Abstract
Recurrent airway obstruction (RAO), known previously as chronic obstructive pulmonary disease (COPD), is a debilitating respiratory condition that significantly contributes to lost training days and illness in racehorses. Herbs are becoming increasingly popular for the prophylaxis or treatment of the clinical signs of RAO despite a paucity of research on efficacy and safety. We evaluated the ability of an herbal composite containing garlic, white horehound, boneset, aniseed, fennel, licorice, thyme, and hyssop to reduce the clinical signs of RAO, hypothesizing that the product would safely reduce signs and would improve the inflammatory cell profile within the lungs. The composite was fed to 6 horses with symptomatic RAO for 21 d in a crossover manner. Ventigraphs were used to record respiratory rate and intrapleural pressure; the proportion of inflammatory cells in fluid aspirated from the trachea was determined. Blood biochemical and hematologic screening was conducted to identify possible adverse effects. Treatment with the composite did not result in statistically significant changes in any of the parameters evaluated. A trend to a decrease in respiratory rate (P = 0.1) and an increase in the proportion of macrophages (P = 0.1) was observed in the horses receiving the herbal composite compared with placebo. These data indicate a potential for the herbal composite to safely reduce the elevated respiratory rate in horses with RAO. Future research with a greater number of horses is warranted to further characterize the effect of this product on horses with RAO.
Résumé
L’obstruction récurrente des voies aériennes (RAO), connue auparavant sous la désignation de maladie pulmonaire obstructive chronique (COPD), est une condition respiratoire débilitante qui contribue de manière significative à la perte de jours d’entraînement et de maladie chez les chevaux de course. Les herbes gagnent en popularité à titre prophylactique ou thérapeutique lors de signes de RAO et ce malgré la rareté des recherches sur leur efficacité et sécurité. Nous avons évalué l’efficacité d’une composition d’herbe contenant de l’ail, du marrube blanc, de l’eupatoire perfoliée, de la graine d’anis, du fenouil, de la réglisse, du thym et du hysope à réduire les signes cliniques de RAO, en assumant que le produit réduirait de manière sécuritaire les signes et améliorerait le profil des cellules inflammatoires dans les poumons. Le composé a été donné à six chevaux symptomatiques de RAO pendant 21 jours selon un mode en croisé. Des pneumographes ont été utilisés afin d’enregistrer le rythme respiratoire et la pression intrapleurale; et les proportions de cellules inflammatoires dans le liquide aspiré à partir de la trachée ont été déterminées. Des profils biochimiques sanguins et hématologiques ont été effectués afin d’identifier des effets non désirés. Le traitement avec le composé d’herbes ne s’est pas soldé par des changements significatifs pour aucun des paramètres étudiés. Une tendance à une diminution du rythme respiratoire (P = 0,1) et une augmentation dans la proportion des macrophages (P = 0,1) a été observée chez les chevaux recevant le composé d’herbes comparativement à ceux recevant un placebo. Ces résultats suggèrent que le composé d’herbes possède un potentiel pour réduire de manière sécuritaire le rythme respiratoire élevé chez les chevaux atteints de RAO. Des recherches supplémentaires utilisant un plus grand nombre de chevaux sont nécessaires pour mieux caractériser l’effet de ce produit chez les chevaux avec RAO.
(Traduit par Docteur Serge Messier)
Introduction
Recurrent airway obstruction (RAO) is a chronic, reversible respiratory dysfunction of mature horses resulting in exercise intolerance. More severe cases present with various degrees of diffuse broncho-constriction, cough, nasal discharge, and increased respiratory effort, coupled with abdominal contraction, increased respiratory rate, or increased audible turbulence during auscultation of the pleural cavity, or a combination of these signs (1). Recurrent airway obstruction occurs most often in horses that are confined in stalls and exposed to environmental challenges such as dust, mold, and fungal spores from feed and bedding, and the risk of RAO developing increases with age (1).
Clinical remission can often be achieved by reducing exposure to environmental irritants through decreasing confinement or by altering the horse’s ability to resist the effects of the irritants through the use of pharmaceuticals. Although the former strategy may well be the more effective at improving lung function, it is often impractical in many professional equine facilities owing to space restrictions and safety concerns for individual horses. Pharmaceutical therapy may include the systemic or aerosol use of anti-inflammatory agents such as dexamethasone and beclomethasone, respectively (2), or the systemic or aerosol use of bronchodilators, such as clenbuterol (3) and albuterol (4), respectively.
Although clenbuterol is effective in reducing clinical signs of RAO and therefore commonly used, it has numerous reported adverse effects, such as inhibition of exercise performance (5). In addition, glucocorticoid use has been associated with an increased risk of laminitis (6) and lung infection (7). Concerns over these adverse effects have contributed to a progressive increase in the use of herbs to treat RAO in horses; however, the clinical and experimental data on efficacy and safety are limited (8).
The usefulness of herbs in treating respiratory disease is well recognized, as evidenced by the wide range of secondary plant metabolites found in conventional treatments for respiratory disease (9). The ingredients in the herbal composite used for the current study, a proprietary formulation (Breathe; Selected Bioproducts, Guelph, Ontario), have been reported in the literature as having positive effects on clinical or pathophysiological indicators of respiratory dysfunction (10–32) (Appendix I). The proportion of each constituent had been determined by the manufacturer over approximately 5 y of use in horses with RAO, such that the final formulation provided the most consistent improvement in clinical signs.
The purpose of this study was to evaluate the effect of the composite on the clinical and pathophysiological signs of RAO in symptomatic horses. It was hypothesized that oral administration of the composite over 21 d to horses with active RAO would reduce their respiratory rate and maximum change in intrapleural pressure (ΔPplmax) and would improve the inflammatory profile of cells obtained from a tracheal aspirate. Furthermore, it was hypothesized that no adverse effects would be identified through hematologic and biochemical studies.
Materials and methods
Horses
Before inclusion in the experiment, the horses displayed “heaves,” as determined by increased intrapleural pressure (> 15 cm H2O), reversible lower airway obstruction, and recurrent airway hyper-responsiveness that was attenuated by bronchodilators or outdoor housing, or both (33). The 6 animals included 2 mares (1 standard-bred and 1 quarterhorse), 3 geldings (1 mixed-breed pony, 1 quarter-horse, and 1 thoroughbred), and 1 stallion (standardbred), aged 12 to 20 y. For environmental challenge, the horses were bedded on dry straw in a research barn at the University of Guelph (Guelph, Ontario), outlet fans in the barn were turned off, all windows were closed to reduce ventilation, and all feed was fed dry, without attempts at reducing dust. This situation was not designed to maximally activate RAO in these horses; rather, the intent was to emulate a typical indoor housing environment to which RAO-affected horses might be exposed, to allow for a realistic evaluation of the effect of the herbal product on the “normal” clinical condition. The horses spent 1 h in a sand paddock per day. During the acclimation and experimental periods, they received twice daily a balanced ration that met their nutritional requirements (34); it consisted of 1.5 kg of concentrate pellets and one-third of a bale of dry hay (80:20 blend of timothy and alfalfa without overt mold contamination). Trace minerals and water were provided ad libitum.
Experimental design
The experiment was designed as a randomized, crossover study, such that each horse received the herbal composite in 1 of the 2 experimental periods, each lasting 21 d. Three horses were assigned, randomly, to each of the treatment and control groups in experimental period 1. Twice a day, each horse received, mixed into the grain ration, 55 g of either the composite or, as placebo control, coarsely chopped alfalfa hay. During a subsequent 14-d washout period, all the horses received an unsupplemented diet and were maintained indoors as previously described. The groups were then reversed, and the trial was repeated in experimental period 2. The trial began at the end of October and ran through the end of November, when the ambient temperature was 15°C to 5°C.
During both experimental periods, on days 0, 7, 14, and 21 the respiratory rate and the ΔPplmax were recorded by means of a Ventigraph (model PG100/REC; Boehringer Ingelheim [Canada], Burlington, Ontario), and tracheal lavage was performed to obtain a cytologic profile of the airways. Jugular venous blood samples were obtained on days 0 and 21 to obtain information on systemic effects of the supplements.
The protocol for animal care and use was approved by the Equine Research Centre Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care (35).
Pulmonary function testing
Esophageal pressure measurements obtained with a Ventigraph correlate well with the ΔPplmax (36) and have been used to calculate the ΔPplmax in horses (8). In this study, the esophageal probe of the Ventigraph was placed through the right nostril of each horse into the esophagus within the thorax, at the position wherein pressure changes correlate to those in the intrapleural space. The horse was given time to return to a normal respiratory rhythm (approximately 10 breaths/min) before Ventigraph measurements were taken over a 5-min period. A blinded analyst determined the respiratory rate and the ΔPplmax.
Tracheal lavage
After completion of the Ventigraph procedure, each horse was sedated with 0.02 mg/kg of xylazine (AnaSed injectable; Lloyd Laboratories, Shenandoah, Iowa, USA) or 0.01 mg/kg of detomidine (Dormosedan; SmithKline Beecham, London, Ontario) and restrained in stocks. An endoscope was passed through the right or left nostril until the pharynx was visible, and the endoscope was guided into the trachea. A sample of tracheal aspirate was obtained by infusing 60 mL of sterile saline (0.9% NaCl) through polyethylene tubing that was passed through the biopsy channel of the endoscope. Approximately 30 mL of aspirate was immediately drawn out of the trachea and collected in sterile polyethylene containers. After each lavage, the endoscope was washed with metrizyme (Metrex Research Division, Sybron Canada Ltd., Morrisburg, Ontario) and rinsed with distilled water, and air was blown through the channel to remove any remaining water. The endoscope was disinfected between sampling days with Glutarex (VWR International, Mississauga, Ontario). Slides were prepared immediately from fresh aspirate with the use of a cytocentrifuge (Shandon Cytospin 11; Shandon Inc., Pittsburgh, Pennsylvania, USA) and stained with Wright’s Hemastain. Differential cell counts were conducted on 100 cells per slide.
Hematologic studies
Jugular venous blood was collected into a sterile Vacutainer (Fisher Scientific, Nepean, Ontario) containing ethylenediamine tetraacetic acid. A complete blood count (CBC) was conducted at the Animal Health Laboratory, University of Guelph, by means of an Advia 120 (Bayer Corporation, Etobicoke, Ontario). Parameters quantified included counts of leukocytes, erythrocytes, and platelets, proportions of segmented neutrophils, lymphocytes, monocytes, eosinophils, and basophils, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, erythrocyte distribution width, mean plasma volume, and total serum protein concentration. The leukocyte differential counts were conducted manually, and morphologic data were determined manually from slides stained with Wright’s Hemastain.
Biochemical studies
Jugular venous blood was collected into a silicone-coated Vacutainer. An equine serum profile (ESP) was conducted at the Animal Health Laboratory by means of a Hitachi 911 Biochemical Analyzer (Boehringer Mannheim, Laval, Quebec). Parameters quantified included concentrations of calcium, phosphorus, magnesium, sodium, potassium, chloride, carbon dioxide, albumin, globulin, urea, creatinine, glucose, cholesterol, total bilirubin, conjugated bilirubin, unconjugated bilirubin, alkaline phosphatase, gamma glutamyltransferase, aspartate aminotransferase, creatine kinase, glutamyl dehydrogenase, and haptoglobin, along with the albumin: globulin ratio, sodium:potassium ratio, and calculated osmolarity.
Analysis of plant material
All dried plant material was obtained from a commercial supplier (Ets Saisse & Fils, Montbrun Les Bains, France) and blended in a mechanical mixer before being packaged in airtight containers by Selected Bioproducts as the herbal composite. Samples of the composite were processed for qualitative determination of flavonoid content.
For extraction of the plant material (37), 1 g of the dry composite was ground in a mortar while 25 mL of MeOH was added. The slurry was transferred to a 50-mL centrifuge tube and sonicated for 30 min in an ultrasonication bath. A 3-mL sample of the resultant extract was mixed with 3 mL of ddH2O and then with 1 mL of CHCl3. Thismixture was vortexed at 13 000 × g for 30 min. A 5-mL sample of the supernatant, taken from the upper layer, was dried in a Savant SpeedVac (GMI; Ramsey, Minnesota, USA) at 40°C for 2 h. The dried residue was dissolved in 100 μL of acetonitrile (ACN)/H2O (10/90). The resulting suspension was centrifuged at 13 000 × g for 30 min and filtered through a 0.45-μm filter.
An XTerra MS C18 liquid chromatography column (Waters Chromatography Division, Millipore Canada, Mississauga, Ontario) was used to analyze salicylic acid and flavonoids in the sample extracts, as described previously (37). The flow rate was 0.4 mL/min. The mobile phase (90% H2O/0.2% formic acid; 10% ACN/0.2% formic acid) was ramped in 10% increments every 5 min for a total of 40 min, to a final composition of 10% H2O/0.2% formic acid and 90% ACN/0.2% formic acid. The injection volume was 10 μL. Detection was by mass spectroscopy with electrospray ionization, negative ions being measured over the range of 100 to 1000 m/z (mass-to-charge ratio).
Statistical analysis
Data are reported as mean (and standard error). The respiratory rate, ΔPplmax, and tracheal-aspirate data were analyzed with a 1-tailed, 2-way repeated-measures analysis of variance (ANOVA). The blood-profile data were analyzed with a 2-tailed, 2-way repeated-measures ANOVA. The Holm–Sidak post-hoc test was used to identify significant differences (P < 0.05) between groups.
Results
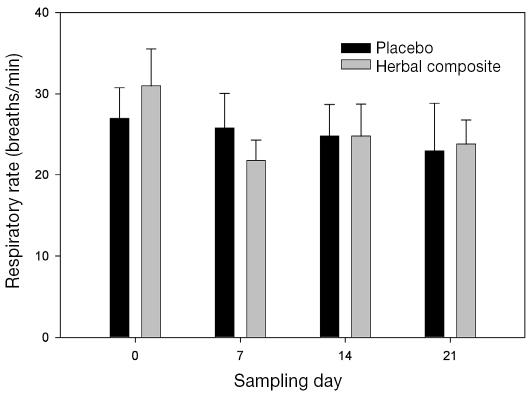
There was a trend to a decrease in respiratory rate when the horses were receiving the herbal composite compared with when they were receiving placebo (P = 0.1) (Figure 1). The mean ΔPplmax was not different at any time during placebo treatment, the mean ranging from 12.7 (5.7) cm H2O on day 0 to 16.3 (6.2) cm H2O on day 21, or during herbal-composite treatment, the mean ranging from 15.0 (6.3) cm H2O on day 0 to 17.0 (7.0) cm H2O on day 21.
Figure 1.
Respiratory rates of horses with recurrent airway obstruction (RAO) during treatment with a placebo or an herbal composite. The decrease from baseline during herbal-composite treatment on all subsequent sampling days was not significant (P = 0.1). There were no differences during placebo treatment.
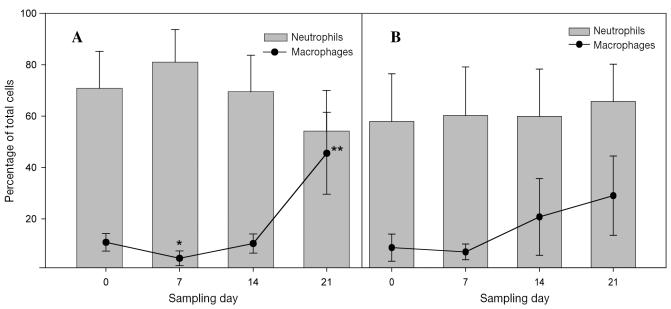
There were no significant differences in the cytologic profile of the tracheal aspirates obtained during herbal-composite treatment compared with those obtained during placebo treatment (Figure 2). During herbal-composite treatment, there was a trend to an increased proportion of macrophages (P = 0.1) and a concurrent, nonsignificant decrease in the proportion of neutrophils.
Figure 2.
Proportions of neutrophils and macrophages in the tracheal aspirates of the horses during herbal-composite (A) and placebo (B) treatment. The single asterisk denotes a significant change from baseline in the proportion of macrophages, the double asterisk a significant change from day 7 in both the proportion of macrophages and the ratio of neutrophils to macrophages. The changes in the proportion of neutrophils were not significant, nor were any of the changes during placebo treatment.
There were no significant differences in any hematologic or biochemical parameters during herbal-composite versus placebo supplementation.
Major flavonoid species identified in the plant material included quercetin, azaleatin, rutin, and kaempferol; minor species detected included quercetin 7-O-glucoside, isovitexin, and isovitexin 2″-O-beta-d-glucoside.
Discussion
The purpose of this study was to obtain preliminary data on the effectiveness of an herbal composite in alleviating the clinical signs of respiratory disease in horses with RAO. The data from this small pilot study demonstrated that horses tended toward a decreased respiratory rate while receiving the herbal composite as compared with a placebo. Although the differences were very small, the number of animals was also small. A greater number of animals is required to measure the benefits of even very potent anti-RAO drugs, such as dexamethasone (38). The statistical power of the tests applied to the data for respiratory rate and ΔPplmax was 0.149 and 0.050, respectively, well below the desired statistical power of 0.8 (39). Given the standard deviations of the mean values for these parameters in our study group (10.4 and 13.5, respectively) and a reported decrease in the values of these parameters of about 30% (8), we would require at least 36 horses in a paired design (or 69 in an unpaired design) to achieve a power of 0.8. We were restricted to a small number of animals for this pilot study, as the high cost of a larger trial must first be justified by some preliminary data, particularly given the nonproprietary nature of the experimental product.
The clinical effectiveness of dietary herbs in general (40), and flavonoids in particular (41), in reducing the incidence and severity of respiratory disease has been well investigated in humans with asthma and in vitro. Other authors have described some benefit of an herbal product containing extract of thyme and primula in horses with RAO, reporting that clinical signs of RAO were not influenced by the product, despite an improvement of about 30% in pulmonary pressure and airway resistance (8). However, that study was designed as a longitudinal study and, as such, could not control for confounding variables such as climatic change, which could have a large influence on pathophysiological indicators of RAO in horses (42). These external factors were controlled for in our study by having an equal number of horses receiving the herbal composite and placebo in the 2 experimental periods.
Supplementation with the herbal composite resulted in a trend to a small decrease in respiratory rate compared with placebo supplementation. Although many factors can contribute to an elevated respiratory rate in RAO-affected horses, including decreased inhibitory function of prostanoids and altered acetylcholine release (43,44), the increase may be mediated, at least in part, by the activation of peroxisome proliferator-activated receptor γ (PPARγ) (45). This nuclear eicosanoid receptor transcription factor is ubiquitously expressed by adipocytes within the wall of the vasculature (46) and in immune cells (47). The protective effect of PPARγ in experimental models of asthma is regulated by the increased concentration of interleukin-10 (IL-10), such that IL-10 behaves as an agonist for PPARγ and enhances its antiasthmatic affect (48). Quercetin, a flavonoid identified in the herbal composite that we used, behaves as a ligand for IL-10 receptors and shows IL-10-like activity (49). Thus, the increasing amount of quercetin within the extracellullar fluid compartment may have increased PPARγ activity in the pulmonary epithelium, contributing to the decrease in respiratory rate in horses receiving the herbal composite compared with when they received placebo.
There is considerable disagreement as to the usefulness of tracheal aspiration as a diagnostic tool in equine airway disease. The results of bronchoalveolar lavage (BAL), a common alternative, have been reported to correlate positively with inflammatory airway disease more readily than the results of tracheal aspiration (50). However, other investigators have found that if only 1 of the techniques is used, accurate diagnosis of inflammatory airway disease is more likely with tracheal aspiration than with BAL (51). For this reason, we chose to use tracheal aspiration to identify changes in cell populations. This small pilot study did not identify significant effects of the herbal composite on the proportion of neutrophils relative to that of macrophages in the tracheal aspirate. However, the proportion of macrophages in the tracheal aspirate increased above the threshold of RAO during herbal supplementation. In contrast, others have reported the mean proportion of macrophages to be clinically normal in horses receiving an herbal composite but representative of RAO in horses receiving a control diet (52). This may indicate a possible effect of this herbal composite on cell populations in the airways of RAO-affected horses. This indication is supported in part by the ability of flavonoids, including quercetin (53), to affect the expression of cell adhesion molecules that regulate homing and extravasation of immune cells from the vasculature into the pulmonary tissues and then provoke departure of these cells back through the draining lymph nodes (54). The small effects observed in our study might have been more pronounced if we had used a combination of tracheal aspiration and BAL, as the combination is reported to be more effective in characterizing inflammatory cell profiles of the lungs of horses than the use of either technique alone (51).
It is likely that compounds in the herbal composite other than flavonoids contribute to effects on airway inflammation. In particular, the composite contains a number of volatile oils known to influence respiratory function (9,55). Further characterization of the herbal composite with respect to volatile constituents is under way in our laboratory.
We conclude that the herbal composite has a broad spectrum of constituent flavonoids that, when fed to horses with symptomatic RAO, contributes to a trend towards attenuation of the elevated respiratory rate and increases the proportion of macrophages in the tracheal aspirate. Further research must use a larger sample and should include BAL as an additional diagnostic technique to more clearly characterize the effect of the composite on RAO-affected horses.
Acknowledgments
This research was conducted with financial assistance from Selected Bioproducts, manufacturer of the herbal composite, and a grant from the National Research Council. Thanks are extended to the owners of the horses for volunteering their participation in the study.
Appendix I
Constituents of the herbal composite and their reported biologic actions related to respiratory health
| In vivo
|
|||
|---|---|---|---|
| Plant material | Human | Animal | In vitro |
| Hyssop officinalis | N/A | N/A | Antiviral (10), antispasmodic (11), antibacterial (12) |
| Allium sativum | Antifungal (13), nonspecific prevention of acute respiratory disease (14) | Antibacterial (15), antiparasitic (16) | Antibacterial (17), anti-inflammatory (18) |
| Pimpinella anisum | N/A | N/A | Smooth muscle relaxant (19), acaricidal (20), antibacterial (21) |
| Marrubium vulgare | N/A | Analgesic (22) | Antispasmodic (23), anti-inflammatory (24) |
| Eupatorium perfoliatum | N/A | N/A | Antibacterial (25) |
| Foeniculum vulgare | N/A | Anti-inflammatory (26), analgesic (27) | Antibacterial (27), antispasmodic (19), acaricidal (20) |
| Glycyrrhiza glabra | Immunomodulator (28) | Antioxidant (29) | Sustains endogenous glucocorticoids in the lung (30) |
| Thymus vulgare | N/A | Treatment of “heaves” in horses (8) | Antibacterial (31), antispasmodic (32) |
N/A — not available
References
- 1.Léguillette R. Recurrent airway obstruction — heaves. Vet Clin Equine. 2003;19:63–86. doi: 10.1016/s0749-0739(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 2.Couetil LL, Art T, de Moffarts B, et al. Effect of beclomethasone dipropionate and dexamethasone isonicotinate on lung function, bronchoalveolar lavage fluid cytology, and transcription factor expression in airways of horses with recurrent airway obstruction. J Vet Intern Med. 2006;20:399–406. doi: 10.1892/0891-6640(2006)20[399:eobdad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Erichsen DF, Aviad AD, Schultz RH, Kennedy TJ. Clinical efficacy and safety of clenbuterol HCl when administered to effect in horses with chronic obstructive pulmonary disease (COPD) Equine Vet J. 1994;26:331–336. doi: 10.1111/j.2042-3306.1994.tb04396.x. [DOI] [PubMed] [Google Scholar]
- 4.Derksen FJ, Olszewski MA, Robinson NE, et al. Aerosolized albuterol sulfate used as a bronchodilator in horses with recurrent airway obstruction. Am J Vet Res. 1999;60:689–693. [PubMed] [Google Scholar]
- 5.Sleeper MM, Kearns CF, McKeever KH. Chronic clenbuterol administration negatively alters cardiac function. Med Sci Sports Exerc. 2002;34:643–650. doi: 10.1097/00005768-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PJ, Slight SH, Ganjam VK, Kreeger JM. Glucocorticoids and laminitis in the horse. Vet Clin North Am Equine Pract. 2002;18:219–236. doi: 10.1016/s0749-0739(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 7.Mair TS. Bacterial pneumonia associated with corticosteroid therapy in three horses. Vet Rec. 1996;138:205–207. doi: 10.1136/vr.138.9.205. [DOI] [PubMed] [Google Scholar]
- 8.van den Hoven R, Zappe H, Zitterl-Eglseer K, Jugl M, Franz C. Study of the effect of Bronchipret on the lung function of five Austrian saddle horses suffering recurrent airway obstruction (heaves) Vet Rec. 2003;152:555–557. doi: 10.1136/vr.152.18.555. [DOI] [PubMed] [Google Scholar]
- 9.Eccles R. Menthol: effects on nasal sensation of airflow and the drive to breathe. Curr Allergy Asthma Rep. 2003;3:210–214. doi: 10.1007/s11882-003-0041-6. [DOI] [PubMed] [Google Scholar]
- 10.Bedoya LM, Palomino SS, Abad MJ, Bermejo P, Alcami J. Screening of selected plant extracts for in vitro inhibitory activity on human immunodeficiency virus. Phytother Res. 2002;16:550–554. doi: 10.1002/ptr.992. [DOI] [PubMed] [Google Scholar]
- 11.Lu M, Battinelli L, Daniele C, Melchioni C, Salvatore G, Mazzanti G. Muscle relaxing activity of Hyssopus officinalis essential oil on isolated intestinal preparations. Planta Med. 2002;68:213–216. doi: 10.1055/s-2002-23139. [DOI] [PubMed] [Google Scholar]
- 12.Marino M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol. 2001;67:187–195. doi: 10.1016/s0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- 13.Davis LE, Shen JK, Cai Y. Antifungal activity in human cerebrospinal fluid and plasma after intravenous administration of Allium sativum. Antimicrob Agents Chemother. 1990;34:651–653. doi: 10.1128/aac.34.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrianova IV, Sobenin IA, Sereda EV, Borodina LI, Studenikin MI. [Effect of long-acting garlic tablets “allicor” on the incidence of acute respiratory viral infections in children. ] Ter Arkh. 2003;75:53–56. [PubMed] [Google Scholar]
- 15.Tsao SM, Hsu CC, Yin MC. Garlic extract and two diallyl sulphides inhibit methicillin-resistant Staphylococcus aureus infection in BALB/cA mice. J Antimicrob Chemother. 2003;52:974–980. doi: 10.1093/jac/dkg476. [DOI] [PubMed] [Google Scholar]
- 16.Nok AJ, Williams S, Chidozie Onyenekwe P. Allium sativum induced death of African trypanosomes. Parasitol Res. 1996;82:634–637. doi: 10.1007/s004360050177. [DOI] [PubMed] [Google Scholar]
- 17.Adeleye IA, Opiah L. Antimicrobial activity of extracts of local cough mixtures on upper respiratory tract bacterial pathogens. West Indian Med J. 2003;52:188–190. [PubMed] [Google Scholar]
- 18.Keiss HP, Dirsch VM, Hartung T, et al. Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr. 2003;133:2171–2175. doi: 10.1093/jn/133.7.2171. [DOI] [PubMed] [Google Scholar]
- 19.Boskabady MH, Khatami A, Nazari A. Possible mechanism(s) for relaxant effects of Foeniculum vulgare on guinea pig tracheal chains. Pharmazie. 2004;59:561–564. [PubMed] [Google Scholar]
- 20.Lee HS. Acaricidal activity of constituents identified in Foeniculum vulgare fruit oil against Dermatophagoides spp. (Acari: Pyroglyphidae) J Agric Food Chem. 2004;52:2887–2889. doi: 10.1021/jf049631t. [DOI] [PubMed] [Google Scholar]
- 21.Tabanca N, Bedir E, Kirimer N, et al. Antimicrobial compounds from Pimpinella species growing in Turkey. Planta Med. 2003;69:933–938. doi: 10.1055/s-2003-45103. [DOI] [PubMed] [Google Scholar]
- 22.Meyre-Silva C, Yunes RA, Schlemper V, Campos-Buzzi F, Cechinel-Filho V. Analgesic potential of marrubiin derivatives, a bioactive diterpene present in Marrubium vulgare (Lamiaceae) Farmaco. 2005;60:321–326. doi: 10.1016/j.farmac.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 23.El-Bardai S, Wibo M, Hamaide MC, Lyoussi B, Quetin-Leclercq J, Morel N. Characterisation of marrubenol, a diterpene extracted from Marrubium vulgare, as an L-type calcium channel blocker. Br J Pharmacol. 2003;140:1211–1216. doi: 10.1038/sj.bjp.0705561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahpaz S, Garbacki N, Tits M, Bailleul F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J Ethnopharmacol. 2002;79:389–392. doi: 10.1016/s0378-8741(01)00415-9. [DOI] [PubMed] [Google Scholar]
- 25.Habtemariam S, Macpherson AM. Cytotoxicity and antibacterial activity of ethanol extract from leaves of a herbal drug, boneset (Eupatorium perfoliatum) Phytother Res. 2000;14:575–577. doi: 10.1002/1099-1573(200011)14:7<575::aid-ptr652>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Choi EM, Hwang JK. Anti-inflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Dadalioglu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem. 2004;52:8255–8260. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- 28.Karimov MM. Use of extracts of Glycerrhiza glabra L. in the correction of some indices of local nonspecific defense in patients with protracted pulmonary pneumonia. Lik Sprava. 2001;5–6:123–125. [PubMed] [Google Scholar]
- 29.Fukai T, Satoh K, Nomura T, Sakagami H. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia. 2003;74:624–629. doi: 10.1016/s0367-326x(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 30.Homma M, Oka K, Niitsuma T, Itoh H. A novel 11 beta- hydroxysteroid dehydrogenase inhibitor contained in saiboku-to, a herbal remedy for steroid-dependent bronchial asthma. J Pharm Pharmacol. 1994;46:305–309. doi: 10.1111/j.2042-7158.1994.tb03799.x. [DOI] [PubMed] [Google Scholar]
- 31.Penalver P, Huerta B, Borge C, Astorga R, Romero R, Perea A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS. 2005;113:1–6. doi: 10.1111/j.1600-0463.2005.apm1130101.x. [DOI] [PubMed] [Google Scholar]
- 32.Meister A, Bernhardt G, Christoffel V, Buschaue A. Antispasmodic activity of Thymus vulgaris extract on the isolated guinea-pig trachea: discrimination between drug and ethanol effects. Planta Med. 1999;65:512–516. doi: 10.1055/s-1999-14006. [DOI] [PubMed] [Google Scholar]
- 33.Robinson NE. International Workshop on Equine Chronic Airway Disease. Michigan State University, 16–18 June 2000. Equine Vet J. 2001;33:5–19. doi: 10.2746/042516401776767412. [DOI] [PubMed] [Google Scholar]
- 34.Subcommittee on Horse Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient Requirements of Horses, 5th revised ed. Washington, DC: National Academy Press, 1989.
- 35.Olfert ED, Cross BM, McWilliams AA, eds. Guide to the Care and Use of Experimental Animals. Volume 1. Ottawa, Ontario: Canadian Council on Animal Care, 1993.
- 36.Greenough A, Morley CJ. Oesophageal pressure measurements in ventilated preterm babies. Arch Dis Child. 1982;57:851–855. doi: 10.1136/adc.57.11.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weckwerth W, Wenzel K, Fiehn O. Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics. 2004;4:78–83. doi: 10.1002/pmic.200200500. [DOI] [PubMed] [Google Scholar]
- 38.Cornelisse CJ, Robinson NE, Berney CE, Kobe CA, Boruta DT, Derksen FJ. Efficacy of oral and intravenous dexamethasone in horses with recurrent airway obstruction. Equine Vet J. 2004;36:426–430. doi: 10.2746/0425164044868413. [DOI] [PubMed] [Google Scholar]
- 39.Livingston EH, Cassidy L. Statistical power and estimation of the number of required subjects for a study based on the t-test: a surgeon’s primer. J Surg Res. 2005;126:149–159. doi: 10.1016/j.jss.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Clement YN, Williams AF, Aranda D, et al. Medicinal herb use among asthmatic patients attending a specialty care facility in Trinidad. BMC Complement. Altern Med. 2005;5:3. doi: 10.1186/1472-6882-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, Wong WS. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am J Respir Crit Care Med. 2003;167:185–192. doi: 10.1164/rccm.200205-420OC. [DOI] [PubMed] [Google Scholar]
- 42.Ward MP, Couetil LL. Climatic and aeroallergen risk factors for chronic obstructive pulmonary disease in horses. Am J Vet Res. 2005;66:818–824. doi: 10.2460/ajvr.2005.66.818. [DOI] [PubMed] [Google Scholar]
- 43.Yu MF, Wang ZW, Robinson NE, Derksen FJ. Modulation of bronchial smooth muscle function in horses with heaves. J Appl Physiol. 1994;77:2149–2154. doi: 10.1152/jappl.1994.77.5.2149. [DOI] [PubMed] [Google Scholar]
- 44.Broadstone RV, LeBlanc PH, Derksen FJ, Robinson NE. In vitro responses of airway smooth muscle from horses with recurrent airway obstruction. Pulm Pharmacol. 1991;4:191–202. doi: 10.1016/0952-0600(91)90011-q. [DOI] [PubMed] [Google Scholar]
- 45.Ward JE, Fernandes DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARgamma ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulm Pharmacol Ther. 2006;19:39–46. doi: 10.1016/j.pupt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator- activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 47.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARγ: differentiation-dependent peroxisomal proliferator-activated receptor gamma expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SR, Lee KS, Park HS, et al. Involvement of IL-10 in peroxisome proliferator-activated receptor gamma-mediated anti-inflammatory response in asthma. Mol Pharmacol. 2005;68:1568–75. doi: 10.1124/mol.105.017160. Epub 2005 Sep 8. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura T, Wang LY, Kusano K, Kitanaka S. Flavonoids that mimic human ligands from the whole plants of Euphorbia lunulata. Chem Pharm Bull (Tokyo) 2005;53:305–308. doi: 10.1248/cpb.53.305. [DOI] [PubMed] [Google Scholar]
- 50.Derksen FJ, Brown CM, Sonea I, Darien BJ, Robinson NE. Comparison of tracheal aspirate and bronchoalveolar lavage cytology in 50 horses with chronic lung disease. Equine Vet J. 1989;21:23–26. doi: 10.1111/j.2042-3306.1989.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 51.Malikides N, Hughes KJ, Hodgson DR, Hodgson JL. Comparison of tracheal aspirates and bronchoalveolar lavage in racehorses. 2. Evaluation of the diagnostic significance of neutrophil percentage. Aust Vet J. 2003;81:685–687. doi: 10.1111/j.1751-0813.2003.tb12540.x. [DOI] [PubMed] [Google Scholar]
- 52.Costa LR, Seahorn TL, Moore RM, Taylor HW, Gaunt SD, Beadle RE. Correlation of clinical score, intrapleural pressure, cytologic findings of bronchoalveolar fluid, and histopathologic lesions of pulmonary tissue in horses with summer pasture- associated obstructive pulmonary disease. Am J Vet Res. 2000;61:167–173. doi: 10.2460/ajvr.2000.61.167. [DOI] [PubMed] [Google Scholar]
- 53.Mochizuki M, Kajiya K, Terao J, et al. Effect of quercetin conjugates on vascular permeability and expression of adhesion molecules. Biofactors. 2004;22:201–204. doi: 10.1002/biof.5520220142. [DOI] [PubMed] [Google Scholar]
- 54.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 55.Inoue K, Takano H, Shiga A, et al. Effects of volatile constituents of a rosemary extract on allergic airway inflammation related to house dust mite allergen in mice. Int J Mol Med. 2005;16:315–319. [PubMed] [Google Scholar]