Abstract
PCR was used to survey bacterial vaginosis flora before and after metronidazole treatment. The species composition for pretreatment patients was variable. Lactobacillus iners was prominent in all patients posttreatment. Atopobium vaginae concentrations were highest for patients who failed or responded incompletely to treatment and lowest for patients who were cured.
Bacterial vaginosis (BV) is the most common cause of vaginal irritation and is associated with adverse pregnancy outcomes (4, 13) and an increased risk of human immunodeficiency virus infection (14, 15). BV results when the normal, predominantly Lactobacillus vaginal flora shifts to one dominated by Gardnerella vaginalis, Mycoplasma hominis, and a variety of anaerobic organisms. However, no specific pathogen has been identified, and the cause of BV is unknown (6). Metronidazole is the most commonly prescribed antibiotic for treatment of BV, but failure and recurrence rates are high (7). Recent cultivation-independent analyses of PCR-amplified 16S rRNA gene sequences reveal that there are bacterial genera associated with BV that were not previously recognized, including a metronidozole-resistant anaerobe, Atopobium vaginae (8, 9, 17, 19). We examined the species composition of the vaginal flora of BV patients before and 1 month after metronidazole treatment by using PCR assays directed toward a broad range of bacterial genera and a quantitative PCR assay targeting A. vaginae.
Clinical assessments of BV were made just prior to treatment and at 4 weeks posttreatment. All six BV patients in the study met all Amsel criteria (2) and had Nugent scores of ≥4 (12). Treatment was a 0.75% topical metronidazole gel applied once daily for 5 days. The study was approved by the Louisiana State University Health Sciences Center institutional review board, and informed consent was obtained from each participant. Vaginal swabs were collected prior to treatment and 30 days posttreatment by using a standard protocol and were stored frozen. To isolate DNA, swabs were agitated in 0.5 ml of molecular-biology-grade water, suspensions were centrifuged, and nucleic acids were isolated from pellets by using an AquaPure genomic DNA kit (Bio-Rad, Hercules, CA). Primers for quantitative PCR assays of A. vaginae 16S rRNA genes were designed using Primrose (3); primers were 5′-GTTAGGTCAGGAGTTAAATCTG-3′ and 5′-TCATGGCCCAGACC-3′. Real-time amplifications were performed on an iCycler (Bio-Rad) using iQ-SYBR Green Supermix (Bio-Rad). Thermal cycling consisted of 95°C for 2.5 min, followed by 40 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 30 s; 10 ng of template DNA was used in each amplification. PCR amplification of vaginal DNA using primers targeting conserved regions of the16S rRNA gene were used to generate clone libraries with a TOPO TA PCR cloning kit (Invitrogen, Carlsbad, CA). Sequencing was performed by an outside contractor (Louisiana State University Health Sciences Center genomics core facility, New Orleans, LA). Sequencher software (GeneCodes, Ann Arbor, MI) was used to analyze sequence data and to group sequences with ≥99% similarity into phylotypes. The BLAST algorithm (1) at the NCBI website and Sequence Match at the RDP II website (10) were used to find 16S rRNA gene sequences in public databases related to each phylotype identified in this study. PCR conditions and reagents (5) and broad-range clone library analyses of vaginal flora have been described previously (9).
The results are summarized in Table 1. Patient 498 was judged a “complete” treatment failure, since neither the Amsel criteria nor the Nugent score had improved at the follow-up visit. Patient 499 failed treatment by Nugent's criterion with a score of 8 but was clinically cured, having none of Amsel's criteria. Patient 500 had a normal Nugent score but was judged a treatment failure based on a persistently elevated vaginal pH. The three remaining patients were judged complete cures.
TABLE 1.
Metronidazole treatment outcome and detection of A. vaginae
| Patient no. | Gram stain outcome (score) | Clinical outcomea | A. vaginae in pretreatment clone library | Quantitative PCR result (CTb)
|
|
|---|---|---|---|---|---|
| Pretreatment | Posttreatment | ||||
| 498 | Failure (8) | Failurec | Yes | 16.2 | 14.7 |
| 499 | Failure (8) | Cure | Yes | 18.5 | 27.0 |
| 500 | Cure (0) | Failured | Yes | 17.7 | 30.2 |
| 505 | Cure (0) | Cure | No | 21.3 | 31.9 |
| 506 | Cure (0) | Cure | No | 19.7 | 34.8 |
| 507 | Cure (0) | Cure | No | 23.1 | 28.8 |
Cure, none of the Amsel criteria met.
PCR cycle at which the specimen crossed the positivity threshold. Lower values indicate higher organism concentrations.
Based on persistent clue cells and discharge.
Based on a pH of >4.7.
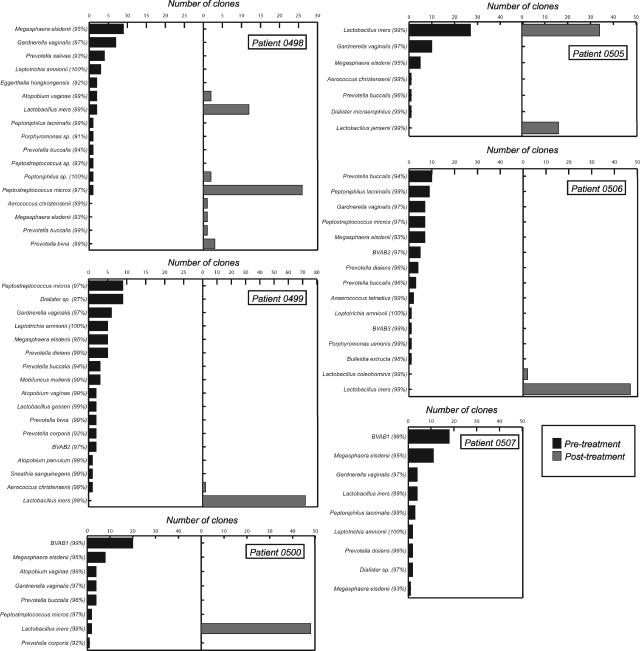
Quantitative PCR indicated that pretreatment A. vaginae concentrations were highest in patients who completely or partially failed treatment (Table 1), and A. vaginae sequences were detected in pretreatment clone libraries of these patients (Table 1; Fig. 1). In contrast, quantitative PCR indicated that A. vaginae concentrations were lowest in patients who were cured, and no A. vaginae sequences were detected in pretreatment clone libraries of any of these cases. It is possible that posttreatment specimens could include DNA from nonviable organisms; however, we think it unlikely that nonviable-organism DNA would persist for a month following treatment. Clearly, more-extensive studies are necessary to assess whether high pretreatment concentrations of individual species, such as A. vaginae, are predictive of adverse treatment outcomes for BV patients, since G. vaginalis and, to a lesser extent, A. vaginae are detectable among patients without BV by species-specific PCR (8). However, it is of interest that quantitative PCR studies of Gardnerella vaginalis and Mycoplasma hominis have already provided evidence that concentrations of individual vaginal species may be more predictive of adverse sequelae than the diagnosis of BV alone (14).
FIG. 1.
Number of clones of each 16S rRNA-defined phylotype detected in PCR-generated libraries using DNA isolated from vaginal swabs of BV patients before (dark gray) and after (light gray) treatment with metronidazole gel. While nearly all the 16S rRNA gene sequences detected in this study were ≥99% similar to those in public databases, in many cases the most similar 16S rRNA gene sequence is that of an uncultivated clone with a cryptic designation. Therefore, the species and strains listed to the left of each bar graph (with percentages of sequence similarity given in parentheses) represent recognized isolates or notable uncultivated BV-associated bacteria (e.g., BVAB1 [8]), and not necessarily the most similar database match to the phylotypes detected in this study.
After treatment, sequence analyses indicated that a single species, Lactobacillus iners, was predominant in all patients, except for the patient who was a complete treatment failure, for whom L. iners sequences were prevalent but not predominant (Fig. 1). Since we routinely detect predominantly Lactobacillus crispatus sequences in patients classified as normal by Nugent's score and Amsel's criteria (data not shown), and normal L. crispatus-dominant vaginal flora is commonly described in the literature (8, 17-19), the predominance of L. iners in “cured” patients was unexpected. Recently, more-refined Gram stain subcategories of vaginal flora have been proposed (16, 18). In this system, L. crispatus is prevalent in specimens with a grade Ia Gram stain and the flora is predominantly L. crispatus as determined by culture. L. iners is rare in grade Ia specimens; however, it is prevalent in grade Ib, a variant of normal, and in grade III, representing BV. The “protective” role of individual vaginal Lactobacillus species is unclear (18). We speculate that L. iners is a transitional species and that an L. crispatus-predominant species composition represents a stable normal flora.
Clone library analyses indicated that, prior to treatment; each BV patient harbored a unique complement of bacterial species (Fig. 1). Evidence of high variability in species composition among BV patients has been well documented by recent extensive PCR analyses of thousands of 16S rRNA gene sequences from dozens of patients (8, 9). We noted that almost all (32 of 35) phylotypes detected in our study were highly related (≥99% sequence similarity) to sequences in GenBank and that most (31 of 35) were from studies of vaginal flora (8, 9, 19). It may be that most, if not all, of the novel species commonly inhabiting the vagina have been described in the published literature. With the exception of Leptotrichia-, Sneathia-, and Porphyromonas-like sequences, all phylotypes in this study clade within the phylum Actinobacteria or Firmicutes, which contain traditional gram-positive actinomycetes and Clostridium-Bacillus species, including Lactobacillus species, respectively. However, some cultivated members of these phyla, such as Megasphaera spp., which are commonly detected in rRNA gene libraries of BV patients, are known to have atypical cell walls, resulting in negative Gram reactions (11). Thus, descriptions of BV as an increase in gram-“negative” species might exaggerate perceptions of phylogenic distance between normal and BV-associated bacterial communities.
Acknowledgments
This study was supported by CDC grant U19AI061972.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Altschul, S., T. Madden, A. Schaeffer, J. Zhang, Z. Zhang, W. Miller, and D. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsel, R., P. A. Totten, C. A. Spiegel, K. C. Chen, D. Eschenbach, and K. K. Holmes. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14-22. [DOI] [PubMed] [Google Scholar]
- 3.Ashelford, K. E., A. J. Weightman, and J. C. Fry. 2002. PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res. 30:3481-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey, J. C., M. A. Klebanoff, J. C. Hauth, S. L. Hillier, E. A. Thom, J. M. Ernest, R. P. Heine, R. P. Nugent, M. L. Fischer, K. J. Leveno, R. Wapner, M. Varner, et al. 2000. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N. Engl. J. Med. 342:534-540. [DOI] [PubMed] [Google Scholar]
- 5.Ferris, M. J., A. Masztal, K. E. Aldridge, J. D. Fortenberry, P. L. Fidel, Jr., and D. H. Martin. 2004. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect. Dis. 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsum, U., A. Hallen, and P. G. Larsson. 2005. Bacterial vaginosis—a laboratory and clinical diagnostics enigma. APMIS 113:153-161. [DOI] [PubMed] [Google Scholar]
- 7.Forsum, U., E. Holst, P. G. Larsson, A. Vasquez, T. Jakobsson, and I. Mattsby-Baltzer. 2005. Bacterial vaginosis—a microbiological and immunological enigma. APMIS 113:81-90. [DOI] [PubMed] [Google Scholar]
- 8.Fredricks, D. N., T. L. Fiedler, and J. M. Marrazzo. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 353:1899-1911. [DOI] [PubMed] [Google Scholar]
- 9.Hyman, R. W., M. Fukushima, L. Diamond, J. Kumm, L. C. Giudice, and R. W. Davis. 2005. Microbes on the human vaginal epithelium. Proc. Natl. Acad. Sci. USA 102:7952-7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchandin, H., E. Jumas-Bilak, B. Gay, C. Teyssier, H. Jean-Pierre, M. S. de Buochberg, C. Carriere, and J. P. Carlier. 2003. Phylogenetic analysis of some Sporomusa sub-branch members isolated from human clinical specimens: description of Megasphaera micronuciformis sp. nov. Int. J. Syst. Evol. Microbiol. 53:547-553. [DOI] [PubMed] [Google Scholar]
- 12.Nugent, R. P., M. A. Krohn, and S. L. Hillier. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 29:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onderdonk, A. B., M. L. Lee, E. Lieberman, M. L. Delaney, and R. E. Tuomala. 2003. Quantitative microbiologic models for preterm delivery. J. Clin. Microbiol. 41:1073-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sha, B. E., M. R. Zariffard, Q. J. Wang, H. Y. Chen, J. Bremer, M. H. Cohen, and G. T. Spear. 2005. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J. Infect. Dis. 191:25-32. [DOI] [PubMed] [Google Scholar]
- 15.Sturm-Ramirez, K., A. Gaye-Diallo, G. Eisen, S. Mboup, and P. J. Kanki. 2000. High levels of tumor necrosis factor-alpha and interleukin-1β in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J. Infect. Dis. 182:467-473. [DOI] [PubMed] [Google Scholar]
- 16.Tohill, B. C., C. M. Heilig, R. S. Klein, A. Rompalo, S. Cu-Uvin, W. Brown, and A. Duerr. 2004. Vaginal flora morphotypic profiles and assessment of bacterial vaginosis in women at risk for HIV infection. Infect. Dis. Obstet. Gynecol. 12:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhelst, R., H. Verstraelen, G. Claeys, G. Verschraegen, J. Delanghe, L. Van Simaey, C. De Ganck, M. Temmerman, and M. Vaneechoutte. 2004. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhelst, R., H. Verstraelen, G. Claeys, G. Verschraegen, L. Van Simaey, C. De Ganck, E. De Backer, M. Temmerman, and M. Vaneechoutte. 2005. Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resembles grade I microflora and revised categorization of grade I microflora. BMC Microbiol. 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou, X., S. J. Bent, M. G. Schneider, C. C. Davis, M. R. Islam, and L. J. Forney. 2004. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 150:2565-2573. [DOI] [PubMed] [Google Scholar]