Abstract
Here we report how variability in the human cytomegalovirus genome sequence may seriously affect the outcome of its molecular diagnosis. A real-time quantitative PCR assay targeting the major immediate-early gene failed to detect the viral load in some, but not all, clinical samples from hematooncological patients. By amplification and sequencing the DNA across the regions targeted by this assay we found a number of nucleotide substitutions which accounted for decreased primer/probe binding. This decreased the sensitivity of the assay up to 1,000-fold.
Human cytomegalovirus (CMV) persists in the host organism for a lifetime and becomes frequently reactivated in immunocompromised patients, such as recipients of bone marrow or solid organ transplants (1, 15, 20). Establishment of a reliable real-time quantitative PCR assay (Q-PCR) for detection of CMV is crucial for successful management of CMV infection. Despite the importance of precise diagnosis of CMV, a generally accepted methodology has not yet been established. Optimal detection of CMV DNA in all clinical samples can be achieved only if primer sequences capable of detecting all the CMV strains are used. A number of different genes are used as a target for quantitative detection of CMV, including the major immediate-early (MIE) gene (5, 10, 12), the phosphoprotein 65 (pp65) gene (6, 7, 18), and the glycoprotein B (gB) gene (9, 11). Some groups use duplex assays to detect two different genes simultaneously (2, 8).
The coding sequence for the MIE gene consists of three exons and has long been assumed to be highly conserved due to the important function that MIE plays in the CMV life cycle and from sequence comparisons of the MIE genes from laboratory strains AD169 and Towne. Later studies described significant sequence variability in some parts of this gene, mainly in its exon 4 (3, 4, 16, 21). Zweygberg Wirgart et al. (21) found the MIE exon 4 sequence to be more variable than the CMV DNA polymerase gene or the glycoprotein B gene. Despite these findings, the MIE gene is still a popular target sequence for routine CMV diagnostics.
In our laboratory, we simultaneously used the Q-PCR assay published by Tanaka et al. (19) that detects a part of exon 4 of the MIE gene (primers RQ1 and RQ2 and RQ probe) and an in-house qualitative PCR assay that detects a region between exon 2 and exon 4 of the same gene (primers CMV 2-4F and CMV 2-4R). Table 1 has further details on the assays and primer/probe sequences. We compared the sensitivities of these two methods by amplifying 10-fold dilutions of 1 × 107 copies of CMV DNA, which had been isolated from the CMV strain AD169 (each dilution was tested in triplicate). The Q-PCR has proven to be about 10 times more sensitive than the qualitative method. The Q-PCR detection limit was 10 copies of CMV DNA apart from 100 copies detected by qualitative PCR.
TABLE 1.
Primers, probes, and PCR specifications used for amplification of various parts of the MIE genea
| Primer/probe designation | Sequence, 5′→3′ | Genomic coordinateb | Cycling conditions |
|---|---|---|---|
| RQ1 | GACTAGTGTGATGCTGGCCAAG | 2719 | 95°C/10 min → 50 times (95°C/15 s → 60°C/1 min) |
| RQ2 | GCTACAATAGCCTCTTCCTCATCTG | 2919 | |
| RQ probe | AGCCTGAGGTTATCAGTGTAATGAAGCGCC | 2758 | |
| CMV 2-4F | GACCCTGATAATCCTGACGA | 1506 | 95°C/10 min → 35 times (95°C/30 s → 53°C/30 s → |
| CMV 2-4R | ATGTGCTCCTTGATTCTATG | 2073 | 72°C/30 s) → 72°C/7 min |
| exon4celyF | GAAATTCACTGGCGCCTTTA | 2101 | 95°C/3 min → 35 times (95°C/1 min → 53°C/1 min → |
| exon4celyR | AGCACCATCCTCCTCTTCCT | 3169 | 72°C/2 min) → 72°C/7 min |
| CMV4F | AGTGAGTTCTGTCGGGTGCT | 2678 | 95°C/3 min → 25 times (95°C/30 s → 53°C/30 s → |
| CMV4R | CCCTCCTCCTCTTCCTCATC | 3075 | 72°C/1 min) → 72°C/7 min |
RQ1, RQ2, and RQ probe, primers and probe for Q-PCR; CMV 2-4F and CMV 2-4R, primers for qualitative PCR; exon4celyF, exon4celyR, CMV4F, and CMV4R, primers for nested PCR and consecutive sequencing.
MIE gene sequence (GenBank accession no. M21295).
Between January 2004 and January 2005 we assayed 1,854 samples from 363 patients including immunocompromised patients with hematological disorders, patients with bone marrow transplants, and/or recipients of peripheral blood stem cells. DNA isolated from 4 × 106 peripheral blood leukocytes (using the QIAamp blood kit [QIAGEN, Germany]) was used as a template. The Q-PCR detected virus reactivation in 64 patients. In contrast, Q-PCR repeatedly failed to detect any viral load in 18 patients who were previously shown as CMV positive using the less-sensitive qualitative method. We hypothesized that such a discrepancy could be explained by primer/probe mismatch due to sequence variability in the MIE exon 4.
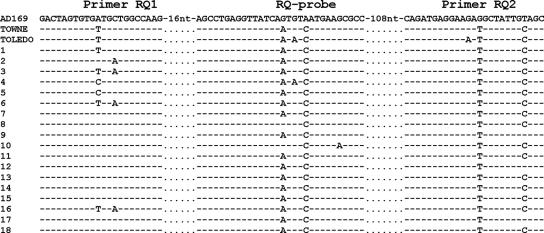
Therefore, we performed a sequence analysis of the Q-PCR target region (nucleotide positions 2719 to 2919, GenBank accession no. M21295) in samples from all 18 patients with false-negative results. We designed two pairs of primers spanning this region and used nested PCR to obtain sufficient amounts of PCR product for sequencing (Table 1 shows primer sequences and locations). Both strands were sequenced using the inner primers and the BigDye Terminator v1.1 Ready Reaction Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) on an ABI PRISM 310 Analyzer. As a control group we sequenced 10 samples in which viral copies were detected by Q-PCR. We used Clustal W (freeware, www.ebi.ac.uk/clustalw/) to align the obtained sequences with the available sequences of laboratory strains AD169, Towne, and Toledo (results are compiled in Fig. 1).
FIG. 1.
Sequence comparison of selected areas of the MIE gene exon 4 of the laboratory strains AD169, Towne, and Toledo with those of clinical isolates. Dashes indicate homology to AD169.
We found a number of nucleotide substitutions in both primers and probe sequence in all 18 false-negative samples. In seven samples, there were substitutions in the forward primer sequence, and all samples had substitutions in the probe and reverse primer regions. When the sequences were compared to the sequences of different laboratory strains, they were found to be more similar to strains Towne and Toledo than to AD169, which served as the template for designing the assay (19). Sequences of all 10 samples of the control group were homologous to the laboratory strain AD169. No association between the sequence of MIE exon 4 and sex, age, clinical diagnosis, and the progression of CMV infection was observed.
We also investigated the effects of primer/probe template mismatches on PCR efficiency. We cloned the PCR products (amplified with primers CMV4F and CMV4R) from AD169 (no mismatch), sample 13 (four mismatches), and sample 3 (six mismatches) into plasmids and used them as templates for real-time PCR (each sample was tested in triplicate). Comparisons of average cycle threshold values for 10-fold dilutions (106 to 102 copies/tube) revealed major differences between plasmid DNA with mismatches and that without mismatches. On average, plasmid derived from sample 13 (four mismatches) was amplified 6.4 cycles later than the mismatch-free plasmid. This represents sensitivity almost 100 times lower (assuming the average 3.32-cycle difference between 10-fold dilutions of standard DNA). Plasmid derived from sample 3 (six mismatches) was amplified about 12.3 cycles later than mismatch-free plasmid. This represents sensitivity over 1,000 times lower.
Genetic variability of CMV has been reported several times (13, 14), and this study documents how seriously this variability can influence PCR results. Considering that CMV is the most frequent cause of life-threatening infections in immunocompromised patients, its diagnosis must be fast and precise. Our data show that the assay published by Tanaka et al. (19) would not detect CMV in 21.9% of positive samples (18/82) from our patients. These samples would have been misjudged as CMV negative without simultaneous use of two PCR assays, and patients could therefore have been at risk of progression of a fatal CMV disease.
Based on our experience and previously published data we strongly recommend not using exon 4 of the MIE gene for routine PCR diagnostics. Schaade et al. (17) suggests that novel real-time PCR assays should be rigorously tested on large panels of viral isolates. However, the existence of rare or newly arising variants cannot be excluded.
Nucleotide sequence accession number.
The GenBank accession number for the MIE gene sequence is M21295.
Acknowledgments
We thank Richard C. Moore for reviewing the English.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Boeckh, M., B. Fries, and W. G. Nichols. 2004. Recent advances in the prevention of CMV infection and disease after hematopoietic stem cell transplantation. Pediatr. Transplant. 8(Suppl. 5):19-27. [DOI] [PubMed] [Google Scholar]
- 2.Boeckh, M., M. Huang, J. Ferrenberg, T. Stevens-Ayers, L. Stensland, W. G. Nichols, and L. Corey. 2004. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J. Clin. Microbiol. 42:1142-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brytting, M., J. Wahlberg, J. Lundeberg, B. Wahren, M. Uhlen, and V. A. Sundqvist. 1992. Variations in the cytomegalovirus major immediate-early gene found by direct genomic sequencing. J. Clin. Microbiol. 30:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, S. 1992. Effect of interstrain variation on diagnostic DNA amplification of the cytomegalovirus major immediate-early gene region. J. Clin. Microbiol. 30:2307-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drago, L., A. Lombardi, E. De Vecchi, G. Giuliani, R. Bartolone, and M. R. Gismondo. 2004. Comparison of nested PCR and real time PCR of herpesvirus infections of central nervous system in HIV patients. BMC Infect. Dis. 4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gault, E., Y. Michel, A. Dehee, C. Belabani, J. C. Nicolas, and A. Garbarg-Chenon. 2001. Quantification of human cytomegalovirus DNA by real-time PCR. J. Clin. Microbiol. 39:772-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griscelli, F., M. Barrois, S. Chauvin, S. Lastere, D. Bellet, and J. H. Bourhis. 2001. Quantification of human cytomegalovirus DNA in bone marrow transplant recipients by real-time PCR. J. Clin. Microbiol. 39:4362-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann, B., V. C. Larsson, C. J. Rubin, F. Sund, B. M. Eriksson, J. Arvidson, Z. Yun, K. Bondeson, and J. Blomberg. 2004. Comparison of a duplex quantitative real-time PCR assay and the COBAS Amplicor CMV Monitor test for detection of cytomegalovirus. J. Clin. Microbiol. 42:1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kearns, A. M., M. Guiver, V. James, and J. King. 2001. Development and evaluation of a real-time quantitative PCR for the detection of human cytomegalovirus. J. Virol. Methods 95:121-131. [DOI] [PubMed] [Google Scholar]
- 10.Leruez-Ville, M., M. Ouachee, R. Delarue, A. S. Sauget, S. Blanche, A. Buzyn, and C. Rouzioux. 2003. Monitoring cytomegalovirus infection in adult and pediatric bone marrow transplant recipients by a real-time PCR assay performed with blood plasma. J. Clin. Microbiol. 41:2040-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, H., J. S. Dummer, W. R. Estes, S. Meng, P. F. Wright, and Y. W. Tang. 2003. Measurement of human cytomegalovirus loads by quantitative real-time PCR for monitoring clinical intervention in transplant recipients. J. Clin. Microbiol. 41:187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitsche, A., N. Steuer, C. A. Schmidt, O. Landt, and W. Siegert. 1999. Different real-time PCR formats compared for the quantitative detection of human cytomegalovirus DNA. Clin. Chem. 45:1932-1937. [PubMed] [Google Scholar]
- 13.Nye, M. B., A. R. Leman, M. E. Meyer, M. A. Menegus, and P. G. Rothberg. 2005. Sequence diversity in the glycoprotein B gene complicates real-time PCR assays for detection and quantification of cytomegalovirus. J. Clin. Microbiol. 43:4968-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pignatelli, S., P. Dal Monte, G. Rossini, and M. P. Landini. 2004. Genetic polymorphisms among human cytomegalovirus (HCMV) wild-type strains. Rev. Med. Virol. 14:383-410. [DOI] [PubMed] [Google Scholar]
- 15.Prentice, H. G., and P. Kho. 1997. Clinical strategies for the management of cytomegalovirus infection and disease in allogeneic bone marrow transplant. Bone Marrow Transplant. 19:135-142. [DOI] [PubMed] [Google Scholar]
- 16.Retiere, C., B. M. Imbert, G. David, P. Courcoux, and M. M. Hallet. 1998. A polymorphism in the major immediate-early gene delineates groups among cytomegalovirus clinical isolates. Virus Res. 57:43-51. [DOI] [PubMed] [Google Scholar]
- 17.Schaade, L., P. Kockelkorn, K. Ritter, and M. Kleines. 2001. Detection of cytomegalovirus DNA in human specimens by LightCycler PCR: melting point analysis is mandatory to detect virus strains with point mutations in the target sequence of the hybridization probes. J. Clin. Microbiol. 39:3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stocher, M., V. Leb, M. Bozic, H. H. Kessler, G. Halwachs-Baumann, O. Landt, H. Stekel, and J. Berg. 2003. Parallel detection of five human herpes virus DNAs by a set of real-time polymerase chain reactions in a single run. J. Clin. Virol. 26:85-93. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka, N., H. Kimura, K. Iida, Y. Saito, I. Tsuge, A. Yoshimi, T. Matsuyama, and T. Morishima. 2000. Quantitative analysis of cytomegalovirus load using a real-time PCR assay. J. Med. Virol. 60:455-462. [DOI] [PubMed] [Google Scholar]
- 20.Zaia, J. A. 2002. Prevention and management of CMV-related problems after hematopoietic stem cell transplantation. Bone Marrow Transplant. 29:633-638. [DOI] [PubMed] [Google Scholar]
- 21.Zweygberg Wirgart, B., M. Brytting, A. Linde, B. Wahren, and L. Grillner. 1998. Sequence variation within three important cytomegalovirus gene regions in isolates from four different patient populations. J. Clin. Microbiol. 36:3662-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]