Abstract
CD38 is a novel multifunctional protein that serves not only as an antigen but also as an enzyme. It catalyzes the metabolism of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate, two structurally and functionally distinct Ca2+ messengers targeting, respectively, the endoplasmic reticulum and lysosomal Ca2+ stores. The protein has recently been crystallized and its three-dimensional structure solved to a resolution of 1.9 Å. The crystal structure of a binary complex reveals critical interactions between residues at the active site and a bound substrate, providing mechanistic insights to its novel multi-functional catalysis. This article reviews the current advances in the understanding of the structural determinants that control the multiple enzymatic reactions catalyzed by CD38.
INTRODUCTION
CD38 is a membrane-bound protein first identified by monoclonal antibody typing of lymphocytes and thus thought of as a lymphocyte-specific antigen (1). Consistently, its expression in lymphocytes shows stage-related variations and ligation by agonistic antibodies against CD38 can trigger a wide range of responses in various types of blood cells (reviewed in 2–4). However, current results show that CD38 is not lymphocyte-specific, but is ubiquitously expressed in virtually all tissues (5,6). It is present not only on cell surfaces but also in various intracellular organelles (7,8), including the nucleus (9–11). The unexpected discovery that CD38 is homologous to ADP-ribosyl cyclase (12) has brought in a new perspective on the cellular function of CD38 and ushered in a new field of investigation. Indeed, it has now been established that CD38 is a multi-functional enzyme catalyzing the metabolism of two distinct Ca2+ messengers, cyclic ADP-ribose (cADPR) (13,14) and nicotinic acid adenine dinucleotide phosphate (NAADP) (15). The former is a novel cyclic nucleotide that modulates the ryanodine receptor and mobilizes the endoplasmic Ca2+ stores (reviewed in 16,17). NAADP is structurally distinct from cADPR and targets separate Ca2+ stores, acidic organelles like lysosomes (18,19). This article focuses on the structural and mechanistic studies aimed at elucidating the multi-functionality of CD38 catalysis.
CD38 IS A MULTI-FUNCTIONAL ENZYME
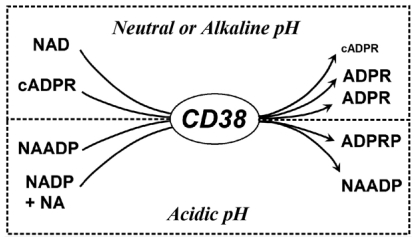
The first indication that CD38 may be an enzyme came from a sequence comparison showing that 86 of the 256 residues of ADP-ribosyl cyclase from Aplysia are identical to CD38 (12). The Aplysia cyclase is the first enzyme that was found to cyclize NAD, a linear molecule, to cADPR, a cyclic product (20). Subsequent studies establish that CD38 indeed catalyzes the cyclization of NAD to produce cADPR (5,21–23). However, unlike the Aplysia cyclase, CD38 cyclizes only a small amount of the substrate, while the majority is hydrolyzed to ADP-ribose instead, providing the first evidence that it is a multifunctional enzyme (Figure 1). Surprisingly, CD38 also uses cADPR, the product, as substrate and catalyzes its hydrolysis to ADP-ribose. This is unexpected because NAD is a linear molecule, structurally distinct from the cyclic and highly compact cADPR, as revealed by X-ray crystallography (14). In fact, CD38 is, so far, the only enzyme ever identified that can hydrolyze cADPR to ADP-ribose.
Figure 1.
The multiple enzymatic reactions catalyzed by CD38. Abbreviations used: cyclic ADP-ribose, cADPR; ADP-ribose, ADPR; nicotinic acid adenine dinucleotide phosphate, NAADP; nicotinic acid, NA; ADP-ribose-2’-phosphate, ADPRP.
The functionality of CD38 turns out to extend much farther, and it can use NADP as substrate as well. In the presence of nicotinic acid, it catalyzes the exchange of the nicotinamide group of NADP with nicotinic acid to produce NAADP (24). Furthermore, most recent results show that CD38 can in fact take NAADP, the product, as substrate and hydrolyze it to ADP-ribose 2’-phosphate (ADPRP) (25). Intriguingly, the two reactions involving NAADP occur only at acidic pH.
With the recent finding of the NAAD-Pase activity, the symmetry is thus complete; at neutral or alkaline pH, CD38 catalyzes the synthesis and hydrolysis of cADPR, while at acidic pH, it catalyzes the synthesis and hydrolysis of NAADP instead. The acidic dependence of the NAADP metabolism and its biological function in targeting the acidic Ca2+ stores in cells (18,19) may not be a simple coincidence, but, instead, may suggest NAADP functioning as a Ca2+ messenger specifically for the acidic organelles of the endocytic pathway in cells.
If it were not because of the biological significance of cADPR and NAADP, this multi-functionality of CD38 could just be an irrelevant curiosity of a promiscuous enzyme. Indeed, accumulated evidence over the past decade has firmly established that cADPR and NAADP are Ca2+ messengers active in a wide range of cellular systems that span three biological kingdoms from protist, plant, to animal, including human (reviewed in 16,17,26,27). The cellular functions they regulate are equally widespread and include fertilization (28–30), T-cell activation (31,32), chemotaxis (33), cell proliferation (34,35), insulin secretion (36), neurite outgrowth (37,38), long-term synaptic depression (39,40), and plant response to stress (41), just to list a few.
CD38 AND ITS HOMOLOGS
CD38 belongs to a multi-member family of proteins that catalyzes the cyclization of NAD to cADPR. The defining member of this family is the Aplysia ADP-ribosyl cyclase (20). It is a soluble protein of 256 residues that is unusually abundant in Aplysia ovotestis (20,42). CD38, in contrast, is a Type II glycosylated membrane protein with a single transmembrane segment near its N-terminus (43). The third member of the family is CD157 (or BST-1), a GPI-anchored antigen (44). Overall, the three proteins share about 25–30% sequence identity. A stretch of nine residues, TLEDTLLGY, present close to the middle of CD38, is highly conserved among the three homologs. Another conservative feature is the positions of the cysteines. The ten cysteines in the cyclase can be perfectly aligned with those in CD38 and CD157. Both CD38 and the cyclase can cyclize NAD into cADPR (reviewed in 45,46), as well as catalyze a base-exchange reaction using NADP as substrate and producing NAADP in the presence of nicotinic acid (24). The cyclase activity of CD157 is much lower in comparison (for example, compare 47 and 48). Whether it can catalyze the base-exchange reaction has not been determined. Because of their function and sequence similarities, these three proteins constitute the ADP-ribosyl cyclase family (46).
A recent study suggests there is a fourth member of the cyclase family, another GPI-anchored protein found in Schistosoma mansoni, a human parasite (49). This protein has 21% sequence identity with human CD38, possesses the TLEDTL-conserved motif and its cysteines also align with the other members of the family. It is also an enzyme, catalyzing both the base-exchange reaction to produce NAADP from NADP and hydrolysis of NAD to ADP-ribose as well. Its cyclase activity, the synthesis of cADPR from NAD, is, however, very low, lower even than that of CD157 (49).
Despite differences in some enzyme characteristics, one consistent feature that is common for all the members of the cyclase family is that they all efficiently catalyze the cyclization of NGD, an analog of NAD, to cyclic GDP-ribose (cGDPR), a fluorescent analog of cADPR that is much more stable to hydrolysis (50). This reaction has thus been used as a simple fluorimetric assay for identifying cyclase homologs and can diagnostically distinguish the unrelated NADases (for example, from Neurospora) that have no cyclase activity at all (51,52).
CRYSTALLOGRAPHY OF CD38 AND HOMOLOGS
The first member of the cyclase family, the Aplysia cyclase, is a soluble protein and large amounts are present in the ovotestis. It is thus also the first to be crystallized and its native structure solved (53). It is a dimer, both in solution and in the crystals, formed from two identical monomers in a head-to-head fashion (53,54). The dimeric structure is physiologically stable because three of its ten helixes and both hydrophobic and ionic residues are involved in forming the contacts (45).
A highly efficient yeast expression system has been developed to enable structure-function studies and to facilitate purification of much of the cyclase for crystallography (55). The cyclase cDNA is spliced behind a secretion signal, the yeast α-factor for mating, and the whole construct put under the control of an alcohol oxidase promoter. When the yeast is presented with methanol as the sole carbon source, the promoter activates the expression of the cyclase and the secretion signal then directs its exportation. In a standard fermentor, as much as 0.5 g/L of cyclase can be secreted by the yeast and thus can easily be purified from the medium (55).
Co-crystallization of the recombinant cyclase with nicotinamide, a substrate for the base-exchange reaction, identifies a deep pocket at the middle cleft of the molecule as the active site. The conserved TLEDTL-segment forms the bottom of the pocket (56). The structures of both the recombinant cyclase produced in the yeast and the native protein are identical in all aspects, including secondary structures and disulfide formation, verifying the yeast expression system. Critical residues at the active site have been analyzed by site-directed mutagenesis. The results show that Glu179 is likely the catalytic residue, because changing it to even a conservative aspartate essentially eliminates all its enzymatic activities. Three other critical residues identified are Trp77, Trp140 and Glu98 (56). It has been proposed that the two tryptophans are responsible for molding the substrate, NAD, a long linear molecule, to a folded conformation such that the two ends can be coupled to form the cyclic product, cADPR (57).
The second member of the cyclase family crystallized was CD157 (58). Like the Aplysia cyclase, it is also a head-to-head dimer. Co-crystallization with various substrates likewise identifies the active site at a similar location. This is possible, presumably, because of the low enzymatic activities of CD157 as compared with the Aplysia cyclase, otherwise the substrates would be converted during the crystallization process. The alignment of the active site pockets of the two molecules shows that they are virtually superimposable (58). The puzzle of why the enzymatic activities of the two proteins are so different remains to be resolved.
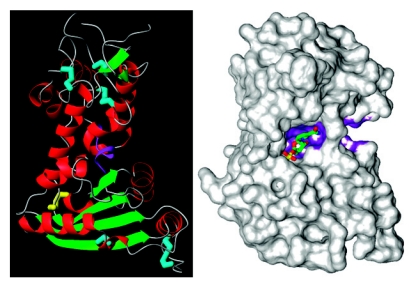
The structure of CD38 turns out to be more difficult to solve and has been accomplished only recently (59). The yeast expression system as described for the large scale production of the Aplysia cyclase is used to express a construct that has the transmembrane segment of CD38 deleted and the glycosylation sites mutated (60). The extra-membrane domain thus produced is fully active enzymatically (61) and is crystallized as head-to-tail dimers (59). The crystal structure of the extra-membrane domain has been solved to 1.9 Å (59). As shown in Figure 2 (left panel), the β-structures are mainly in the C-domain, while the helixes are in the N-domain. The secondary structures of CD38 and the Aplysia cyclase are thus very similar (53). Also shown in the Figure is that all twelve cysteines are paired up as disulfides (cyan). The pair that is not conserved with the Aplysia cyclase is colored yellow. The conserved TLEDTL-motif segment is in the middle of the protein and is colored in purple.
Figure 2.
Crystal Structures of CD38. Left panel. Color codes for the secondary structures are: green, β-structures; red, α-helix; cyan, conserved disulfide bonds; yellow, unique disulfide bond; purple, TLEDTL-conserved motif. Right panel. The van der Waal’s surface of CD38 is shown. The bound substrate molecule at the active site is nicotinamide mononucleotide. The surface of the TLEDTL-conserved motif is colored purple.
The high enzymatic activities of CD38 prelude co-crystallization with substrates. To circumvent this problem, an inactive mutant of CD38 is used instead (25). Site-directed mutagenesis studies show that Glu226 is most likely the catalytic residue, because any modification renders CD38 catalytically inactive (61). This residue is equivalent to the catalytic Glu179 of the Aplysia cyclase. To prevent the residual enzyme activity of the inactive mutant from converting the substrate, pre-formed crystals of E226G, with Glu226 changed to glycine, is incubated briefly with a substrate analog of NAD, nicotinamide mononucleotide (NMN), to allow its diffusion into the active site. NMN is a good analog because CD38 can readily hydrolyze it to ribose-5-phosphate and ara-F-NMN is a potent inhibitor of its enzymatic activities (62). Figure 2 (right panel) shows a surface map of E226G with a molecule of NMN bound to its active site pocket at the middle of the protein (25). The surface of the conserved TLEDTL-segment is colored purple and forms the bottom of the pocket. A surface potential map of CD38 is shown in Figure 3. The active site (yellow) is mostly negatively charged (red) with its abundance of acidic residues. The N-terminal region which interacts with the membrane is mostly positively charged (blue). This could provide preferential interaction with negatively charged phospholipids in the membrane. In the Figure, the transmembrane segment is modeled arbitrarily as a helix, while the N-terminal segment is depicted as a coil.
Figure 3.
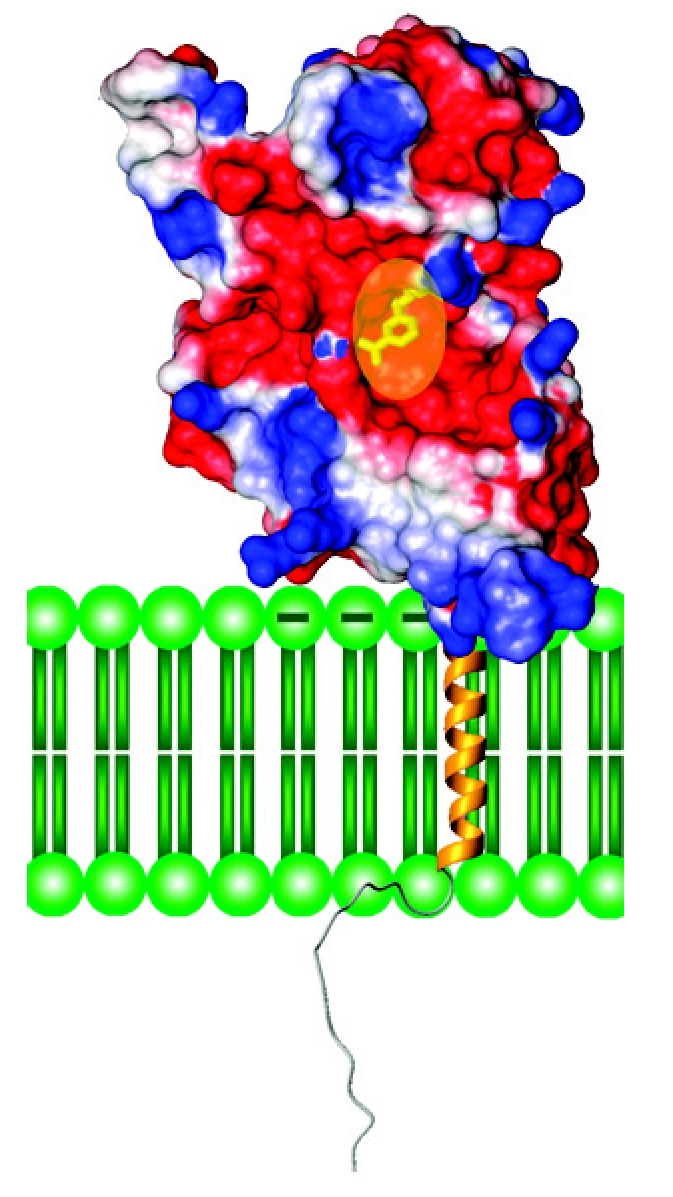
A model of native CD38 embedded in a membrane. The surface potential of the extra-membrane domain is based on crystallography, with the negative charged regions colored red, while positively charged are blue. The transparent oval (yellow) indicates the location of the active site pocket. The transmembrane segment is modeled arbitrarily as a helix (gold), while the N-terminal tail as a random coil. Phospholipids of the membrane are colored green.
STRUCTURE AND ENZYMATIC FUNCTIONS OF CD38
The high resolution structure (1.9 Å) of the apoprotein of CD38 and the success of obtaining binary substrate-enzyme complexes represent major advances, providing not only a high resolution view of the molecule but also setting the stage for pinpointing the structural determinants that control its novel multi-functionality.
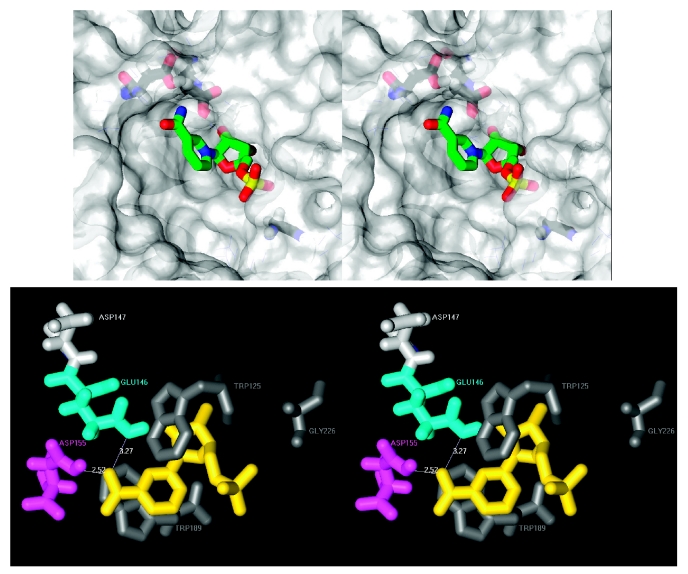
The first question that has been addressed recently is the unusual acidic dependency of the NAADP synthesis and hydrolysis reactions catalyzed by CD38 (25). The abundance of acidic residues at the active site pocket suggests the possibility that they may exert electrostatic repulsion to the nicotinic acid group of NAADP that is negatively charged at neutral pH (pKa ≈ 5), inhibiting its binding to the site and thus restricting its hydrolysis only at acidic pH when both the group and the residues are protonated. Figure 4 shows the stereo and closed up view of the active site (25). It can be seen that the nicotinamide group of NMN, a substrate analog of NAADP, is indeed bound very close to two acidic residues, Glu146 (3.27 Å) and Asp155 (2.52 Å). Two tryptophans (Trp125 and Trp189) are also close to the substrate. Mutagenesis studies confirm that both residues are critical for the enzymatic activities of CD38 (61). They are equivalent to Trp77 and Trp140 of the Aplysia cyclase, which also are critical for the enzymatic activities of the cyclase. The ring of Trp189 in CD38 is parallel to and stacks with the nicotinamide ring of NMN, indicating the residue is crucial in coordinating the binding of the substrate. Also shown in the Figure is the residue Gly226, which, in this mutant protein, replaces the catalytic Glu226 of the wildtype.
Figure 4.
Stereo views of the active site of CD38. Top panel. A close-up surface view is shown with a molecule of nicotinamide mononucleotide (NMN) bound to the active site pocket. Color coding for its atoms: green, carbon; blue, nitrogen; yellow, phosphorus; red, oxygen. Hydrogen atoms are not shown for clarity. The van der Waal’s surface are colored transparent white, allowing the critical residues to be seen. Color code for atoms of the residues: black, carbon; cyan, nitrogen; red, oxygen; white, hydrogen. Bottom panel. Critical residues and NMN (yellow) are shown. Numbers are distances in Angstroms. Glu146 is colored cyan; Asp155, magenta; Asp147, light gray; and dark gray for Trp125, Trp189, and Gly226.
The close contacts between Glu146 and Asp155, which are negatively charged at neutral pH, and the nicotinamide group of NMN indicate that, if the substrate were NAADP instead, the electrostatic repulsion of the residues to the charged nicotinic acid group would prevent its binding to the active site and, therefore, its hydrolysis. This provides structural evidence for the inability of CD38 to hydrolyze NAADP at neutral pH (25). Changing Glu146 to a neutral residue, such as glycine or alanine, or Asp155 to glutamine or asparagine, eliminates the electrostatic repulsion between the active site residues and the substrate and, indeed, restores the ability of CD38 to catalyze the hydrolysis of NAADP even at alkaline pH. The adjacent residue of Glu146 is another acidic residue, Asp147. As shown in Figure 4, it is, however, much farther from the nicotinamide ring of the bound NMN substrate and is also pointing away from the substrate. It is thus expected to have much less electrostatic effect on the substrate binding. This is indeed the case as changing Asp147 to valine, also a neutral residue, has very little effect on the acidic dependence of the NAADPase activity.
Both Glu146 and Asp155 also are important in controlling the acidic dependency of the synthesis of NAADP via the base-exchange reaction. Changing them to neutral residues likewise allows the reaction that normally occurs only at acidic pH to proceed even at alkaline pH (25). This indicates that nicotinic acid, the co-substrate of the base-exchange reaction, most likely also binds at the same location close to Glu146 and Asp155 during the reaction and its repulsion at neutral pH by these residues, again, account for the inhibition of the reaction.
The above findings in CD38 actually extend to the Aplysia cyclase as well. Incubation of the cyclase with NAADP results in its cyclization to cyclic ADP-ribose-2’-phosphate (cADPRP) and this reaction, similar to the NAADPase reaction of CD38, occurs only at acidic pH (25). Changing Glu98, which is equivalent to Glu146 of CD38, to a neutral residue allows not only the cyclization of NAADP but also the synthesis of NAADP via the base-exchange reaction to proceed at alkaline pH (25).
That both the hydrolysis and synthesis reactions occur at the same site and are controlled by the same glutamate residue (Glu146 in CD38 and Glu98 in cyclase) is consistent with the unified mechanism previously proposed for the multi-functional catalysis of the cyclase family (57). Accordingly, the tryptophans at the active site pocket mold the linear substrate, NAD, NADP, or NAADP, to a cyclic conformation. The binding is controlled by the glutamate residue such that negatively charged molecules like NAADP, can bind, and thus serve as a substrate, only when it is protonated at acidic pH. Catalytic attack by another glutamate, Glu226 of CD38 or Glu179 of the cyclase, releases the nicotinamide (NAD or NADP as substrate) or the nicotinic acid group (NAADP as substrate) and forms an activated intermediate. Nucleophilic attack of the intermediate by either water or nicotinic acid respectively leads to either hydrolysis or synthesis via base-exchange. The latter can occur only at acidic pH because the access of nicotinic acid to the intermediate is, likewise, electrostatically repelled by the controlling glutamate residue that is negatively charged at neutral or alkaline pH.
Finally, cyclization occurs if the intermediate is attacked intra-molecularly by the adenine group of the substrate, which can readily occur at neutral or alkaline pH as the adenine ring is uncharged and not repulsed by the negative charge of the controlling glutamate residue.
PHYSIOLOGICAL FUNCTIONS OF CD38
The enzymatic activities of CD38 characterized in vitro are physiologically relevant. Studies using knockout mice convincingly establish that CD38 is responsible for regulating cADPR levels in vivo, because most tissues in the CD38 knockout mice show large decreases in endogenous cADPR levels (33,63). A notable exception is in the brain, which shows only about 40% decrease, suggesting, in this case, another cyclase may be present to compensate for the CD38 deficiency. Indeed, cyclase activities with characteristics different from CD38 and CD157 have been found in the brain of the CD38 knockout mice (64). In contrast, in the pancreas, a large decrease in endogenous cADPR is seen in the knockout mice [65]. In the islets, the disruption of CD38 impairs glucose-induced increases of cADPR, intracellular Ca2+ and insulin secretion (66). In the acinar cells, the acetylcholine-induced cADPR elevation is eliminated, leading to significant alterations of the Ca2+ signaling patterns (65). These results indicate that CD38 is responsible not only for maintaining the basal cADPR levels in cells and tissues, but also for transducing stimulus signals of glucose and acetylcholine for example, through the cADPR-dependent Ca2+ signaling pathway. Likewise, in neutrophils, cADPR production by CD38 is found to regulate both intracellular Ca2+ release and extracellular Ca2+ influx during chemotaxis and is required for bacterial clearance in vivo (33). CD38 knockout mice also show other defects, including disordered osteoclast formation and function (67), altered airway responsiveness (68), impairment of dendritic cell trafficking, and reduced humoral immune response (69), inhibition of α-adrenoceptor stimulated contraction in aorta (70) and cardiac hypertrophy (71). The physiological functions of CD38 are clearly widespread.
Whether CD38 is responsible for metabolism of NAADP in vivo has only just begun to be explored. This is partly due to the lack of a convenient assay for endogenous NAADP. The development of a highly sensitive fluorimetric cycling assay using coupled enzyme systems should facilitate the progress (32,72). Recent results on the knockout mice presented in an international meeting on CD38 (Torino, Italy, 2006) do indicate that it is likely to be responsible for mediating the agonist-induced elevation of NAADP in the pancreatic acinar cells (Cancela and Galione, unpublished).
As described above, CD38 catalyzes not only the synthesis of cADPR and NAADP, but also their hydrolysis. In fact, cADPR is resistant to all nucleotide phosphodiesterases as well as phosphatases tested and CD38 is the only known enzyme that can specifically hydrolyze cADPR (73). In contrast, there are two known pathways for the degradation of NAADP. It is sensitive to many nucleotide phosphodiesterases and phosphatases (32,72). As described above, CD38 also can hydrolyze it to ADPRP. This activity is specific for NAADP but not other common nucleotides. With its hydrolytic activities toward cADPR, NAD, NADP, and NAADP, CD38 appears to be a degradation enzyme specific for purine nucleotides. It remains to be established whether CD38 is responsible for degrading cADPR and NAADP in vivo. Nevertheless, it is clearly advantageous if both the synthesis and degradation of these novel messengers are catalyzed by a single enzyme. This would ensure that the degradation pathway for the two Ca2+ messengers would be at the precise location of their synthesis, facilitating their efficient removal after their signaling functions are completed.
Clearly, the most intriguing question that remains to be elucidated is how these multiple functions of CD38 are regulated. The availability of the high resolution structure and the success of obtaining binary substrate complexes should now set the stage for pinpointing the structural determinants of regulation. Indeed, the solution of the unusual pH-dependency described above represents the first step toward that final goal.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci. 1980;77:1588–92. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malavasi F, et al. CD38: a multi-lineage cell activation molecule with a split personality. Int J Clin Lab Res. 1992;22:73–80. doi: 10.1007/BF02591400. [DOI] [PubMed] [Google Scholar]
- 3.Malavasi F, et al. Human CD38: a glycoprotein in search of a function. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 4.Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J. 1996;10:1408–17. doi: 10.1096/fasebj.10.12.8903511. [DOI] [PubMed] [Google Scholar]
- 5.Takasawa S, et al. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J Biol Chem. 1993;268:26052–4. [PubMed] [Google Scholar]
- 6.Fernandez JE, et al. Analysis of the distribution of human CD38 and of its ligand CD31 in normal tissues. J Biol Regul Homeostatic Agents. 1998;12:81–91. [PubMed] [Google Scholar]
- 7.Yamada M, Mizuguchi M, Otsuka N, Ikeda K, Takahashi H. Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res. 1997;756:52–60. doi: 10.1016/s0006-8993(97)00117-0. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, et al. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J. 2002;16:302–14. doi: 10.1096/fj.01-0705com. [DOI] [PubMed] [Google Scholar]
- 9.Adebanjo OA, et al. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nature Cell Biol. 1999;1:409–14. doi: 10.1038/15640. [DOI] [PubMed] [Google Scholar]
- 10.Khoo KM, et al. Localization of the cyclic ADP-ribose-dependent calcium signaling pathway in hepatocyte nucleus. J Biol Chem. 2000;275:24807–17. doi: 10.1074/jbc.M908231199. [DOI] [PubMed] [Google Scholar]
- 11.Yalcintepe L, et al. Nuclear CD38 in retinoic acid-induced HL-60 cells. Exper Cell Res. 2005;303:14–21. doi: 10.1016/j.yexcr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 12.States DJ, Walseth TF, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:1608–15. [PubMed] [Google Scholar]
- 14.Lee HC, Aarhus R, Levitt D. The crystal structure of cyclic ADP-ribose. Nature Struct Biol. 1994;1:143–4. doi: 10.1038/nsb0394-143. [DOI] [PubMed] [Google Scholar]
- 15.Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem. 1995;270:2152–7. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 16.Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–64. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 17.Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Ann Rev Pharmacol Toxicol. 2001;41:317–45. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 18.Churchill GC, et al. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–8. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki M, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–40. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 20.Lee HC, Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;2:203–9. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HC, et al. Production and hydrolysis of cyclic ADP-ribose at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993;191:639–45. doi: 10.1006/bbrc.1993.1265. [DOI] [PubMed] [Google Scholar]
- 22.Howard M, et al. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–9. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Jacobson EL, Jacobson MK. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993;261:1330–3. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- 24.Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem. 1995;270:30327–33. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- 25.Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine NAADP synthesis and hydrolysis activities. J Biol Chem. 2006;21:21. doi: 10.1074/jbc.M604370200. [DOI] [PubMed] [Google Scholar]
- 26.Lee HC. (2002) Cyclic ADP-ribose and NAADP. Structures, Metabolism and Functions Dordrecht: Kluwer Academic Publishers.
- 27.Lee HC. Nicotinic acid adenine dinu-cleotide phosphate (NAADP)-mediated calcium signaling. J Biol Chem. 2005;280:33693–6. doi: 10.1074/jbc.R500012200. [DOI] [PubMed] [Google Scholar]
- 28.Churchill GC, et al. Sperm deliver a new second messenger: NAADP. Curr Biol. 2003;13:125–8. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee HC, Aarhus R, Walseth TF. Calcium mobilization by dual receptors during fertilization of sea urchin eggs. Science. 1993;261:352–5. doi: 10.1126/science.8392749. [DOI] [PubMed] [Google Scholar]
- 30.Galione A, et al. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993;261:348–52. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- 31.Guse AH, et al. Regulation of calcium signaling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–3. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 32.Gasser A, Bruhn S, Guse AH. Second messenger function of nicotinic acid adenine dinucleotide phosphate (NAADP) revealed by an improved enzymatic cycling assay. J Biol Chem. 2006;281:16906–13. doi: 10.1074/jbc.M601347200. [DOI] [PubMed] [Google Scholar]
- 33.Partida-Sanchez S, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nature Med. 2001;7:1209–16. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 34.Zocchi E, et al. Stroma-generated cyclic ADP-ribose stimulates the expansion of early human hemopoietic progenitors by a paracrine interaction. FASEB J. 2001;15:29. doi: 10.1096/fj.00-0803fje. [DOI] [PubMed] [Google Scholar]
- 35.Bruzzone S, De Flora A, Usai C, Graeff R, Lee HC. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated proliferation of human peripheral blood mononuclear cells. Biochem J. 2003;375:395–403. doi: 10.1042/BJ20030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamoto H, Takasawa S. Recent advances in the Okamoto Model: The CD38-Cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in {beta}-Cells. Diabetes. 2002;51:S462–73. doi: 10.2337/diabetes.51.2007.s462. [DOI] [PubMed] [Google Scholar]
- 37.Brailoiu E, et al. Messenger-specific role for NAADP in neuronal differentiation. J Biol Chem. 2006;281:15923–8. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- 38.Brailoiu E, et al. NAADP potentiates neurite outgrowth. J Biol Chem. 2005;280:5646–50. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- 39.Reyes-Harde M, Empson R, Potter BVL, Galione A, Stanton PK. Evidence of a role for cyclic ADP-ribose in long-term synaptic depression in hippocampus. Proc Natl Acad Sci. 1999;96:4061–6. doi: 10.1073/pnas.96.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes-Harde M, Potter BVL, Galione A, Stanton PK. Induction of hippocampal LTD requires nitric-oxide-stimulated PKG activity and Ca2+ release from cyclic ADP-ribose-sensitive stores. J Neurophysiol. 1999;82:1569–76. doi: 10.1152/jn.1999.82.3.1569. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, et al. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–30. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 42.Hellmich MR, Strumwasser F. Purification and characterization of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991;2:193–202. doi: 10.1091/mbc.2.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–5. [PubMed] [Google Scholar]
- 44.Itoh M, et al. Molecular cloning of murine BST-1 having homology with CD38 and Aplysia ADP-ribosyl cyclase. Biochem Biophys Res Commun. 1994;203:1309–17. doi: 10.1006/bbrc.1994.2325. [DOI] [PubMed] [Google Scholar]
- 45.Lee HC. Enzymatic functions and structures of CD38 and homologs. Chem Immunol. 2000;75:39–59. doi: 10.1159/000058774. [DOI] [PubMed] [Google Scholar]
- 46.Lee HC, Munshi CB, Graeff R. 2002. ADP-ribosyl cyclase-A family of cADPR and NAADP metabolizing enzymes. In Cyclic ADP-ribose and NAADP. Structures, Metabolism and Functions, ed. H. C. Lee, pp. 23–43. Dordrecht: Kluwer Academic Publishers.
- 47.Yamamoto-Katayama S, et al. Site-directed removal of N-glycosylation sites in BST-1/CD157: effects on molecular and functional heterogeneity. Biochem J. 2001;357:385–92. doi: 10.1042/0264-6021:3570385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graeff R, Munshi C, Aarhus R, Johns M, Lee HC. A single residue at the active site of CD38 determines its NAD cyclizing and hydrolyzing activities. J Biol Chem. 2001;276:12169–73. doi: 10.1074/jbc.M011299200. [DOI] [PubMed] [Google Scholar]
- 49.Goodrich SP, et al. Production of calcium-mobilizing metabolites by a novel member of the ADP-ribosyl cyclase family expressed in Schistosoma mansoni. Biochemistry. 2005;44:11082–97. doi: 10.1021/bi050704r. [DOI] [PubMed] [Google Scholar]
- 50.Graeff RM, Mehta K, Lee HC. GDP-ribosyl cyclase activity as a measure of CD38 induction by retinoic acid in HL-60 cells. Biochem Biophys Res Commun. 1994;205:722–7. doi: 10.1006/bbrc.1994.2725. [DOI] [PubMed] [Google Scholar]
- 51.Graeff RM, Walseth TF, Hill HK, Lee HC. Fluorescent analogs of cyclic ADP-ribose: synthesis, spectral characterization, and use. Biochemistry. 1996;35:379–86. doi: 10.1021/bi952083f. [DOI] [PubMed] [Google Scholar]
- 52.Lee HC, Graeff R, Walseth TF. Cyclic ADP-ribose and its metabolic enzymes. Biochimie. 1995;77:345–55. doi: 10.1016/0300-9084(96)88145-4. [DOI] [PubMed] [Google Scholar]
- 53.Prasad GS, et al. Crystal structure of Aplysia ADP ribosyl cyclase, a homologue of the bifunctional ectozyme CD38. Nature Struct Biol. 1996;3:957–64. doi: 10.1038/nsb1196-957. [DOI] [PubMed] [Google Scholar]
- 54.Munshi C, Baumann C, Levitt D, Bloomfield VA, Lee HC. The homodimeric form of ADP-ribosyl cyclase in solution. Biochim Biophys Acta. 1998;1388:428–36. doi: 10.1016/s0167-4838(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 55.Munshi C, Lee HC. High-level expression of recombinant Aplysia ADP-ribosyl cyclase in Pichia Pastoris by fermentation. Prot Express Purif. 1997;11:104–10. doi: 10.1006/prep.1997.0773. [DOI] [PubMed] [Google Scholar]
- 56.Munshi C, et al. Characterization of the active site of ADP-ribosyl cyclase. J Biol Chem. 1999;274:30770–7. doi: 10.1074/jbc.274.43.30770. [DOI] [PubMed] [Google Scholar]
- 57.Lee HC. A unified mechanism for enzymatic synthesis of two calcium messengers, cyclic ADP-ribose and NAADP. Biol Chem. 1999;380:785–93. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto-Katayama S, et al. Crystallographic studies on human BST-1/CD157 with ADP-ribosyl cyclase and NAD glycohydrolase activities. J Mol Biol. 2002;316:711–23. doi: 10.1006/jmbi.2001.5386. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q, et al. Crystal structure of human CD38 extracellular domain. Structure. 2005;13:1331–9. doi: 10.1016/j.str.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Munshi CB, Fryxell KB, Lee HC, Branton WD. Large scale production of human CD38 in yeast by fermentation. Methods Enzymol. 1997;280:318–30. doi: 10.1016/s0076-6879(97)80123-1. [DOI] [PubMed] [Google Scholar]
- 61.Munshi C, et al. Identification of the enzymatic active site of CD38 by site-directed mutagenesis. J Biol Chem. 2000;275:21566–71. doi: 10.1074/jbc.M909365199. [DOI] [PubMed] [Google Scholar]
- 62.Sauve AA, Deng HT, Angeletti RH, Schramm VL. A covalent intermediate in CD38 is responsible for ADP-ribosylation and cyclization reactions. J Am Chem Soc. 2000;122:7855–9. [Google Scholar]
- 63.Graeff R, Lee HC. 2002. Novel cycling assays for cADPR and NAADP. In Cyclic ADP-ribose and NAADP. Structures, Metabolism and Functions, ed. H. C. Lee, pp.101–20. Dordrecht: Kluwer Academic Publishers
- 64.Ceni C, et al. Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38 deficient mice. J Biol Chem. 2003;278:40670–8. doi: 10.1074/jbc.M301196200. [DOI] [PubMed] [Google Scholar]
- 65.Fukushi Y, et al. Identification of cyclic ADP-ribose-dependent mechanisms in pancreatic muscarinic Ca2+ signaling using CD38 knockout mice. J Biol Chem. 2001;276:649–55. doi: 10.1074/jbc.M004469200. [DOI] [PubMed] [Google Scholar]
- 66.Kato I, et al. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i and insulin secretion. J Biol Chem. 1999;274:1869–72. doi: 10.1074/jbc.274.4.1869. [DOI] [PubMed] [Google Scholar]
- 67.Sun L, et al. Disordered osteoclast formation and function in a CD38 (ADP-ribosyl cyclase)-deficient mouse establishes an essential role for CD38 in bone resorption. FASEB J. 2003;17:369–75. doi: 10.1096/fj.02-0205com. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande DA, et al. Altered airway responsiveness in CD38 deficient mice. Am J Respir Cell Mol Biol. 2005;32:149–56. doi: 10.1165/rcmb.2004-0243OC. [DOI] [PubMed] [Google Scholar]
- 69.Partida-Sanchez S, et al. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38; Impact on the development of humoral immunity. Immunity. 2004;20:279–91. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- 70.Mitsui-Saito M, Kato I, Takasawa S, Okamoto H, Yanagisawa T. CD38 gene disruption inhibits the contraction induced by alpha-adrenoceptor stimulation in mouse aorta. J Vet Med Sci. 2003;65:1325–30. doi: 10.1292/jvms.65.1325. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi J, et al. Deficit of CD38/cyclic ADP-ribose is differentially compensated in hearts by gender. Biochem Biophys Res Commun. 2003;312:434–40. doi: 10.1016/j.bbrc.2003.10.143. [DOI] [PubMed] [Google Scholar]
- 72.Graeff R, Lee HC. A novel cycling assay for nicotinic acid-adenine dinucleotide phosphate with nanomolar sensitivity. Biochem J. 2002;367:163–8. doi: 10.1042/BJ20020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Graeff R, Lee HC. A novel cycling assay for cellular cyclic ADP-ribose with nanomolar sensitivity. Biochem J. 2002;361:379–84. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]