Abstract
Rotavirus P[8]G9 was recognized as the most widespread genotype during a sentinel-based survey in Italy; phylogenetic analysis of the VP7 and VP4 genes showed that Italian isolates constituted a closely related genetic cluster distinct from the other G9 strains recently isolated in other European countries, America, and Asia.
Rotavirus, the most common cause of gastroenteritis in infants and young children, presents an outer capsid composed of the VP4 and VP7 structural proteins, which expose the major antigen determinants capable of inducing neutralizing antibodies. The antigenic and molecular characteristics of VP4 and VP7 allow subdivision into two serogenotypes, P and G.
The different approaches followed for the development of the two new rotavirus vaccines—a monovalent live attenuated vaccine containing a P1A[8]G1 strain (Rotarix; GlaxoSmithKline Biologicals) and a pentavalent bovine-human G1 to G4, P[8] reassortant vaccine (RotaTeq; Merck and Sanofi Pasteur MSD)—and the lack of clear data about the heterotypic protection both underline the importance of virological surveillance and strain characterization (11, 18, 22).
Virological surveillance is spotty and largely incomplete, and in most regions of the world, including developed countries, the epidemiological picture is based mainly on specimens collected from hospitalized children (6, 9, 17, 20, 24). Data about genotype circulation in the general outpatient pediatric population are largely missing.
In this article, the genotype distribution of rotavirus strains detected in stools collected from children ranging in age from newborn to 5 years during a large community surveillance is presented, and the molecular characterization of emerging types is shown in relation to the epidemiological aspects.
In Italy, primary health care is provided by community pediatricians who monitor physical and psychosocial growth and development, promote age-appropriate screening, establish the first contact with the patient for diagnosis and treatment of acute and chronic disorders, and coordinate the management of health problems requiring multiple professional services. Each pediatrician surveys about 800 children ranging in age from newborn to 14 years.
The sentinel-based network for rotavirus surveillance was organized in 2004 with 10 primary care pediatricians who surveyed globally 3,611 children, ranging in age from newborn to 5 years, living in Leghorn, Italy. Stratification by age range formed groups of 741, 747, 711, 708, and 705 children in the ranges of newborn to 11 months, 12 to 23 months, 24 to 35 months, 36 to 47 months, and 48 to 59 months, respectively. This sample corresponds to 24% of the entire population of newborns to 5-year-olds in the district. Pediatricians reported on a weekly basis the number of new cases of gastroenteritis, defined as the occurrence of three or more watery stools in a period of 24 h (22). The survey lasted for 12 months, from April 2005 to April 2006. Stool samples were collected from every child with gastroenteritis, stored at −20°C, and sent to the Department of Health Sciences, University of Genoa, where they were tested for rotavirus by real-time PCR (Fastset Rotavirus; Arrows Diagnostics, Italy).
Two epidemic peaks were observed during the spring of 2005 and the 2006 winter-spring season, when the gastroenteritis incidence reached 9 and 5 cases/1,000 children ranging from newborns to 5-year-olds per week, respectively, with >40% of samples positive for rotavirus.
Positive samples were P and G genotyped using a primer-specific seminested multiplex PCR, as previously described and recently modified (13). Molecular characterization of G9 strains was performed by partial sequence analysis of the VP7 (843 bp; nucleotides 5 to 848) and VP4 (801 bp; nucleotides 39 to 840) genes using the primers described by Iturriza-Gomara et al. (13).
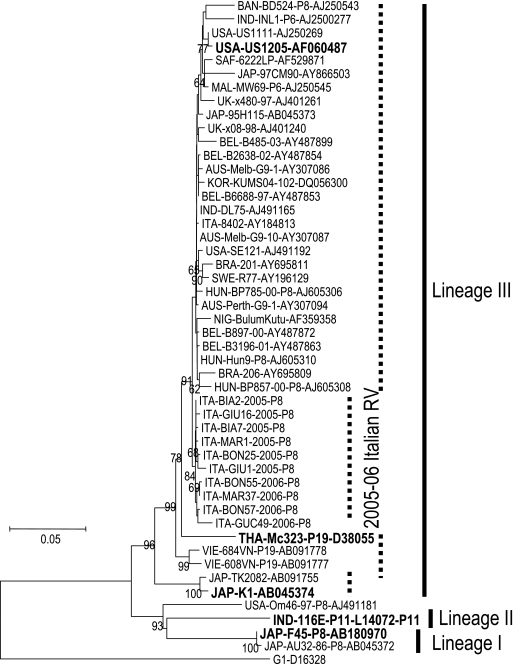
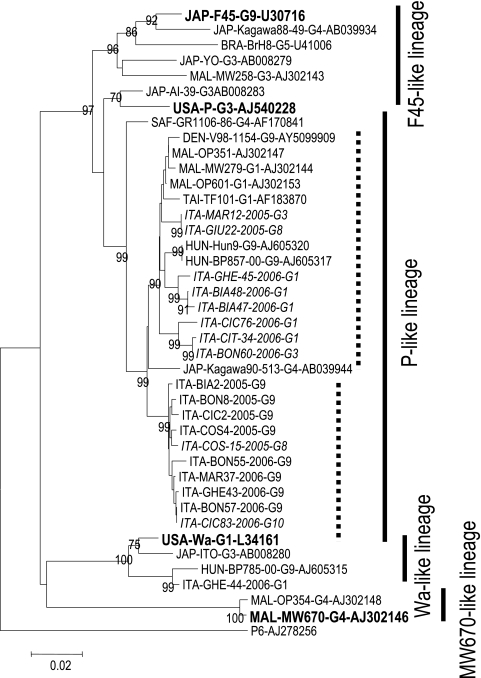
During the period from 4 April 2005 to 2 April 2006, a total of 597 specimens were collected; of those, 2 were not sufficient for RNA extraction, so 595 specimens (99.7%) were analyzed. A total of 203 out of 595 (34.1%) tested positive for rotavirus, and 72.9% were typeable. The most prevalent types identified included P[8]G9 (42.6% of the typeable samples), P[8]G1 (16.2%), P[8]G3 (14.2%), and P[8]G8 (8.8%). Less common types were P[4]G2, P[8]G4, and P[8]G10, found in 5.4%, 5.4%, and 2.7% of the typeable rotavirus samples, respectively. Seven (4.7%) mixed infections were observed (G1 plus G9 and G1 plus G3). A subset of 25 P[8]G9 strains and 10 P[8], non-G9 viruses was randomly selected and characterized by sequence analysis of the VP7 and VP4 genes. Phylogenetic analysis with MEGA software was performed on partial VP7 and VP4 nucleotide sequences and on the deduced amino acid sequences. The resulting neighbor-joining phylogenetic trees, including reference strains and selected viruses isolated in different areas of the world, are shown in Fig. 1 and 2.
FIG. 1.
Neighbor-joining phylogenetic tree of the VP7 gene, including G9 Italian strains, reference strains (boldfaced), and selected viruses isolated in different areas of the world.
FIG. 2.
Neighbor-joining phylogenetic tree of the VP4 gene including Italian strains, reference strains (boldfaced), and selected viruses isolated in different areas of the world.
VP7 nucleotide sequence comparison and phylogenetic reconstruction indicated that the Italian rotavirus strains formed a closely related cluster belonging to lineage III. Lineage III includes at least three other clusters: the P[19]G9 strains identified in Thailand in 1989 (e.g., THA-Mc323) and in Vietnam in the late 1990s (e.g., VIE-684VN), the viruses detected in Japan and other Far Eastern countries in the same period and at the beginning of 2000 (e.g., JAP-K1), and the strains recently isolated in other European countries, North and South America, Australia, and Asia (6, 14, 20). The rotavirus strains detected during this survey clustered separately from the most frequently isolated G9 strains (bootstrap P value, 0.84). In particular, they do not appear to be related to Italian strains, collected in Southern Italy during the winter of 1999 to 2000, that appeared to be very similar to Japanese strain 95H115, and they are also different from rotaviruses isolated during the following season (winter 2001 to 2002), represented by strain ITA-84/02 in the phylogenetic tree in Fig. 1 (3, 16).
The VP7 sequence of 2005-to-2006 Italian isolates showed 97.7 to 98.8% nucleotide identity to reference strain US1205, with nucleotide differences from other US1205-like viruses at positions 150, 567, and 600 (A150G, G567A, and G600A). The strain most frequently isolated during the 2005 spring epidemic (BIA2) showed an amino acid sequence identical to that of reference strain US1205, whereas other viruses detected during the 2005 epidemic peak showed amino acid mutations at positions 260 (I260L) and 218 (V218G), in antigenic site C, and at position 247 (R247K), close to antigenic site F (for antigenic site definition, see reference 14). Rotavirus GIU1, detected in a sample collected at the end of the spring epidemic, bore the amino acid change T243N, relative to reference strain US1205, in a position very close to antigenic site F. The sequences of the strains detected during the 2006 winter and spring seasons showed 0.3 to 1.2% and 0.4 to 0.8% nucleotide and amino acid differences from the 2005 viruses, respectively, and all of them presented a common mutation at position 315 (A315G). Another nucleotide mutation at position 161 (G161A), which determines an amino acid change at position 54 (G54E), was found in the virus detected during the spring of 2006.
Like the data obtained by molecular characterization of the VP7 gene, phylogenetic analysis of VP4 sequences indicated that all Italian G9 viruses formed a separate cluster (bootstrap P value, 0.99) inside the P-like lineage (Fig. 2), showing a 94.3 to 94.9% nucleotide identity to the P reference strain. The assignment of Italian rotaviruses to the P-like lineage is supported by nonsynonymous findings at positions 494, 576, and 593 and silent nucleotide changes at positions 87, 105, 126, 141, 171, 297, 588, 609, 672, and 676, which are highly conserved among members of this lineage (6). All Italian G9 strains shared nucleotide differences at positions 153, 263, and 561, resulting in an amino acid change at position 88 with respect to other viruses belonging to the P-like lineage. The VP4 sequences of other P[8] strains exhibiting G1, G3, and G8 genotypes fell into a P-like cluster that also included strains recently isolated in other European countries (Hungary, Denmark, and Italy), Africa (Malawi), and southern Asia (Taiwan and Japan) (3-6, 10). Of note, the Southern Italian G9 strains mentioned above and circulating during the 1999-to-2000 season belonged to this cluster and were in fact very closely related to Malawian strain OP351 (Fig. 2). They showed complete nucleotide identity, suggesting a clonal origin (data not shown) (3). Conversely, VP4 sequences from Italian G1 strains circulating in the past 2 decades showed high heterogeneity, belonging to two different lineages: the Wa-like lineage included viruses collected in the years 1986 to 2002, and the P-like lineage included strains isolated in the years 1995 to 2004 (5).
Among Italian non-G9 strains characterized in this study, only rotavirus P[8]G8 COS15-2005, which was very close to P[8]G9 COS4-2005, and rotavirus P[8]G1 GHE44-2006 did not fall into the P-like cluster that also included strains recently isolated in Europe, Africa, and southern Asia; the latter strain belonged to the Wa-like lineage.
The P[8]G10 strains showed a nucleotide sequence identical to that of the P[8]G9 BON57-2006 strain, circulating during the same period, during the 2006 winter peak. Interestingly, the VP4 sequences of P[8]G1, P[8]G3, and P[8]G8 strains exhibited higher heterogeneity than that of P[8]G9; comparison of the most prevalent G1 and G9 VP4 sequences indicated that G1 sequences showed eight- or fourfold-higher p distances than G9 sequences, considering all G1 strains or only G1 strains belonging to the P-like lineage, respectively (p distance ± standard error, 0.048 ± 0.005 for all G1 strains, 0.022 ± 0.004 for G1 strains belonging to the P-like lineage, and 0.006 ± 0.002 for G9 strains).
In 2006, the extents of nucleotide and amino acid changes were 0.4 to 0.8% and 0.4 to 1.7%, respectively, compared to those of viruses detected during the previous season. Viruses detected during the 2006 winter and spring seasons presented amino acid changes at positions 31 (T31P), close to epitope 1; 61 (V61G), in antigenic site 3; and 73 (L73P) (for antigenic site definition, see reference 15).
In conclusion, this survey provided both a precise picture of circulating rotaviruses, through the typing of about one-quarter of circulating strains in a medium-size town over 1 year, and the characterization by sequence analysis of the genes encoding the two outer layer proteins of the most prevalent and emerging P[8]G9 type. The P[8]G9 strains isolated during the 1-year survey formed a closely related cluster clearly separated from the other strains recently isolated in other European countries, America, and Asia with regard to both the VP7 and the VP4 gene.
Even though we surveyed a relatively small area for only 1 year, our findings suggest (i) the circulation of G9 strains belonging to the same cluster for two seasons without introduction or cocirculation of other variants, as opposed to G1, G3, and G8 viruses, which showed high heterogeneity; (ii) the possibility of VP4 exchange between G9 and G8 strains and G9 and G10 strains that circulated during the spring of 2005 and the winter of 2006, respectively; and (iii) a continuous genetic drift, which determined the emergence of mutants characterized by amino acid changes over the VP4 and VP7 antigenic sites. The drifts we observed consisted of 0.4 to 0.8% and 0.4 to 1.7% amino acid substitutions in the VP7 and VP4 genes, a substitution rate similar to that observed for the hemagglutinin of influenza B viruses (ranging from 0.3 to 0.5% change/year) and lower than that of influenza A/H3N2 viruses (mean, 3.2% amino acid substitution/year in the past decade) (1, 2). The role of the observed amino acid changes in the emergence of potential escape mutants with respect to the G9 candidate vaccine or previous G9 infection has yet to be understood. Indeed, recent data by Hoshino et al. showed that antibodies to lineage I viruses, the oldest G9 strains isolated chronologically, neutralized lineage III viruses, which emerged 10 or more years later, as efficiently as they neutralized homologous strains (12). In addition, consistent homotypic protection over time has been shown between vaccine and circulating strains belonging to the same genotype, in particular with regard to the G1 component of rhesus-human quadrivalent, human monovalent, and human-bovine pentavalent vaccines against severe diarrhea, although field trials were conducted 15 to 20 years after the isolation of vaccine strains (7, 8, 19, 21, 23).
The observations emerging from this study enrich the data on the sequence diversity observed within the modern G9 lineage over the past decade, contributing to a better understanding of the evolution of rotaviruses (6) and emphasizing the importance of virological surveillance of circulating rotaviruses to detect new variants and possible antigenic changes, whose potential effect on vaccine effectiveness should be carefully evaluated.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this work have been deposited in GenBank under accession numbers AF150311 to AF150339.
Acknowledgments
Contributing members of the Pediatric Leghorn Group are Luigi Boni, Renato Cicchiello, Filippo Citti, Alessandro Costagliela, Andrea Ghelardini, Franco Giuntini, Marco Gucci, Alessandro Marini, and Patrizia Tamburini.
This work was performed at the Department of Health Sciences, University of Genoa, Genoa, Italy.
This study was funded by a grant from sanofi pasteur MSD (Italy).
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Ansaldi, F., P. D'Agaro, D. de Florentiis, S. Puzelli, Y. P. Lin, I. Donatelli, R. Gasparini, P. Crovari, A. Hay, and C. Campello. 2003. Molecular characterization of influenza B viruses circulating in Northern Italy during the 2001-2002 epidemic season. J. Med. Virol. 70:463-469. [DOI] [PubMed] [Google Scholar]
- 2.Ansaldi, F., G. Icardi, R. Gasparini, C. Campello, S. Puzelli, A. Bella, I. Donatelli, S. Salmaso, and P. Crovari. 2005. New A/H3N2 influenza variant: a small genetic evolution but a heavy burden on the Italian population during the 2004-2005 season. J. Clin. Microbiol. 43:3027-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arista, S., G. M. Giammanco, S. De Grazia, C. Migliore, V. Martella, and A. Cascio. 2004. Molecular characterization of the genotype G9 human rotavirus strains recovered in Palermo, Italy, during the winter of 1999-2000. Epidemiol. Infect. 132:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arista, S., G. M. Giammanco, S. De Grazia, C. Colomba, and V. Martella. 2005. Genetic variability among serotype G4 Italian human rotaviruses. J. Clin. Microbiol. 43:1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arista, S., G. M. Giammanco, S. De Grazia, S. Ramirez, C. Colomba, A. Cascio, and V. Martella. 2006. Heterogeneity and temporal dynamics of evolution of G1 human rotaviruses in a settled population. J. Virol. 80:10724-10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banyai, K., J. R. Gentsch, R. Schipp, F. Jakab, E. Meleg, I. Mihaly, and G. Szucs. 2005. Dominating prevalence of P[8], G1 and P[8], G9 rotavirus strains among children admitted to hospital between 2000 and 2003 in Budapest, Hungary. J. Med. Virol. 76:414-423. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein, D. I., V. E. Smith, J. R. Sherwood, G. M. Schift, D. S. Sander, D. R. Springs, and R. L. Ward. 1998. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine 16:381-387. [DOI] [PubMed] [Google Scholar]
- 8.Clark, H. F., F. E. Borian, K. Modesto, and S. A. Plotkin. 1990. Serotype 1 reassortant of bovine rotavirus WC3, strain WI79, induces a polytypic antibody response in infants. Vaccine 8:327-332. [DOI] [PubMed] [Google Scholar]
- 9.Cubitt, W. D., A. D. Steele, and M. Iturriza. 2000. Characterisation of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6]. J. Med. Virol. 61:150-154. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, T. K., J. Eugen-Olsen, A. G. Pedersen, K. Molbak, K. Rostgaard, and N. M. Nielsen. 2005. Characterization of rotavirus strains in a Danish population: high frequency of mixed infections and diversity within the VP4 gene of P8 strains. J. Clin. Microbiol. 43:1099-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentsch, J. R., A. R. Laird, B. Bielfelt, D. D. Griffin, U. D. Parashar, J. S. Bresee, B. Jiang, and R. I. Glass. 2005. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J. Infect. Dis. 192:S146-S159. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino, Y., R. W. Jones, J. Ross, S. Honma, N. Santos, J. R. Gentsch, and A. Z. Kapikian. 2004. Rotavirus serotype G9 strains belonging to VP7 gene phylogenetic sequence lineage 1 may be more suitable for serotype G9 vaccine candidates than those belonging to lineage 2 or 3. J. Virol. 78:7795-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iturriza-Gomara, M., G. Kang, and J. Gray. 2004. Rotavirus genotyping: keeping-up with an evolving population of human rotaviruses. J. Clin. Virol. 31:259-365. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood, C., N. Bogdanovic-Sakran, E. Palombo, P. Masendycz, G. Barnes, and R. Bishop. 2003. Genetic and antigenic characterization of rotavirus serotype G9 strains isolated in Australia between 1997 and 2001. J. Clin. Microbiol. 41:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs-Nolan, J., D. Yoo, and Y. Mine. 2003. Fine mapping of sequential neutralization epitopes on the subunit protein VP4 of human rotavirus. Biochem. J. 376:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martella, V., V. Terio, S. Arista, G. Elia, M. Corrente, A. Madio, A. Cirani, and C. Buonavoglia. 2004. Nucleotide variation in the VP7 gene affects PCR genotyping of G9 rotavirus identified in Italy. J. Med. Virol. 72:143-148. [DOI] [PubMed] [Google Scholar]
- 17.Oh, D. Y., G. Gaedicke, and E. Schreier. 2003. Viral agents of acute gastroenteritis in German children: prevalence and molecular diversity. J. Med. Virol. 71:82-93. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Schael, I., M. Blanco, M. Vilar, D. Garcia, L. White, A. Z. Kapikian, and J. Flores. 1990. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. J. Clin. Microbiol. 28:553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Schael, I., M. J. Guntinas, M. Perez, V. Pagone, W. Cunto, Y. Hoshino, and A. Z. Kapikian. 1997. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N. Engl. J. Med. 337:1181-1187. [DOI] [PubMed] [Google Scholar]
- 20.Rahman, M., J. Matthijnssens, T. Goegebuer, K. De Leener, L. Vanderwegen, I. van der Donck, L. Van Hoovels, S. De Vos, T. Azim, and M. Van Ranst. 2005. Predominance of rotavirus G9 genotype in children hospitalized for rotavirus gastroenteritis in Belgium during 1999-2003. J. Clin. Virol. 33:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Palacios, G. M., I. Perez-Schael, F. R. Velazquez, H. Abate, T. Breuer, S. C. Clemens, B. Cheuvart, F. Espinoza, et al. 2006. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 354:11-22. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez, F. R., D. O. Matson, J. J. Calva, L. Guerrero, A. L. Morrow, S. Carter-Campbell, R. I. Glass, M. K. Estes, L. K. Pickering, and G. M. Ruiz-Palacios. 1996. Rotavirus infections in infants as protection against subsequent infections. N. Engl. J. Med. 335:1022-1028. [DOI] [PubMed] [Google Scholar]
- 23.Vesikari, T., D. O. Matson, P. Dennehy, P. Van Damme, M. Santosham, Z. Rodriguez, M. J. Dallas, J. F. Heyse, et al. 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 354:23-33. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelmi, I., C. Mier, E. Roman, J. Colomina, J. Prat, A. Sanchez-Fauquier, et al. 1999. The molecular epidemiology of the rotavirus in Spanish children. Enferm. Infecc. Microbiol. Clin. 17:509-514. (In Spanish.) [PubMed] [Google Scholar]