Abstract
A young woman developed multiple abscesses in her transplanted kidney. Amplification of the 16S rRNA gene with subsequent sequencing revealed Ureaplasma urealyticum as the infectious agent. Microbiological diagnosis and sensitivity testing led to therapy with levofloxacin, resulting in rapid recovery of the patient.
CASE REPORT
A 19-year-old young woman presented with abdominal pain, dysuria, macrohematuria, loss of weight (5 kg during the previous 6 weeks), and general fatigue. She had a history of juvenile nephronophthisis (diagnosed at the age of 9 years), leading to kidney transplantation at the age of 11. Transplant function was excellent, and primary immunosuppression therapy included tacrolimus and prednisolone. The patient was diagnosed with a posttransplant lymphoproliferative disease 6 years later. Histology showed a B-cell lymphoma, and immunosuppression therapy was reduced and switched to sirolimus (5 mg/kg/day) and prednisolone (5 mg/day). The patient also received four doses of rituximab (600 mg each). The posttransplant lymphoproliferative disease regressed rapidly, and the patient had no further evidence of recurrence during 2 years of follow-up. One month prior to presentation, a unilateral ovariectomy was performed because of a bleeding ovarian cyst. The patient had never experienced urinary tract infections.
Laboratory investigations showed an elevated serum creatinine level (1.63 mg/dl; previous baseline, 0.8 mg/dl), a slightly elevated C-reactive protein (CRP) level (2.1 mg/dl), and massive leucocyturia (1,000 white blood cells [WBC]/μl). The differential WBC analysis showed a shift to the left (rods, 11%; segmented neutrophils, 63%; lymphocytes, 34%; eosinophils, 2%; basophils, 1%), but the total WBC count was not elevated (9.15/nl); the hemoglobin level was 9.0 mg/dl. Immunosuppression therapy at this time consisted of sirolimus (trough level, 17 ng/ml; highly elevated) and prednisolone (5 mg/day).
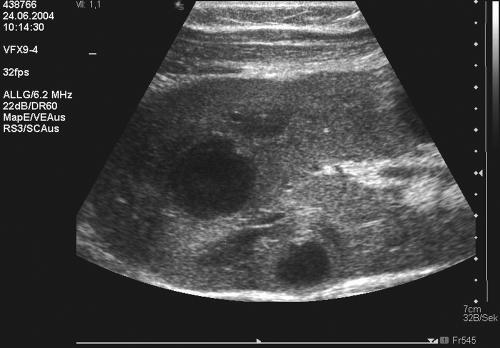
The ultrasound of the kidney transplant showed multiple abscesses (maximal diameter, 0.5 cm) which were diffusely distributed throughout the whole kidney (Fig. 1).
FIG. 1.
Ultrasound image showing extensive intrarenal abscess formations in the transplanted kidney.
With the diagnosis of transplant pyelonephritis with intrarenal bacterial abscess formations, the patient was treated with ampicillin (120 mg/kg/day) and ceftazidime (90 mg/kg/day) for 3 weeks. However, the patient did not respond to this therapy and developed persistent hyperthermia up to 40°C with little response to antipyretics. The CRP level rose to 10 mg/dl, and the intrarenal abscess formations showed an increase in volume up to Ø of 2 cm, while the serum creatinine increased to 2.9 mg/dl; urine output was sufficient at all times. After 10 days of treatment, the general condition of the young woman had not improved, and antibiotic therapy was supplemented with imipenem/cilastin (40 mg/kg/day).
Repeated cultures of blood and urine remained sterile with conventional culture methods. Puncture of three abscesses resulted in purulent material. On microbiological examination, cultures for aerobic and anaerobic bacteria, fungi, and mycobacteria, as well as PCR for Mycobacterium tuberculosis complex (COBAS AMPLICOR; Roche diagnostics) were negative. Subsequently, DNA extracted from the two aspirates was submitted to eubacterial amplification of the 16S rRNA gene using primers TPU1 (corresponding to complementary positions 8 to 27 in the Escherichia coli 16S rRNA gene (4) and RTU 3 (corresponding to complementary positions 519 to 536 in the Escherichia coli 16S rRNA gene (4), as described earlier (13). Sequencing of the obtained PCR products resulted in 434- or 432-bp fragments with highest homology to Ureaplasma urealyticum 16S rRNA genes of the former T960 biovar complex in both materials (100% identity to U. urealyticum serovars 2, 4, 5, 7, 8, 9, 10, 11, 12, and 13 (16). For prevention of cross-contamination, all molecular assays were performed in a separate molecular diagnostic unit, following the guidelines of good laboratory practice, including strict separation of DNA extraction, pre- and postamplification analysis, and UDP prophylaxis.
Subsequently, vaginal, cervical, and urethral smears were successfully cultured for Ureaplasma, using specified culture techniques (“Mycoplasma Duo”; Bio-Rad). For approximate quantification, serial dilutions were inoculated in the Mycoplasma Duo plates according to the manufacturer's recommendations. Color changes of phenol red, indicating hydrolysis of urea, were documented. This assay allows discrimination between ≥104 color-changing units (CCU) per ml, ≤103 CCU/ml, or negative samples. Species identification of the isolates was again confirmed using 16S rRNA gene sequencing. Urine samples were negative for Ureaplasma, both in cultures and by PCR (Table 1).
TABLE 1.
Diagnostic studies
| Datea | Material tested | Result by culturec
|
Result by PCRc
|
||||
|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Mycobacteria | U. urealyticum | MTBCf | U. urealyticumg | ||
| 24.06. | MSUb | Neg | Neg | ND | ND | ND | ND |
| 01.07. | Abscess aspirate | Negd | Neg | Neg | ND | Neg | Pos |
| 22.07. | Urethra smear sample | NDe | ND | ND | Pos; CCU, ≥104/ml | ND | Pos |
| Vagina smear sample | ND | ND | ND | Pos; CCU, ≥104/ml | ND | Pos | |
| Cervix smear sample | ND | ND | ND | Pos; CCU, ≤103/ml | ND | Pos | |
| 28.07. | Abscess (1) aspirate | Neg | Neg | ND | Neg | ND | Pos |
| Abscess (2) aspirate | Neg | Neg | ND | Neg | ND | Pos | |
| Abscess (3) aspirate | Neg | Neg | ND | Neg | ND | Pos | |
Expressed as day.month.
MSU, midstream urine.
Pos, positive; Neg, negative.
Negative for aerobic and anaerobic bacteria.
ND, not determined.
Mycobacterium tuberculosis complex.
Pos, positive by PCR and sequencing.
A second puncture remained culture negative for Ureaplasma, although eubacterial PCR and sequencing again verified the diagnosis.
Antimicrobial susceptibility testing was performed using the E-test (AB Biodisk, Solna, Sweden) on selective agar plates containing urea and phenol red (Mycoplasma/Ureaplasma selective agar; Oxoid, Wesel, Germany) under CO2 incubation. Agar plates were inoculated with a high concentration of Ureaplasma (approximately 105 CCU/ml), because at lower bacterial count, end-point selection is critical. The medium color change from yellow to red, indicating Ureaplasma growth, was clearly visible after 24 h. Furthermore, growth was documented as observed after 4 days using a stereomicroscope, with drug MICs as shown in Table 2 (20).
TABLE 2.
E-tests for U. urealyticum isolate and MIC ranges for Ureaplasma spp.a
| Antibiotic | MIC (μg/ml) for U. urealyticum isolate | MIC range (μg/ml) for Ureaplasma spp. |
|---|---|---|
| Doxycycline | <0.016 | 0.02-1 |
| Erythromycin | 12 | 0.02-4 |
| Azithromycin | 0.125 | 0.5-4 |
| Ciprofloxacin | 4 | 0.1-16 |
| Levofloxin | 0.125 | 0.2-1 |
| Moxifloxacin | 0.5 | 0.12-0.5 |
Data from reference 20 (Waites et al., 2001).
After the diagnosis, therapy was switched to levofloxacin (10 mg/kg/day), followed by rapid recovery of the patient with a decrease of inflammatory parameters (CRP, 0.75 mg/dl after 8 weeks), renal functional parameters (creatinine level, 0.8 mg/dl after 8 weeks), and disappearance of abscesses on ultrasound examination. It is important to state that fluoroquinolones are the only bactericidal antimicrobial drugs for treatment of systemic Ureaplasma infections, which is especially crucial for immunocompromised patients.
Follow-up cultures and swabs showed no evidence of further Ureaplasma growth.
Viral and bacterial infections are a frequent complication after renal transplantation, requiring close surveillance of immunosuppressive therapy and a high level of suspicion in the presence of unusual symptoms.
The present case is remarkable for three reasons. (i) U. urealyticum has never been reported previously as a cause of abscess formation in a transplanted kidney. To our knowledge, the complication of intrarenal abscess has been described only once previously, occurring in a female adult patient 10 years after renal transplantation due to infection with E. coli (15). Renal abscesses seem to be infrequent both in adults (9) and in children (3, 5, 17) and may be caused by a variety of gram-positive and gram-negative organisms. Ureaplasma is a weak pathogen and is frequently found in the urogenital tracts of healthy asymptomatic adults. It has long been suspected that Ureaplasma could be of pathogenic significance for immunocompromised patients, i.e., patients after renal transplantation. Early reports have found no difference in the prevalence of Ureaplasma colonization in healthy individuals compared to the prevalence seen with hemodialysis and renal transplant patients (2). In a study of 123 patients with a functioning renal transplant, the colonization rate was 11% and colonization was not associated with a decline of function of the grafted kidney (1). In immunocompetent individuals, Ureaplasma has an etiologic role in male urethritis and in females during periods of pregnancy, as well as in newborns (8). However, Ureaplasma may occasionally cause more-severe disease, i.e., pneumonia or arthritis, in patients with immunodeficiencies; nevertheless, the occurrence of major infectious complications with Mycoplasma and Ureaplasma seems to be low both in patients with hypogammaglobulinemia and in those with human immunodeficiency virus infection (18). In the present case, U. urealyticum could clearly be demonstrated as the causative agent involved in intrarenal abscess formation. However, it cannot be decided whether the spread of infection was by the ascending route or by hematogenous seed. The patient denied any previous or recent sexual activity.
(ii) Several reports now indicate that immunosuppression with so-called target-of-rapamycin inhibitors, such as sirolimus or everolimus, may be associated with an increased rate of bacterial infections, including infections by opportunistic organisms (10, 11). The rate of overall bacterial infection was significantly higher in everolimus-treated transplant patients than in azathioprine-treated patients (6). Rapamycin has potent antimigratory and antiproliferative effects and is associated with a dysregulation of the innate immune response, including a decrease in interleukin-10 gene transcription (12). Thaunat et al. reported two cases of pulmonary infection caused by Mycobacterium xenopi during sirolimus therapy (19). Furthermore, sirolimus may increase the susceptibility to bacterial infection by inhibiting the oxidative burst potential of circulating neutrophils (7). In the present case, the sirolimus level was highly elevated. We suspected erroneous intake of sirolimus in this otherwise highly compliant individual. Alternatively, the previous treatment with rituximab may have been implicated in a higher susceptibility to bacterial infections, although during 2 years of follow-up and at presentation, lymphocyte blood counts and T-cell subsets were normal.
(iii) This report also demonstrates the power of molecular-biological methods in diagnosing unusual bacterial infections. Eubacterial PCR amplification of the 16S rRNA genes in clinical samples with subsequent sequencing of the PCR product is laborious and carries a high risk of contamination. Furthermore, it cannot differentiate between live or dead bacteria (14). It should therefore not be regarded as a routine method but should be restricted to culture-negative samples that were obtained under sterile conditions. However, in the presented case, PCR yielded the univocal diagnosis of U. urealyticum as a causative agent, which could not be obtained with any other technique. The involvement of another bacterial species is almost excluded, since PCR products of different samples resulted in clear sequencing results.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Birch, D. F., A. J. D'Apice, and K. F. Fairley. 1981. Ureaplasma urealyticum in the upper urinary tracts of renal allograft recipients. J. Infect. Dis. 144:123-127. [DOI] [PubMed] [Google Scholar]
- 2.Bollmann, R., W. Kohler, and M. Mehl. 1984. Cultural and serologic studies of the colonization of mycoplasmas in hemodialysis and kidney transplant patients. Z. Urol. Nephrol. 77:671-677. (In German.) [PubMed] [Google Scholar]
- 3.Brook, I. 1994. The role of anaerobic bacteria in perinephric and renal abscesses in children. Pediatrics 93:261-264. [PubMed] [Google Scholar]
- 4.Brosius, J., M. L. Palmer, P. J. Kennedy, and H. F. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dembry, L. M., and V. T. Andriole. 1997. Renal and perirenal abscesses. Infect. Dis. Clin. N. Am. 11:663-680. [DOI] [PubMed] [Google Scholar]
- 6.Eisen, H. J., E. M. Tuzcu, R. Dorent, J. Kobashigawa, D. Mancini, H. A. Valantine-von Kaeppler, R. C. Starling, K. Sorensen, M. Hummel, J. M. Lind, K. H. Abeywickrama, and P. Bernhardt. 2003. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N. Engl. J. Med. 349:847-858. [DOI] [PubMed] [Google Scholar]
- 7.Gee, I., A. K. Trull, S. C. Charman, and G. J. Alexander. 2003. Sirolimus inhibits oxidative burst activity in transplant recipients. Transplantation 76:1766-1768. [DOI] [PubMed] [Google Scholar]
- 8.Horner, P., B. Thomas, C. B. Gilroy, M. Egger, and D. Taylor-Robinson. 2001. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin. Infect. Dis. 32:995-1003. [DOI] [PubMed] [Google Scholar]
- 9.Hoverman, I. V., L. O. Gentry, D. W. Jones, and W. G. Guerriero. 1980. Intrarenal abscess. Report of 14 cases. Arch. Intern. Med. 140:914-916. [DOI] [PubMed] [Google Scholar]
- 10.Hymes, L. C., and B. L. Warshaw. 2005. Sirolimus in pediatric patients: results in the first 6 months post-renal transplant. Pediatr. Transplant. 9:520-522. [DOI] [PubMed] [Google Scholar]
- 11.Ibáñez, J. P., M. L. Monteverde, J. Goldberg, M. A. Diaz, and A. Turconi. 2005. Sirolimus in pediatric renal transplantation. Transplant. Proc. 37:682-684. [DOI] [PubMed] [Google Scholar]
- 12.Jøorgensen, P. F., J. E. Wang, M. Almlof, R. Solberg, C. Okkenhaug, T. Scholz, C. Thiemermann, S. J. Foster, and A. O. Aasen. 2001. Sirolimus interferes with the innate response to bacterial products in human whole blood by attenuation of IL-10 production. Scand. J. Immunol. 53:184-191. [DOI] [PubMed] [Google Scholar]
- 13.Moter, A., C. Hoenig, B. K. Choi, B. Riep, and U. B. Gobel. 1998. Molecular epidemiology of oral treponemes associated with periodontal disease. J. Clin. Microbiol. 36:1399-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters, R. P., M. A. van Agtmael, S. A. Danner, P. H. Savelkoul, and C. M. Vandenbroucke-Grauls. 2004. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 4:751-760. [DOI] [PubMed] [Google Scholar]
- 15.Rao, M. M., P. H. Vaska, L. A. Albertyn, and T. H. Mathew. 1983. Intrarenal abscess in a transplant organ. J. Urol. 130:971-972. [DOI] [PubMed] [Google Scholar]
- 16.Robertson, J. A., G. W. Stemke, J. W. Davis, Jr., R. Harasawa, D. Thirkell, F. Kong, M. C. Shepard, and D. K. Ford. 2002. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int. J. Syst. Evol. Microbiol. 52:587-597. [DOI] [PubMed] [Google Scholar]
- 17.Sood, S. K., D. Mulvihill, and R. S. Daum. 1989. Intrarenal abscess caused by Klebsiella pneumoniae in a neonate: modern management and diagnosis. Am. J. Perinatol. 6:367-370. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Robinson, D., P. M. Furr, and A. D. Webster. 1986. Ureaplasma urealyticum in the immunocompromised host. Pediatr. Infect. Dis. 5:S236-S238. [DOI] [PubMed] [Google Scholar]
- 19.Thaunat, O., E. Morelon, M. Stern, P. Buffet, C. Offredo, M. F. Mamzer-Bruneel, and H. Kreis. 2004. Mycobacterium xenopi pulmonary infection in two renal transplant recipients under sirolimus therapy. Transpl. Infect. Dis. 6:179-182. [DOI] [PubMed] [Google Scholar]
- 20.Waites, K., C. M. Bebear, J. A. Robertson, D. F. Talkington, and G. E. Kenny. 2001. Antimicrobial susceptibility testing, p. 16. In Cumitech 34, Laboratory diagnosis of mycoplasmal infections. Coordinating ed., F. S. Nolte. ASM Press, Washington, DC.