas spp. Some Vibrio species are spot indole negative. The organism is sensitive to the vibriostatic compound O/129 (aeromonads are resistant), positive for the string test, and grows at higher salt concentrations but does not grow at 0% NaCl (as was seen in our patient) (10, 11). Commercial biochemical test kits (e.g., API 20E) may differentiate Vibrio vulnificus from Aeromonas or Plesiomonas spp., but several reports show evidence of an inability to accurately differentiate these organisms (1, 8-10, 20). A useful alternative to biochemical methods in the identification of the cause of necrotizing fasciitis is that of bacterial 16S rRNA gene sequencing analysis, which has the ability to accurately confirm the identity of V. vulnificus and differentiate it from other organisms such as Aeromonas and Plesiomonas (2, 9, 21, 22, 24, 25). Antibiotics and surgical debridement are mandatory early in the course of severe infection in order to reduce morbidity and mortality. A broad-spectrum cephalosporin (ceftazidime or cefotaxime) plus doxycycline or minocycline is considered the first-line antimicrobial agent, with fluoroquinolones being second-line drugs (13, 19, 28).
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Abbott, S. L., L. S. Seli, M. Catino, Jr., M. A. Hartley, and J. M. Janda. 1998. Misidentification of unusual Aeromonas species as members of the genus Vibrio: a continuing problem. J. Clin. Microbiol. 36:1103-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aznar, R., W. Ludwig, R. I. Amann, and K. H. Schleifer. 1994. Sequence determination of rRNA genes of pathogenic Vibrio species and whole-cell identification of Vibrio vulnificus with rRNA-targeted oligonucleotide probes. Int. J. Syst. Bacteriol. 44:330-337. [DOI] [PubMed] [Google Scholar]
- 3.Bednov, Y., and E. Beckman. 1997. Vibrio vulnificus, p. 889-896. In D. H. Connor, F. W. Chandler, and D. A. Schwartz (ed.), Pathology of infectious diseases, 6th ed., vol. I. Appleton and Lange, Stamford, CT. [Google Scholar]
- 4.Borenstein, M., and F. Kerdel. 2003. Infections with Vibrio vulnificus. Dermatol. Clin. 21:245-248. [DOI] [PubMed] [Google Scholar]
- 5.Chiang, S. R., and Y. C. Chuang. 2003. Vibrio vulnificus infection: clinical manifestations, pathogenesis, and antimicrobial therapy. J Microbiol. Immunol. Infect. 36:81-88. [PubMed] [Google Scholar]
- 6.CLSI. 2006. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, M45-A, no. 26, vol. 25. Clinical Laboratory Standards Institute, Wayne, PA.
- 7.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M7-A7, no. 2, vol. 26. Clinical Laboratory Standards Institute, Wayne, PA.
- 8.Colodner, R., R. Raz, I. Meir, T. Lazarovich, L. Lerner, J. Kopelowitz, Y. Keness, W. Sakran, S. Ken-Dror, and N. Bisharat. 2004. Identification of the emerging pathogen Vibrio vulnificus biotype 3 by commercially available phenotypic methods. J. Clin. Microbiol. 42:4137-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalsgaard, A., I. Dalsgaard, L. Hoi, and J. L. Larsen. 1996. Comparison of a commercial biochemical kit and an oligonucleotide probe for identification of environmental isolates of Vibrio vulnificus. Lett. Appl. Microbiol. 22:184-188. [DOI] [PubMed] [Google Scholar]
- 10.Farmer, J. J., and J. M. Janda. 2005. Vibrionaceae, p. 491-555. In D. J. Brenner, N. R. Krieg, J. T. Staley, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2. Springer-Verlag, New York, NY. [Google Scholar]
- 11.Farmer, J. J., J. M. Janda, and K. Birkhead. 2003. Vibrio, p. 701-718. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 12.Forbes, B. A., D. F. Sahm, and A. S. Weissfeld. 2002. Vibrio, Aeromonas, Pleisiomonas, Shigelloides, and Chromobacterium violaceum, p. 423-433. In B. A. Forbes, D. F. Sahm, and A. S. Weissfeld (ed.), Bailey & Scott's diagnostic microbiology, 11th ed. Mosby, Inc., St. Louis, MO.
- 13.Gilbert, D. N., R. C. Moellering, G. M. Eliopoulos, and M. A. Sande. 2005. The Sandford guide to antimicrobial therapy, 25th ed. Antimicrobial Therapy, Inc., Sperryville, VA.
- 14.Gulig, P. A., K. L. Bourdage, and A. M. Starks. 2005. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 43:118-131. [PubMed] [Google Scholar]
- 15.Haq, S. M., and H. H. Dayal. 2005. Chronic liver disease and consumption of raw oysters: a potentially lethal combination—a review of Vibrio vulnificus septicemia. Am. J. Gastroenterol. 100:1195-1199. [DOI] [PubMed] [Google Scholar]
- 16.Harwood, V. J., J. P. Gandhi, and A. C. Wright. 2004. Methods for isolation and confirmation of Vibrio vulnificus from oysters and environmental sources: a review. J. Microbiol. Methods 59:301-316. [DOI] [PubMed] [Google Scholar]
- 17.Helms, S. D., J. D. Oliver, and J. C. Travis. 1984. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect. Immun. 45:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.H'Ng, M. W., W. Y. Chew, and B. K. Tan. 2005. Necrotizing fasciitis caused by Vibrio vulnificus: a review of four cases in a Singapore tertiary hospital. J. Trauma 59:482-485. [DOI] [PubMed] [Google Scholar]
- 19.Hsueh, P. R., C. Y. Lin, H. J. Tang, H. C. Lee, J. W. Liu, Y. C. Liu, and Y. C. Chuang. 2004. Vibrio vulnificus in Taiwan. Emerg. Infect. Dis. 10:1363-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israil, A. M., M. C. Balotescu, I. Alexandru, and G. Dobre. 2003. Discordancies between classical and API 20E microtest biochemical identification of Vibrio and Aeromonas strains. Bacteriol. Virusol. Parazitol. Epidemiol. 48:141-143. (In Romanian.) [PubMed] [Google Scholar]
- 21.Muldrew, K. L., J. F. Simpson, C. W. Stratton, and Y. W. Tang. 2005. Molecular diagnosis of necrotizing fasciitis by 16S rRNA gene sequencing and superantigen gene detection. J. Mol. Diagn. 7:641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagao, M., Y. Shimizu, Y. Kawada, H. Baba, K. Yamada, K. Torii, and M. Ohta. 2006. Two cases of sucrose-fermenting Vibrio vulnificus infection in which 16S rDNA sequencing was useful for diagnosis. Jpn. J. Infect. Dis. 59:108-110. [PubMed] [Google Scholar]
- 23.Neil, M. A., and C. J. Carpenter. 2005. Other pathogenic vibrios, p. 2545-2548. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. II. Elsevier, Inc., Philadelphia, PA. [Google Scholar]
- 24.Rosche, T. M., Y. Yano, and J. D. Oliver. 2005. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 49:381-389. [DOI] [PubMed] [Google Scholar]
- 25.Ruimy, R., V. Breittmayer, P. Elbaze, B. Lafay, O. Boussemart, M. Gauthier, and R. Christen. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 44:416-426. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro, R. L., S. Altekruse, L. Hutwagner, R. Bishop, R. Hammond, S. Wilson, B. Ray, S. Thompson, R. V. Tauxe, and P. M. Griffin for the Vibrio Working Group. 1998. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988-1996. J. Infect. Dis. 178:752-759. [DOI] [PubMed] [Google Scholar]
- 27.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 28.Tang, H.-J., M.-C. Chang, W.-C. Ko, K.-Y. Huang, C.-L. Lee, and Y.-C. Chuang. 2002. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob. Agents Chemother. 46:3580-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang, W. M., K. K. Fung, V. C. Cheng, and L. Lucke. 2006. Rapidly progressive necrotising fasciitis following a stonefish sting: a report of two cases. J. Orthop. Surg. (Hong Kong) 14:67-70. [DOI] [PubMed] [Google Scholar]
- 30.Tang, Y.-W., A. Von Graevenitz, M. G. Waddington, M. K. Hopkins, D. H. Smith, H. Li, C. P. Kolbert, S. O. Montgomery, and D. H. Persing. 2000. Identification of coryneform bacterial isolates by ribosomal DNA sequence analysis. J. Clin. Microbiol. 38:1676-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulusarac, O., and E. Carter. 2004. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South. Med. J. 97:163-168. [DOI] [PubMed] [Google Scholar]
- 32.Vecer, J., H. Kubatova, P. Hanek, A. Esnerova, H. Klazarova, and K. M. Lochmann. 2000. Severe sepsis with organ failure in necrotizing fasciitis of the right leg caused by infection with Vibrio vulnificus. Vnitr Lek 46:423-425. (In Czech.) [PubMed] [Google Scholar]
- 33.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]