Abstract
The increasing number of clinical cases of inclusion body hepatitis (IBH) associated with fowl adenoviruses (FAdVs) is a growing concern in different parts of the world, including Canada. After an outbreak of IBH in a 10-d-old pullet broiler breeder flock, we serologically monitored the flock from 8 to 46 wk of age, using the agar gel precipitation test (AGPT) offered by diagnostic laboratories and an FAdV group-specific enzyme-linked immunosorbemt assay (ELISA) developed earlier. In addition, we tested 1-d-old progeny for possible vertical transmission of FAdV when the breeder flock approached the peak of egg production by performing virus isolation and polymerase chain reaction (PCR) procedures on target organs. As in previous studies comparing the 2 tests, ELISA was more sensitive than AGPT. With ELISA, a few birds had weakly positive results at 8 wk of age, and all the birds had strongly positive results from 12 wk of age until the end of the study. This group-specific ELISA is therefore a sensitive and practical way to monitor FAdV antibodies in commercial flocks. None of the 1-d-old chicks tested were positive by PCR, nor was FAdV isolated from the same tissues, indicating an absence of transmission of infectious virus to the progeny. The lack of virus production and transmission could be due to the presence of high antibody titers in the layers.
Résumé
L’augmentation du nombre de cas cliniques d’hépatite à corps d’inclusions (IBH) associés avec l’adénovirus aviaire (FAdV) est une préoccupation grandissante dans différentes parties du monde, incluant le Canada. Suite à un épisode d’IBH chez des poulettes de 10 jours d’âge dans un troupeau d’oiseaux reproducteurs, un suivi sérologique du troupeau a été effectué de l’âge de 8 semaines jusqu’à 46 semaines utilisant un test de précipitation en agar (AGPT) offert par des laboratoires de diagnostic et une épreuve immunoenzymatique (ELISA) spécifique au groupe FAdV développée antérieurement. De plus, la progéniture a été testée pour vérifier une possible transmission verticale lorsque le troupeau reproducteur approcha le pic de production d’œufs, en effectuant une épreuve d’isolement viral et une réaction d’amplification en chaîne par la polymérase (PCR) sur les organes cibles d’oiseaux âgé de 1 jour. Tout comme lors d’études antérieures comparant les deux épreuves, l’ELISA était plus sensible que l’AGPT. Avec l’épreuve ELISA quelques oiseaux ont montré une réaction faiblement positive à 8 semaines d’âge, et tous les oiseaux présentaient des résultats fortement positifs à partir de 12 semaines d’âge jusqu’à la fin de l’étude. L’épreuve ELISA spécifique de groupe est ainsi sensible et pratique pour surveiller les anticorps anti-FAdV dans les troupeaux commerciaux. Aucun des poussins de 1 jour testés ne s’est avéré positif par PCR de même que pour l’isolement de FAdV à partir des mêmes tissus, indiquant ainsi l’absence de transmission du virus infectieux à la progéniture. L’absence de production virale et de transmission pourrait être due à la présence de titres d’anticorps élevés chez les pondeuses.
(Traduit par Docteur Serge Messier)
Introduction
Inclusion body hepatitis (IBH) is usually seen in chickens 3 to 6 wk old. The 1st sign is often a sudden increase in mortality rate in the flock, reaching sometimes 5% to 10%; the rate then returns to normal after 5 to 7 d. The mortality pattern, the age of the flock, and the gross and histopathological findings post mortem are characteristic for the disease, and fowl adenoviruses (FAdVs) can be isolated from the affected birds (1–4). Immunosuppressive agents such as infectious bursal disease virus and chicken infectious anemia virus are thought to predispose chickens to IBH (5–7).
Fowl adenoviruses are ubiquitous and often of low virulence. To date, 11 serotypes in the genus Aviadenovirus of the family Adenoviridae have been recognized. All FAdVs share a group-specific antigen. Certain FAdVs are more pathogenic than others: serotype 4 has been involved in hydropericardium syndrome, and serotype 8 has been implicated in peracute IBH (1,2,5,8). Recently the increased number of FAdV-associated outbreaks of IBH has renewed interest in investigating these viruses, disease pathogenesis, and diagnostic options (9–12).
Serologic surveys of adenovirus infections have been performed at a certain age of chickens and representing a given geographic area. Fowl-adenovirus-specific antibodies are commonly found in breeder and layer flocks, and exposure to multiple serotypes is documented (13–16). The agar gel precipitation test (AGPT) is a widely used serologic test for the detection of FAdV antibodies, since it is fast and economical. However, it has several disadvantages, such as lack of sensitivity (16), and the detection of infection might be delayed in fowl since precipitins may develop only after a 2nd exposure to FAdV (17). The AGPT has a satisfactory sensitivity when performed on serum from commercial flocks that are often exposed to multiple adenovirus serotypes, but it is less sensitive in detecting adenovirus infections in specific-pathogen-free (SPF) flocks (4). The sensitivity of AGPT can be increased by using antigen pooled from different FAdV serotypes (18).
Enzyme-linked immunosorbent assays (ELISAs) for the detection of group-specific or type-specific antibodies to adenoviruses have been described (19–21). These tests are typically more sensitive than the AGPT and represent the method of choice to screen SPF flocks for the presence of adenovirus antibodies. Cross-reactivity between various FAdV serotypes, as reported by Calnek et al (19), is a basis for sensitive detection of exposure to heterologous FAdV serotypes.
Adenoviruses are known to spread very efficiently horizontally and can cause persistent and latent infections (16,22). Periodic virus excretion has been shown for several flocks (17), especially when the local immunity wanes, 8 to 12 wk after the initial infection (4). Furthermore, virus excretion is particularly important around the peak of egg production (16).
We reported a case of IBH in southwestern Ontario in a young broiler breeder pullet flock (23). An adenovirus was isolated from the liver of affected birds. Through analysis by virus neutralization and restriction fragment length polymorphism of the DNA, the isolate was identified as a serotype 2 FAdV of species D. The source and the route of introduction of the virus to the flock could not be established with certainty.
The objectives of the present study were as follows: 1) to monitor serologically the specific breeder flock that experienced IBH at an early age; 2) to compare the commercially available AGPT that uses FAdV-1 as the antigen with an FAdV group-specific ELISA; and 3) to test 1-d-old progeny for possible vertical virus transmission by polymerase chain reaction (PCR) and virus isolation.
Materials and methods
Flock
The flock monitored in this study was a pullet broiler breeder flock located in Ontario, Canada, that experienced IBH at 10 d of age. The mortality rate over the 5-d-long outbreak was 2.1% (23). The flock performed well thereafter during the raising and egg-production periods. The cockerels raised in the next-door barn on the same premises were not affected. Pullets and cockerels were moved to the laying facility when they were 20 wk old.
Serologic testing
A blood sample was collected from each of 15 randomly selected female chickens and each of 15 randomly selected male chickens monthly from 8 to 15 wk of age and from each of 15 randomly selected chickens monthly from 20 to 46 wk of age. The serum samples were tested for FAdV-specific antibodies by AGPT and ELISA, and some randomly selected samples were tested by plaque-reduction assay. The FAdV-1 antigen for the AGPT was purchased from Charles River SPAFAS (North Franklin, Connecticut, USA), and the test was performed in the Animal Health Laboratory, University of Guelph, Guelph, Ontario.
The ELISA was done as previously described (21). Briefly, microtiter plates (Becton-Dickinson, Franklin Lakes, New Jersey, USA) were coated with FAdV-9 antigen treated with N-lauroyl sarcosine at a concentration of 200 ng/well and blocked with 3% bovine serum albumin. The bound antibody was detected by alkaline-phosphatase-labeled goat IgG conjugate against chicken immunoglobulin (Kirkegaard and Perry Laboratories, Gaithersburg, Maryland, USA) and Sigma Fast p-nitrophenyl phosphate (Sigma Chemical Company, St. Louis, Missouri, USA). Optical density (OD) was read in a Bio-Tek ELISA microplate reader (Bio-Tek Industries, Atlanta, Georgia, USA) at 405 nm. The sample-to-positive (S/P) ratio for each readings. Serum was sample was calculated from the mean OD405 diluted to 1/100 for the ELISA and for the plaque-reduction assay, whereas it was undiluted for the AGPT.
To detect virus-neutralizing antibodies, the plaque-reduction assay was done as previously described (23), with use of the FAdV-2 virus isolated from the flock during the IBH outbreak and a serotype 8 FAdV. Briefly, 100 plaque-forming units of the virus in 100 μL of cell culture medium were mixed with 100 μL of some of the diluted serum samples collected at 12 and 33 wk. The mixture was incubated for 1 h, and the samples were then added to chicken hepatoma cells (CH-SAH cell line [24]). After a 1-h adsorption period, the cells were covered with a 0.7% agarose overlay. The plaques were visualized by addition of a 2nd overlay after 3 d of incubation at 37°C in 5% CO2 containing 0.02% neutral red. A sample that inhibited the formation of more than 50% of the plaques compared with the negative-control serum sample was considered positive.
Samples for PCR and virus isolation
The progeny of the breeder flock at the beginning of its peak production (28 wk old) were tested for possible vertical transmission of FAdVs. Fifty 1-d-old chicks were euthanized, and liver, spleen, cecal tonsils, and kidneys were collected from every chick. For 25 chicks the individual organs were kept separately, whereas for the remaining 25 chicks the 4 organs were pooled. All samples were kept frozen at −70°C until PCR and virus isolation were performed.
DNA extraction and PCR
Half a gram of tissue was homogenized and incubated in 600 μL of digestion buffer (0.5 mg of proteinase K, 0.5% sodium dodecyl sulfate, 5 mM of Tris-HCl, and 5 mM of ethylene diamine tetraacetic acid [EDTA], pH 8.0) at 56°C with gentle agitation overnight. The DNA was precipitated after 2 phenol–chloroform extractions, and the washed pellet was resuspended in 30 μL of water.
The procedures for PCR and agarose gel electrophoresis were as previously described (21). The primers, designed on the basis of the FAdV-9 nucleotide sequence within the pVI open reading frame (25), were 5′-TCGTCGTCGTGAGCAGTTGTC-3′ and 3′-TAGGAGCGGTAGTGGGAACGGA-5′. In brief, PCR was done in a 50-μL final volume in 1X PCR buffer containing 2 U of Taq DNA polymerase (Invitrogen, Grand Island, New York, USA), 2.5 mM of M MgCl2, 0.2 mM of each deoxyribonucleotide triphosphate, 0.2 μ of each primer, and 5 μL of DNA. The parameters of cycling in a GeneAmp PCR System 2400 thermocycler (Applied Biosystems, Foster City, California, USA) were 3 min of denaturation at 94°C, followed by 35 cycles of 30 s denaturation at 94°C, annealing at 62°C for 30 s, extension at 72°C for 30 s, and a final 8-min elongation. For analysis, 10 μL of the PCR product was electrophoresed in a 1% agarose gel in TAE buffer (40 mM of Tris, 5 mM of sodium acetate, and 1 mM of EDTA). The DNA bands were visualized with 0.2 mg/mL ethidium bromide in a GelDoc system (Bio-Rad Laboratories, Hercules, California, USA). The 1-kb DNA ladder was from Invitrogen.
Virus isolation
For virus isolation from liver, spleen, cecal tonsils, and kidneys of the 1-d-old chicks, the tissues were homogenized in Dulbecco’s Modified Eagle’s Medium/F12 containing 100 U/mL of penicillin and 100 μg/mL of streptomycin and were then centrifuged at 750 × g for 10 min at 4°C. The supernatant was passed through a 0.45-μm filter and then inoculated into CH-SAH cells cultured in 6-well plates (24). The cultures were incubated until a cytopathic effect (CPE) was observed or for 5 d when no CPE was visible earlier. A sample was considered negative when, after 3 blind passages, no CPE was observed.
Results
Serologic monitoring
Table I summarizes the results of AGPT and ELISA testing for FAdV-specific antibodies in the serum of the pullet broiler breeder flock affected by IBH at 10 d of age. With AGPT, most of the samples collected at 8 and 12 wk of age were negative, whereas most of those collected at 20 wk of age and on were positive. With ELISA, most of the samples collected at 8 wk of age were also negative, the remaining 3 being only weakly positive; however, from 12 wk on, all samples were strongly positive. The values with ELISA were practically constant until 46 wk (the last testing time), although there was a spike to a higher S/P ratio at week 33 (Figure 1).
Table I.
Proportions of serum samples positive for antibodies specific to fowl adenoviruses by an agar gel precipitation test (AGPT) or an enzyme-linked immunosorbent assay (ELISA) in a broiler breeder flock, the pullet flock having been affected by inclusion body hepatitis at 10 d of age
| Age (wk) | Pullets
|
Cockerels
|
Layer flock
|
|||
|---|---|---|---|---|---|---|
| AGPT | ELISA | AGPT | ELISA | AGPT | ELISA | |
| 8 | 0/15 | 3/15 | 2/15 | 0/15 | N/A | N/A |
| 12 | 0/15 | 15/15 | 0/15 | 15/15 | N/A | N/A |
| 15 | 7/15 | 15/15 | 4/15 | 15/15 | N/A | N/A |
| 20 | N/A | N/A | N/A | N/A | 13/15 | 15/15 |
| 24 | N/A | N/A | N/A | N/A | 10/13a | 15/15 |
| 29 | N/A | N/A | N/A | N/A | 15/15 | 15/15 |
| 33 | N/A | N/A | N/A | N/A | 12/15 | 15/15 |
| 38 | N/A | N/A | N/A | N/A | 15/15 | 15/15 |
| 46 | N/A | N/A | N/A | N/A | 14/15 | 15/15 |
N/A — not applicable: the males and females were moved together to a laying facility at 20 wk of age.
The volume of 2 samples was not sufficient for testing.
Figure 1.

Mean sample-to-positive (S/P) ratios, and standard errors of optical densities of bound antibody specific to fowl adenoviruses (FAdVs), as determined in an enzyme-linked immunosorbent assay, in a broiler breeder flock whose pullets were affected by inclusion body hepatitis at 10 d of age.
To answer the question whether the rise in antibody titer at 12 wk was due to reinfection with the same virus serotype that had been found at 14 d of age in the flock or was due to a different serotype, we performed a virus neutralization test with the serum samples collected from 8 pullets and 8 cockerels at 12 wk and with 8 samples collected at 33 wk. All the 12-wk samples neutralized the originally isolated virus. In addition, 1 cockerel sample and 1 pullet sample neutralized a serotype 8 Ontario field isolate of FAdV. Moreover, all the 33-wk samples neutralized both viruses.
Testing for vertical transmission
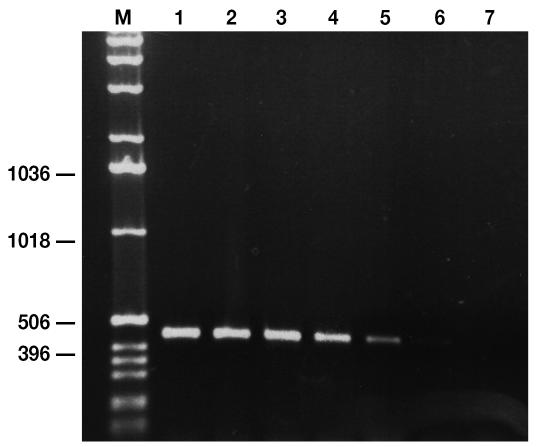
The PCR primers amplified a product of 451 base pairs from the positive control containing FAdV-9 (21). The size of the DNA fragment was indistinguishable by agarose gel analysis when the virus isolated earlier from the breeder flock was the template (Figure 2, lane 1). The sensitivity of the PCR was established by amplifying 10-fold dilutions of purified DNA: 1 pg was the smallest amount of DNA detectable (Figure 2, lane 6). The primers were able to detect different FAdV serotypes and field isolates (data not shown).
Figure 2.
Agarose gel of polymerase chain reaction products amplified with the use of serial 10-fold dilutions of FAdV DNA as the template and stained with ethidium bromide. The sizes of the DNA fragments, in base pairs, are indicated on the left. Lanes 1 to 7 represent 100 ng, 10 ng, 1 ng, 100 pg, 10 pg, 1 pg, and 100 fg of DNA, respectively. M — 1-kb DNA ladder.
After validation of the PCR detection system, we tested liver, spleen, cecal tonsil, and kidney tissues of 1-d-old chickens hatched from eggs collected from 28-wk-old layers for potential vertical transmission of FAdV from the breeder flock. None of the 125 samples of extracted DNA (processed separately for each tissue from 25 chicks and pooled for each of another 25 chicks) produced a DNA band visible in agarose gel after ethidium bromide staining. For almost all of the tissues the DNA extraction and PCR amplification process was done twice.
Virus isolation was attempted with all of the individual and pooled tissues. After 3 blind passages, no CPE, such as cellular rounding, clumping, or detaching from the surface, was observed for any of the samples.
Discussion
In this broiler breeder flock affected with IBH caused by a serotype 2 FAdV at an early age, only a few of the earliest blood samples, collected at week 8, were positive by ELISA, and the results were very close to the cut-off point. By week 12, all samples were strongly positive, and the antibody levels remained high up to the end of the study, at 46 wk. This suggests that seroconversion occurred during the 4-wk interval from 8 to 12 wk of age. Since all 16 of the randomly selected and tested serum samples from 8 pullets and 8 cockerels (from the available 30 samples) collected at 12 wk neutralized the virus isolated at 14 d of age from the flock, seroconversion was probably due to reactivation of the virus or reinfection with a virus of the same serotype. However, 1 cockerel sample and 1 pullet sample from 12 wk also neutralized a serotype 8 Ontario field isolate of FAdV. Moreover, all 8 of the randomly selected (from the available 15) and tested serum samples from week 33 neutralized both viruses, which indicates that another virus, a serotype 8 FAdV, was probably introduced into the flock around 8 to 10 wk of age. Virus isolation was not attempted during the laying period; therefore, the presence and type of virus cannot be identified with certainty.
Clemmer (26) reported that birds infected with the CELO virus were resistant to reinfection with the same serotype 45 d after the primary infection. However, our results are more in agreement with those of Yates et al (27), who were able to reinfect chickens with the same strain after 8 wk. Our results also support the finding of Dawson et al (20) and Calnek et al (19) that the ELISA is more sensitive than the AGPT performed with monovalent FAdV-1 antigen, particularly soon after infection.
We used virus isolation and PCR procedures to determine whether FAdVs were transmitted vertically at the start of peak egg production (at 28 wk of age). None of the tissue samples from 50 1-d-old progeny of the infected breeder flock gave positive results by PCR, and no virus was isolated from the same tissues. Since the primers used in this study can amplify DNA from various sero-types of FAdV, it is not likely that the negative results were due to PCR performance. The PCR was sensitive enough to detect 1 pg of viral DNA, but the possibility that the viral load in the samples was below the detection limit cannot be excluded. However, this was not likely, because this PCR has been shown to be sufficiently sensitive to detect vertical transmission and establishment of latent infection with FAdV in chickens (22). The high titer of neutralizing antibodies may have prevented virus excretion in the pullet flock. It is also possible that our sample size was not high enough to detect a few positive samples. As well, chicks hatched from eggs at a later age could have been tested to determine if transmission did occur later.
The ELISA test used in this study appears to have good potential for practical application to monitor for the presence of adenovirus antibodies in commercial flocks. The test is easily conducted, is broad spectrum since it is not serotype-specific, and is considerably more sensitive than the AGPT in early stages of infection. Our data provide evidence of exposure to FAdV but no evidence of maintenance or vertical transmission in birds with a strong immune response to FAdV.
Acknowledgments
This work was supported by the Poultry Industry Council and the Ontario Ministry of Agriculture, Food and Rural Affairs.
References
- 1.Christensen NH, Saifuddin M. A primary epidemic of inclusion body hepatitis in broilers. Avian Dis. 1989;33:622–630. [PubMed] [Google Scholar]
- 2.Cowen BS. Inclusion body hepatitis anaemia and hydropericardium syndrome: aetiology and control. World Poult Sci J. 1992;48:247–254. [Google Scholar]
- 3.McFerran JB, McCracken RM, Connor TJ, Evans RT. Isolation of viruses from clinical outbreaks of inclusion body hepatitis. Avian Pathol. 1976;5:315–324. doi: 10.1080/03079457608418201. [DOI] [PubMed] [Google Scholar]
- 4.McFerran JB, Smyth JA. Avian adenoviruses. Rev Sci Tech. 2000;19:589–601. [PubMed] [Google Scholar]
- 5.Dohms JE, Metz A. Stress — mechanisms of immunosuppression. Vet Immunol Immunopathol. 1991;30:89–109. doi: 10.1016/0165-2427(91)90011-z. [DOI] [PubMed] [Google Scholar]
- 6.McCracken RM, Adair BM. Avian adenoviruses. In: McFerran JB, McNulty MS, eds. Virus Infections of Birds. Amsterdam: Elsevier Science Publishers BV, 1993:123–144.
- 7.Toro HC, Gonzales C, Cerda L, Hess M, Reyes E, Geissea C. Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/hydropericardium syndrome. Avian Dis. 2000;44:51–58. [PubMed] [Google Scholar]
- 8.Anjum AD, Sabri MA, Iqbal Z. Hydropericardium syndrome in broiler chickens in Pakistan. Vet Rec. 1989;124:247–248. doi: 10.1136/vr.124.10.247. [DOI] [PubMed] [Google Scholar]
- 9.El-Attrache J, Villegas P. Genomic identification and characterization of avian adenoviruses associated with inclusion body hepatitis. Avian Dis. 2001;45:780–787. [PubMed] [Google Scholar]
- 10.Erny KM, Barr DA, Fahey KJ. Molecular characterization of highly virulent fowl adenoviruses associated with outbreaks of inclusion body hepatitis. Avian Pathol. 1991;20:597–606. doi: 10.1080/03079459108418799. [DOI] [PubMed] [Google Scholar]
- 11.Jiang P, Ojkic D, Tuboly T, Huber P, Nagy É. Application of the polymerase chain reaction to detect fowl adenoviruses. Can J Vet Res. 1999;63:124–129. [PMC free article] [PubMed] [Google Scholar]
- 12.Meulemans G, Boschmans M, van den Berg TP, Decaesstecker M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001;30:655–660. doi: 10.1080/03079450120092143. [DOI] [PubMed] [Google Scholar]
- 13.Adair BM, McFerran JB, Calvert VM. Development of a microtitre fluorescent antibody test for serological detection of adenovirus infection in birds. Avian Pathol. 1980;9:291–300. doi: 10.1080/03079458008418414. [DOI] [PubMed] [Google Scholar]
- 14.Cowen B, Calnek BW, Hitchner SB. Broad antigenicity exhibited by some isolates of avian adenovirus. Am J Vet Res. 1977;38:959–962. [PubMed] [Google Scholar]
- 15.Grimes TM, Culver DH, King DJ. Virus-neutralizing antibody titers against 8 avian adenovirus serotypes in breeder hens in Georgia by a microneutralization procedure. Avian Dis. 1977;21:220–229. [PubMed] [Google Scholar]
- 16.McFerran JB, Adair BM. Group I adenovirus infections. In: Saif YM, ed. Diseases of Poultry. Ames, Iowa: Iowa State University Press, 2003:214–227.
- 17.McFerran JB. Immunity to adenoviruses. In: Rose ME, Payne LN, Freeman BM, eds. Avian Immunology. Edinburgh: British Poultry Science, 1981:187–203.
- 18.Cowen BS. A trivalent antigen for the detection of type I avian adenovirus precipitin. Avian Dis. 1987;31:351–354. [PubMed] [Google Scholar]
- 19.Calnek BW, Shek WR, Menendez NA, Stiube P. Serological cross-reactivity of avian adenovirus serotypes in an enzyme-linked immunosorbent assay. Avian Dis. 1982;26:897–906. [PubMed] [Google Scholar]
- 20.Dawson GJ, Orsi LN, Yates VJ, Chang PW, Pronovost AD. An enzyme-linked immunosorbent assay for detection of antibodies to avian adenovirus and avian adenovirus-associated virus in chickens. Avian Dis. 1980;24:393–402. [PubMed] [Google Scholar]
- 21.Ojkic D, Nagy É. Antibody response and virus distribution in chickens inoculated with wild-type and recombinant fowl adenovirus. Vaccine. 2003;22:42–48. doi: 10.1016/s0264-410x(03)00544-9. [DOI] [PubMed] [Google Scholar]
- 22.Grgic H, Philippe C, Ojkic D, Nagy É. Study of vertical transmission of fowl adenoviruses. Can J Vet Res. 2006;70:230–233. [PMC free article] [PubMed] [Google Scholar]
- 23.Philippe C, Grgic H, Nagy É. Inclusion body hepatitis in young broiler breeders associated with a serotype 2 adenovirus in Ontario, Canada. J Appl Poultry Res. 2005;14:588–593. [Google Scholar]
- 24.Alexander HS, Huber P, Cao J, Krell PJ, Nagy É. Growth characteristics of fowl adenovirus type 8 in a chicken hepatoma cell line. J Virol Methods. 1998;74:9–14. doi: 10.1016/s0166-0934(98)00062-7. [DOI] [PubMed] [Google Scholar]
- 25.Ojkic D, Nagy É. The complete nucleotide sequence of fowl adenovirus type 8. J Gen Virol. 2000;81:1833–1837. doi: 10.1099/0022-1317-81-7-1833. [DOI] [PubMed] [Google Scholar]
- 26.Clemmer DL. Experimental enteric infection of chickens with an avian adenovirus (strain 93) Proc Soc Exp Biol Med. 1965;118:943–948. doi: 10.3181/00379727-118-30013. [DOI] [PubMed] [Google Scholar]
- 27.Yates VJ, Rhee YO, Fry DE. Serological response in chickens exposed to a type 1 avian adenovirus alone or in combination with the adeno associated virus. Avian Dis. 1977;21:408–414. [PubMed] [Google Scholar]