Abstract
Understanding ecosystem processes as they relate to wildfire and vegetation dynamics is of growing importance as fire frequency and extent increase throughout the western United States. However, the effects of severe, stand-replacing wildfires are poorly understood. We studied inorganic nitrogen pools and mineralization rates after stand-replacing wildfires in the Greater Yellowstone Ecosystem, Wyoming. After fires that burned in summer 2000, soil ammonium concentration peaked in 2001 (33 mg NH4-N· kgsoil−1); soil nitrate increased subsequently (2.7 mg NO3-N·kgsoil−1 in 2003) but was still low. However, annual net ammonification rates were largely negative from 2001 to 2004, indicating ammonium depletion. Thus, although net nitrification rates were positive, annual net nitrogen mineralization (net ammonification plus net nitrification) remained low. Aboveground net primary production (ANPP) increased from 0.25 to 1.6 Mg·ha−1·yr−1 from 2001 to 2004, but variation in ANPP among stands was not related to net nitrogen mineralization rates. Across a broader temporal gradient (stand age zero to >250 yr), negative rates of net annual ammonification were especially pronounced in the first postfire year. Laboratory incubations using 15N isotope pool dilution revealed that gross production of ammonium was reduced and ammonium consumption greatly exceeded gross production during the initial postfire years. Our results suggest a microbial nitrogen sink for several years after severe, stand-replacing fire, confirming earlier hypotheses about postdisturbance succession and nutrient cycling in cold, fire-dominated coniferous forests. Postfire forests in Yellowstone seem to be highly conservative for nitrogen, and microbial immobilization of ammonium plays a key role during early succession.
Keywords: nitrification, nitrogen mineralization, Rocky Mountains, Pinus contorta, lodgepole pine
Enhanced understanding of ecosystem processes as they relate to wildfire and vegetation dynamics is of growing importance, particularly as fire frequency and extent increase throughout the western U.S. in response to recent changes in climate (1, 2). Fire seasons during the past two decades have been among the most severe and expensive on record, and widespread media attention on wildfire has elevated public awareness and catalyzed management and policy responses (2–4). Nonetheless, the effects of severe, stand-replacing wildfire on vegetation and ecosystem processes are poorly understood. Prior studies have characterized the range and complexity of natural fire regimes in conifer forests (5–7) and elucidated many ecological roles of natural wildfires (e.g., refs. 8 and 9). However, most research on fire and ecosystem processes has focused on low-intensity surface fires or prescribed burns that typically do not kill the mature trees rather than on severe, stand-replacing fires.
Substantial nutrient losses can follow forest disturbances, as was demonstrated by deforestation experiments conducted by Bormann and Likens (10) in eastern deciduous forests of New Hampshire. The removal of vegetation by disturbance increases soil temperature and moisture, thereby enhancing conditions for mineralization (conversion of organic to inorganic forms of a given nutrient) at a time when biomass of vegetation, and therefore plant uptake, is reduced (11, 12). If mineralization exceeds immobilization and plant uptake, mobile inorganic nutrients (e.g., nitrate) can be lost from the disturbed forest and affect the chemistry of nearby streams (13, 14). Not all disturbed forests lose nutrients (15, 16), but there is concern that more severe disturbances, especially intense wildfire (e.g., ref. 17) may result in substantial nutrient losses. Data on nutrient pools and cycling rates are needed to determine whether and when postdisturbance nutrient losses are likely after severe wildfire.
Natural stand-replacing fire regimes (infrequent, high-severity fires that kill the canopy trees) dominate the extensive boreal forests of North America, Fennoscandia, and Asia and many coniferous forests of the Northern Rocky Mountains (6). Fire return intervals are long, ranging from ≈60 yr in jack pine (Pinus banksiana) to several centuries in some spruce-fir (Picea-Abies) communities. Climate, particularly severe regional drought, sets the stage for occasional years of extensive conflagrations, which account for most of the cumulative area burned (5, 18–20). Stand-replacing fires alter vegetation and soils immediately and directly, but they also produce a mosaic of burn severities and postfire vegetation (21–23) that structures the landscape for decades or centuries (24–27). Many of these coniferous forests are thought to be nitrogen (N) limited, yet recent reviews have revealed a surprising paucity of data on N dynamics after severe stand-replacing wildfire (28–30). This lack of information represents an important gap in current knowledge (31).
Ecologists have often studied N dynamics in response to disturbances because plant production is frequently limited by N, and N can be a sensitive indicator of ecosystem function (11, 12, 15, 16, 32). Fire volatilizes N from vegetation and litter, reducing N storage in the burned ecosystem but often increasing mineralization of the remaining organic matter. Postfire studies of N pools in a variety of ecosystems have shown 2- to 26-fold increases in soil ammonium (NH4+) that are relatively short lived (<2 yr) and followed by 2- to 5-fold increases in soil nitrate (NO3−) concentrations (28, 33). The relatively few studies of N pools after stand-replacing fire report similar results (30). In contrast, studies of postfire N mineralization rates have yielded conflicting results, with both increases and decreases reported (28), but data for rates after severe, stand-replacing fire are scarce. Because severe fires have profound effects on biotic and abiotic conditions, postfire changes in N mineralization rates might be even greater than those observed after fires that do not kill the canopy trees (30). However, whether postfire mineralization rates will be higher or lower after stand-replacing fire is unclear. Higher N mineralization rates might occur in response to release of available N from previously inaccessible forms; a pulse of organic N as roots decompose after vegetation is killed; warmer postfire soil temperatures that increase the rates of microbially mediated processes such as decomposition and nutrient release (34); and elevated nitrification in the presence of charcoal (35). However, lower N mineralization rates might be expected if losses of total N to combustion are large, thus depleting N stocks; if microbial biomass is reduced substantially after fire (36) or the ratio of fungal to bacterial biomass changes; or if loss of canopy trees and other vegetation, particularly the relatively N-rich foliage and twigs, reduces the quantity and quality of inputs of microbial carbon (C) substrates needed for mineralization (32). Empirical measurements for the early years after stand-replacing fire are clearly needed, yet few studies have focused on vegetation and N dynamics during that time.
The first several years after a stand-replacing fire in the Northern Rocky Mountains are characterized by rapid development of herbaceous vegetation (22, 23, 37). Net primary production is negligible initially, but graminoids and forbs increase quickly. These “N-extravagant plants” (sensu Chapman et al.; ref. 38) are predicted to access N after it has been mineralized from the pool of soil organic matter (SOM), often preferring nitrate to ammonium, and they produce high-quality litter inputs. The influence of tree seedlings during these first few years is minimal, but tree productivity increases with succession and often exceeds herbaceous productivity within 10 yr (39). This transition to conifer dominance corresponds to a shift from “N-extravagant” to “N-conservative plants” (38). The conifers typically depend on mycorrhizal fungi for nutrient uptake and are predicted to access at least some organic N rather than depending entirely on mineralized N, but they generally take up ammonium preferentially to nitrate. The conifers store nutrients very efficiently and produce low-quality litter (higher C:N ratio; ref. 40). Thus, we might anticipate high N turnover rates and microbial control of N cycling during early postfire succession, and low N turnover rates and plant (conifer) control of N cycling in a mature forest (38, 41).
We studied inorganic N pools and N mineralization rates after severe, stand-replacing fire in lodgepole pine (Pinus contorta var. latifolia) forests in the Northern Rocky Mountains. Looking first at early postfire succession (1–4 yr since fire), we asked how N pools and N mineralization rates vary among stands after severe stand-replacing wildfire, and whether total aboveground net primary production (ANPP) was correlated with N pools or inorganic N availability. We expected a pulse of NH4+ followed by pulse of NO3− and elevated rates of N mineralization, with higher N mineralization rates in areas of greater burn severity (see Methods for fire-severity classes used here; all were burned to mineral soil). We also expected nitrification to represent a small fraction of net N mineralization, as expected in N-limited conifer forests (42) and N mineralization rates (or at least nitrification rates) to be positively associated with ANPP. Looking next across a wide range of postfire stand ages (0–250 yr), we asked how N availability varies with time since severe, stand-replacing fire. We expected to see the greatest soil N pools and N mineralization rates during the early postfire years. To further explore components of net N mineralization, we conducted 15N isotope pool dilution studies to estimate gross production and consumption of ammonium across a range of times since fire. The pool dilution assay allows processes that collectively result in the net production of ammonium to be estimated separately, potentially enhancing our ability to explain the field-based measurements.
Our research was conducted in the Greater Yellowstone Ecosystem (GYE) in northwest Wyoming [supporting information (SI) Fig. 7], where stand-replacing fires have occurred at 100- to 500-yr intervals throughout the Holocene (43–45). Atmospheric deposition of N throughout the GYE is negligible (www.epa.gov/castnet/charts/yel408tn.gif). For vegetation and N dynamics during early postfire succession (1–4 yr since fire), we conducted studies from 2001 to 2004 in 10 stands located in two severe fires (Glade and Moran fires) that burned 1,300 ha during summer 2000. To evaluate N dynamics with increasing time since fire, we augmented our studies with data from mature forests (five stands >250 yr since fire), the 1988 Yellowstone fires, and a recent fire in 2003. The 1988 Yellowstone fires burned ≈35% of the GYE, and we studied N mineralization at 10 yr (3 stands) and 15 yr (14 stands) postfire. The 2003 East Fire was ignited by lightning in August and burned 7,300 ha through mature forests. We initiated studies (3 stands) within this burn in September 2003, as soon as access was permitted.
Results
Early Postfire Vegetation and N Availability.
Glade and Moran fires of 2000.
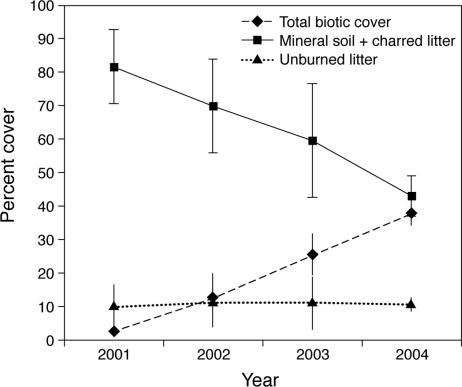
Herbaceous vegetation increased as expected during early postfire succession (Fig. 1 and SI Table 2). Total biotic cover averaged only 2% in 2001 but increased to 38% by 2004. Biotic cover was dominated by graminoids (largely Carex geyeri, Carex rossii, and Calamagrostis rubescens), which increased from 1.5% in 2001 to nearly 20% in 2004. Forbs also increased in cover from 0.5% in 2001 to nearly 17% in 2004; shrub cover was generally low but also increasing. Percent cover of lodgepole pine seedlings was low throughout this time period (<0.5% average each year) and did not change significantly through time. Although postfire treefall was not yet high, the percent cover of coarse wood tripled to 7.9% in 2004 after ranging from 2.5% to 2.9% in 2001–2003. On average, there was significantly more newly deposited, unburned litter (20% vs. 4.6%), less exposed mineral soil (28% vs. 41%), and greater lodgepole pine seedling cover (0.35% vs. 0.02%) in severe-surface burns, which killed but did not consume live tree foliage, than in crown fires, in which foliage was consumed. Cover of potential N fixing species was relatively low. Cover of Ceonothus velutinus, which establishes from a soil seed bank after fire, was greater in crown fire than in surface fire (2.4% vs. 0.3%, respectively). In contrast, cover of Lupinus argenteus, which has no seedbank and typically resprouts after fire, was greater in severe-surface fire than in crown fire (6.5% vs. 2.5%, respectively).
Fig. 1.
Changes in aboveground cover after severe stand-replacing fire in the GYE during the summer of 2000. Means are from 10 plots; error bars are ± 2 SE.
During the first 4 yr postfire, ANPP increased significantly from a mean of 0.25 Mg·ha−1·yr−1 in 2001, to 1.0 Mg·ha−1·yr−1 in 2002, to 1.6 Mg·ha−1·yr−1 in both 2003 and 2004. Most (65–77%) of the ANPP was from graminoids, with forbs as the next major contributor; shrubs and trees contributed a very small fraction. ANOVA revealed significant differences in ANPP among years but not between the two sites or the two fire-severity classes.
Mineral soils were somewhat acidic, with pH averaging 5.6, and had relatively low SOM (4%) and total N (0.10%) (SI Table 3). None of the general soil characteristics we analyzed varied with fire severity, and only two varied between the two sites; soil calcium was higher at the Moran site than at Glade (P = 0.0468), and exchangeable phosphorus (P) was marginally higher at Moran (P = 0.06).
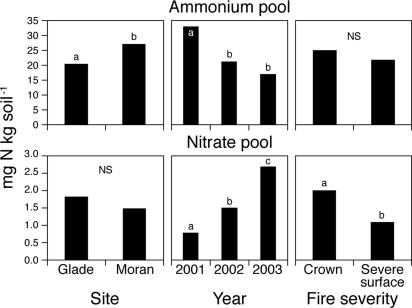
N pools.
The soil pool of inorganic N (NO3− + NH4+) declined with time since fire, from a mean of 33.8 mg N·kgsoil−1 in 2001 (1 yr after fire) to 19.8 mg N·kgsoil−1 in 2003. Most of the N pool was in the form of NH4+, although the relative proportion of NH4+ declined from 98% in 2001 to 86% in 2003. ANOVA revealed significant influences of year, site and fire severity on the NH4+ and NO3− pools, but there were no interactions among these main effects (Table 1). The pool size of NH4+ was greatest in 2001, declining by ≈30% in subsequent years, and was greater at Moran than at Glade (Fig. 2). However, NH4+ pool size did not differ between fire-severity classes (Table 1 and Fig. 2). Pool sizes of NO3− were always low (< 3.0 mg N·kgsoil−1), but they did increase significantly each year. In contrast to NH4+ pools, NO3− pools did not differ between sites but differed between fire severities (higher in crown fire than in severe-surface fire; Fig. 2).
Table 1.
Summary of ANOVA results for nitrogen pools and net mineralization rates in 10 stands studied in the Glade and Moran fires that burned during summer 2000
| Response | Site | Year | Fire severity | Model r2 | P |
|---|---|---|---|---|---|
| NH4+ pool | * | ** | NS | 0.62 | 0.0076 |
| NO3− pool | NS | *** | ** | 0.74 | 0.0003 |
| Net ammonification | NS | NS | NS | – | NS |
| Net nitrification | ** | P = 0.06 | NS | 0.55 | 0.0293 |
| Net N mineralization | NS | NS | NS | – | NS |
Analyses tested for effects of site (Glade vs. Moran fires), fire severity (crown vs. severe surface fire), and year (2001 to 2004). For all models, n = 40 and df = 12, 27. Entries indicate significant main effects: *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant. There were no significant interactions between main effects.
Fig. 2.
Extractable soil nitrate and ammonium pools measured 1, 2, and 3 yr after stand-replacing fire (n = 10 plots) by time since fire, site, and fire severity. Means with the same letter do not differ from each other (Tukey's HSD test, P < 0.05).
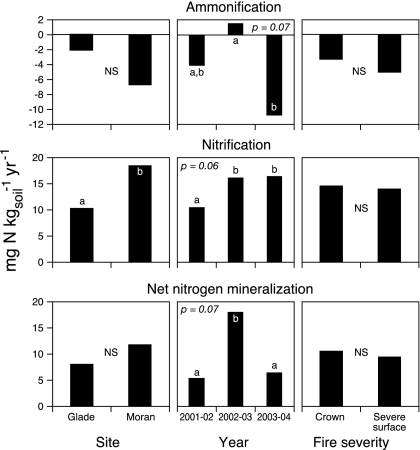
Net N mineralization rates.
Annual net ammonification rates were negative or near zero throughout this study (Fig. 3), indicating a net depletion of ammonium, with the lowest values observed in 2003–2004. There was substantial variation in ammonification among the 10 stands, especially during the first 2 yr [e.g., range of −36 to 16.3 mg N·kgsoil−1 in 2001–2002, and −9.8 to 9.1 in 2002–2003, with coefficients of variation (CV) ≈300%]. However, ANOVA revealed no differences attributable to site or fire-severity class (Table 1). In contrast, nitrification rates were positive during each year of this study (Fig. 3) and were considerably less variable among the 10 stands (e.g., range of 0.9–25.8 mg N·kgsoil−1 in 2001–2002, and 6.0–36.7 in 2002–2003, with CVs ≈65%). Nitrification rates differed by year and between the two sites (Fig. 3), with higher rates observed at Moran, which notably had greater pools of extractable NH4+. As with ammonification, nitrification rates did not vary by fire-severity class. Annual net N mineralization (ammonification plus nitrification) did not differ between sites or fire-severity classes but was highest in the 2002–2003 incubation (Fig. 3 and Table 1).
Fig. 3.
Net annual nitrification, ammonification, and net N mineralization from 1-year in situ resin core incubations, as measured during the second, third, and fourth years after stand-replacing fire (n = 10 plots) by time since fire, site, and fire severity. Means with the same letter do not differ from each other (Tukey's HSD test, P < 0.05).
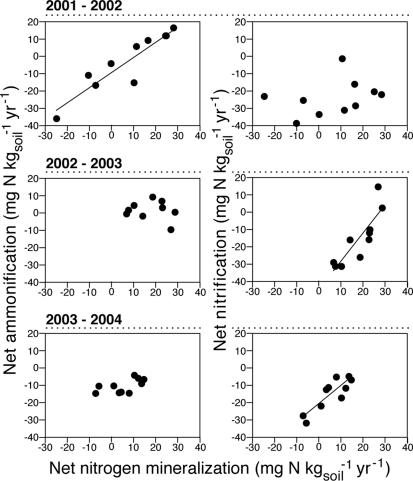
The relationship between nitrification and net N mineralization (net ammonification plus net nitrification) shifted during these initial postfire years (Fig. 4). In the second year after fire (2001–2002), nitrification was not correlated with net N mineralization rates, but net ammonification rates were correlated with net N mineralization (r = 0.91, P = 0.0002). During the third and fourth years after fire, nitrification showed a strong positive, linear relationship with net N mineralization (for 2002–2003, r = 0.86, P = 0.0015; for 2003–2004, r = 0.86, P = 0.0012) (Fig. 4). Furthermore, because net ammonification rates were largely negative, nitrification comprised ≥100% of net N mineralization rate during these second through fourth years after fire.
Fig. 4.
Relative net ammonification and net nitrification from 1-yr in situ incubations using resin cores for the second, third, and fourth years after stand-replacing fire (n = 10 plots).
There were few significant correlations between N mineralization rates and N pools. Ammonification rates were negatively correlated with initial NH4+ pool sizes in 2001–2002 (r = −0.90, P = 0.0004) and 2003–2004 (r = −0.87, P = 0.0009) but showed no significant correlation in 2002–2003. Nitrification showed a marginally significant positive correlation with the NH4+ pool in 2002–2003 (r = 0.62, P = 0.056) but no other correlations with NH4+ or NO3− pools. Net N mineralization was negatively correlated with NH4+ pool size in only 2001–2002 (r = −0.72, P = 0.0182).
N availability and ANPP.
There were no significant relationships between ANPP and either N pools or rates of nitrification, ammonification, and net N mineralization during this early postfire period (all P > 0.05).
Variation in N Pools and Mineralization Rates with Time Since Fire (0 to >250 yr).
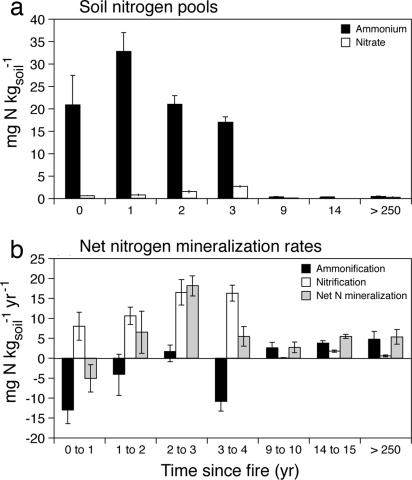
When placed within a longer temporal context, N pools clearly showed an immediate but transient increase in the first few years after stand-replacing fire (Fig. 5a). The pool of extractable inorganic N was elevated within a month after fire, with ammonium showing the greatest response and reaching a peak 1 yr after fire. Nitrate pools were an order of magnitude lower than ammonium pools, but they increased during the first 4 yr after fire with a peak occurring 2 yr later than the peak in ammonium. Extractable NO3− and NH4+ in the soil were both negligible by the 10th year after stand-replacing fire and remained low in 15-yr-old and mature forests (Fig. 5a).
Fig. 5.
Soil N and time since fire. (a) Mean (± 2 SE) pools of soil ammonium and nitrate with time since stand-replacing fire. (b) Mean (± 2 SE) annual net nitrification, net ammonification, and net N mineralization rates with time since stand-replacing fire.
Net N mineralization rate was negative during the initial postfire year, increased for the next few years, then declined (Fig. 5b). Net ammonification was strongly negative, especially during the year immediately after the 2003 stand-replacing fire (Fig. 5b). Although nitrification rate was positive during that initial postfire year, the net depletion of ammonium resulted in negative net annual N mineralization, or a net depletion of inorganic N (Fig. 5b). Nitrification rates were positive and increased 3- to 5-fold during the first 4 yr after fire, but the absolute rates remained low. By 10 yr after fire, ammonification rates were positive, although moderate, and ammonification accounted for most of the net N mineralization observed (Fig. 5b). Ammonification was positive and nitrification was negligible in forests that had not burned for >250 yr.
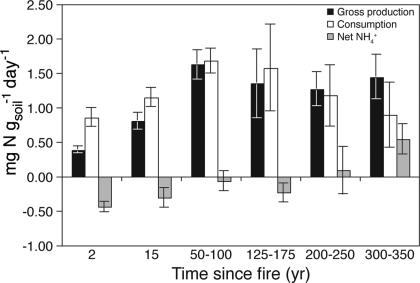
Pool dilution studies revealed that consumption of ammonium exceeded gross production in soils sampled 2 and 15 yr since fire, suggesting substantial microbial immobilization (Fig. 6). Gross production and consumption were roughly comparable during midsuccessional stand ages, and production exceeded consumption only in the oldest stands. However, gross production of ammonium was lower at 2 and 15 yr since fire relative to gross production in the older stands (Fig. 6).
Fig. 6.
Gross production, consumption, and net production of ammonium determined by 15N isotopic pool dilution with time since stand-replacing fire.
Discussion
Our results provide new insights into the consequences of natural stand-replacing fire in conifer forests of the Northern Rocky Mountains, confirming some prior expectations about soil N pools (30) and mineralization rates (15) and suggesting that these systems may have effective mechanisms for N conservation after severe fire. Furthermore, the data also suggest that important qualitative changes in N cycling occur during the first decade after fire, coinciding with rapid change in postfire vegetation and increased productivity of the re-establishing stand of conifers.
Soil ammonium concentrations increased and were followed by increases in soil nitrate, as observed after fire in many systems (30), but the pools did not predict mineralization rates. The elevated nitrate concentrations also were still quite low (12:210), as observed in other acidic conifer forests (46) and even after other disturbances. For example, in logged Douglas-fir (Pseudotsuga menziesii) forests in western Oregon, nitrate concentrations increased 30-fold but were still less than precipitation inputs and essentially inconsequential (47). After their initial but transient increase, pools of NH4+ and NO3− both declined with time since fire, as expected.
Our results indicate substantial microbial immobilization of ammonium N after severe, stand-replacing fire in the GYE, despite the elevated concentrations of soil NH4+. These results support hypotheses posed decades ago for postdisturbance N cycling in fire- or cold-dominated coniferous forests where vegetation is likely to be growing under N deficiency (15, 42). Vitousek and Mellilo (42) posited that ammonium immobilization by microbes would be of particular importance after disturbance in such systems and when large quantities of N-poor wood remain after disturbance. The conifer forests of the GYE meet both of these criteria, and we observed negative ammonification in situ. In our laboratory incubations, gross production of ammonium was low, and consumption exceeded gross production for at least 15 yr after fire. Moreover, our results are consistent with recent studies exploring postdisturbance N cycling; e.g., fire had a net negative influence on N mineralization in jack pine (48). After clearcutting in loblolly pine (Pinus taeda), immobilization was the major sink for inorganic N, as regrowing vegetation accounted for only 9–13% of labeled N uptake (32).
Nitrification rates, although they increased after fire, remained relatively low. Although NH4+ availability is thought to be the most important direct determinant of nitrification rate (12:208), high postfire pools of NH4+ were not associated with higher nitrification in our study. However, nitrification rates were higher in the Moran fire where SOM in comparable unburned was greater than at Glade (10.3% vs. 3.1%) and postfire NH4+ pools were higher, suggesting the potential importance of both C and NH4+. The negative net ammonification rates in the presence of high soil NH4+ pools suggest that nitrifiers could still be substrate limited. Nitrification may cease if the NH4+ supply declines below some threshold or if microbial assimilation of NH4+ preempts uptake by nitrifiers (49), as our data suggest. Factors other than NH4+ supply may also limit nitrification in coniferous forest soils (50, 51). Low pH has been associated with low rates of nitrification (52, 53), although Booth et al. (54) did not find a significant effect of pH. Nitrification rates are typically slow in dry soils (55), and soils in the GYE remain dry during much of the growing season. Mineralization also has been shown to increase with rising temperatures (56), which typify recently burned forests, but assimilation may increase more than mineralization (57, 58). Thus, the elevated postfire soil temperatures may enhance ammonium immobilization, thereby reducing nitrification.
Vitousek and Mellilo (42) suggested three other potential mechanisms that could cause nitrification to remain low after disturbance. First, the nitrifier population size may be low in the undisturbed forest because they are outcompeted for ammonium; after disturbance, it may still take time for them to increase, as their growth rates are relatively low. Studies have suggested nitrifiers are more susceptible to fire than are other soil groups (41), which may further reduce their postfire populations. Second, nitrification may be inhibited by secondary plant compounds. However, recent studies suggest the presence of charcoal in the soils may neutralize this effect for several decades after fire (59) and thus facilitate nitrification (35). Third, nitrification could be prevented by competition between nitrifiers and other soil organisms for P. We cannot evaluate this potential competition rigorously, but total soil P was not a significant factor in exploratory analyses (data not shown).
Nitrification is a very small fraction of net mineralization in many temperate conifer forests, often 0–4%, whereas it is typically near 100% in tropical forests (12:209). Relative nitrification was high during the early postfire years in the GYE, with a strong linear relationship between nitrification and net N mineralization observed during the third and fourth years after fire. Hart et al. (49) found a strong linear relationship between nitrification and mineralization in forest soils of the Pacific Northwest, and Yermakov and Rothstein (48) found similar results in postfire jack pine. The high relative nitrification during early succession may reflect the dominance of herbaceous vegetation and the turnover of N remaining in the mineral soil after fire. However, further study is needed to understand the variability in space and time of postfire nitrification.
Net N mineralization rates are controlled by the availability of dissolved organic N, inorganic N, activity of soil microbes and their relative demands for C and N. The high variability in ammonification rates among recently burned stands of the same age likely reflects spatial heterogeneity in the amount and quality of organic matter and the size and composition of the postfire microbial community. This variability dampens substantially as succession proceeds; e.g., variability in net N mineralization declined through time in a chronosequence of harvested Douglas-fir stands (60). Postfire nitrification was much less variable than ammonification, suggesting the potential for broader scale factors to constrain nitrification rates. In general, the relatively low SOM characteristic of lodgepole pine forests in the GYE probably constrains net N mineralization rates. In a burned black spruce (Picea mariana) stand in Alaska, net N mineralization rates were approximately six times higher during the second year postfire than we measured in the Glade and Moran fires (61). Net in situ annual ammonification in the black spruce system was high and positive (39 mg N·kgsoil−1·yr−1), in contrast to our study, and SOM was 18.6% in the black spruce stands, approximately five times greater than in the burned stands we studied.
In addition to microbial immobilization, vegetation regrowth is another potential sink for postfire N. Fast-growing vegetation can be effective at retaining nutrients during early succession, although our methods excluded N uptake by vegetation. Prescott et al. (40) found that rates of N uptake by ground vegetation and shrubs in a clear-cut site were comparable with rates in mature forests. However, biomass and productivity were quite low in the Glade and Moran fires for the first 4 yr, and postfire vegetation is unlikely to act as a significant N sink until tree productivity increases substantially. Over the long term, plant–soil feedbacks develop between postfire vegetation and soil microbial communities, and Hart et al. (41) suggest that these feedbacks are key to the sustainability of these forests.
A positive relationship between ANPP and N mineralization has been observed across a range of temperate forest types (62), but we found no relationship between these two processes when analyzing them within stands during the first 4 yr after stand-replacing fire. Furthermore, there was no relationship between total foliar N and net N mineralization in the Glade and Moran fires in 2002, 2 yr postfire (63). The absence of a relationship between ANPP and N mineralization may reflect a lack of N limitation during this time, insufficient plant biomass to detect a relationship, insufficient time for feedbacks between vegetation and soils to have developed, or the use by plants of an alternative source of N (such as organic N; ref. 64). Furthermore, plants that resprouted after fire may rely initially on nutrients stored in roots and rhizomes.
The lack of relationship between ANPP and N mineralization is also consistent with microbial control of the N cycle during early postfire succession when conifer productivity is negligible. In most stands, lodgepole pine productivity will dominate ANPP by 10 yr after fire (39). Lodgepole pine is characterized by high nutrient use efficiency, and it produces litter of low quality (high C:N ratio). Levitt (65) found a strong negative relationship between ANPP and N availability in 17-yr-old postfire stands, suggesting that the conifers had become a strong N sink by that time. N availability was positively related to the abundance and quality of herbaceous litter (65), as Scott and Binkley (66) also found. Collectively, our results are consistent with the study of Chapman et al. (38), which suggested a shift from N-extravagant to N-conservative plants during the first two decades after fire, and with the negative feedback between nutrient use efficiency and N mineralization proposed by Tateno and Chapin (67). We hypothesize that postfire N dynamics are initially controlled by microbes, with the alterations in plant community composition and productivity [particularly the shift in balance between overstory and understory vegetation (41)] governing the long-term effects of fire on soil processes and N cycling.
Methods for measuring net rates of N mineralization all have some weaknesses, and the net N mineralization values reported here provide a comparative index of N availability rather than a direct measure. The resin core measurements are made in situ, but the soil was disturbed and separated from plant roots, and the amount of N that is available to plants may be underestimated (e.g., refs. 12 and 68). Furthermore, the conceptual framework underpinning studies of N mineralization has changed substantially in recent years. Plants have been shown to use organic N as a source of N, especially in low-N systems (e.g., refs. 69 and 70), indicating that plants can compete successfully with microbes at least some of the time. Recognition of the importance of organic N uptake has identified depolymerization of organic N compounds in the litter and soil into bioavailable forms (e.g., dissolved organic N) as a key process regulating N cycling and placed constraints on the interpretation of net N mineralization assays (71). We did not estimate either availability or uptake of organic N in this study, but pilot data from an isotope tracer study indicated uptake of intact glycine by 2-yr-old lodgepole pine seedlings in the Glade fire (E.A.H.S. and K.L.M., unpublished data). However, the 15N isotope-pool dilution methods compliment N mineralization assays by separating gross production of NH4+ from its consumption, thereby estimating the actual rate at which NH4+ is being made available, and providing important insights into N cycling (54). In our study, the net N mineralization measurements and the pool dilution assays both suggest the importance of microbial immobilization of NH4+ during early (<15 yr since fire) postfire succession.
In conclusion, this study suggests that microbial immobilization is likely to play a key role in conservation of N after severe stand-replacing fire in subalpine lodgepole pine forests. Enhanced microbial immobilization of NH4+ would help minimize off-site losses of N after fire (11, 14), as would slow rates of nitrification because NO3− is much more mobile in soils than NH4+. Indeed, we found negligible concentrations of NO3− and NH4+ in streamwater and snowmelt in burned areas of Yellowstone (72), and nutrient concentrations in Yellowstone's lakes were not elevated after the 1988 fires (73). Thus, postfire losses of N may be minimal in this ecosystem, in contrast to large nitrate losses after disturbances in the more fertile, eastern deciduous forests (10, 74). Our data suggest that the fire-dominated conifer forests of the GYE are highly conservative for N, and the important N sinks in this landscape likely shift from microbial immobilization to plant uptake as postfire succession proceeds.
Methods
Early Postfire Succession and N Availability: Glade and Moran Fires of 2000.
The Glade and Moran fires burned in Grand Teton National Park (GTNP) and the adjacent Rockefeller Parkway (administered by GTNP). The 1,280-ha Glade fire was located just south of Yellowstone National Park (YNP) and burned in 120- and 150-yr-old lodgepole pine (Pinus contorta var. latifolia) forests that developed after stand-replacing fires in 1856 and 1879. The substrate consisted of Quaternary rhyolite bedrock and rhyolite-dominated glacial deposits, and soils were mostly Typic Cryumbrepts and Dystric Chryocrepts. The 840-ha Moran fire on the western shore of Jackson Lake burned in mixed forests of lodgepole pine, Engelmann spruce (Picea engelmannii), and subalpine fir (Abies lasiocarpa) that had not burned previously for at least 200 yr. The substrate consisted of glacial moraine deposits, containing material from Precambrian crystalline rocks as well as Paleozoic sedimentary rocks; soils were mostly Typic Chryocrepts. Both sites were at ≈2,150 m elevation on gently rolling topography. The nearest weather station is at Moran, WY, ≈25 km away in the same mountain valley as the two study sites. This station has recorded average air temperatures of −9.6°C in December and 16°C in July, and average annual precipitation of 575 mm. Although one flank of the Glade fire was actively suppressed to protect park developments, most of the area (including our study site) burned without interference. Because fire retardant typically contains inorganic N, we were careful to select a study area where no fire retardant was applied. The Moran fire burned entirely without interference and with no retardant application.
In both the Glade and Moran fires, we established five permanently marked 50-m × 50-m (0.25-ha) plots in July 2001 (SI Fig. 7). All 10 were in stand-replacing burns in which all trees were killed and the litter layer was consumed; two plots at each site were in areas of crown fire and three were in areas of severe-surface fire. Fire-severity classes followed Turner et al. (22, 37). In areas of crown fire, the needles of canopy trees were completely consumed by fire, the soil organic layer was almost entirely consumed, and soil was bare with no litter. In areas of severe surface burn, the canopy trees were also killed by fire but the needles were not consumed; the soil organic layer was almost entirely consumed, but there were dead needles that fell from the canopy after the fire on the soil surface. Soils had essentially no O horizon. Except for these differences in fire severity, all five stands at a site were similar with respect to topography and prefire vegetation structure. Plots were oriented toward true north, separated by at least 200 m, and initially positioned by extending a central 50-m transect due north from a random starting point.
Cover, ANPP, and Soil.
To characterize abiotic and biotic cover characteristics in the vicinity of the N mineralization measurements (described below), we recorded percent cover surrounding each resin core (n = 20 per stand) in July 2001, 2002, 2003, and 2004 within a 0.25-m2 circular frame. Abiotic cover categories included exposed mineral soil, rock, coarse wood, unburned litter (which was deposited after the fires, e.g., newly fallen pine needles), and charred litter. For biotic cover, percent cover of two potential N fixers, Lupinus argenteus and Ceonothus velutinus, was recorded to species; all other vegetation was recorded to functional group (forb, graminoid, shrub, and tree seedling). Cover summed to 100% in each frame.
We estimated postfire ANPP for each plot by species during the summers of 2001–2004 using methods of Turner et al. (39). Briefly, aboveground plant cover was recorded to species in 0.25-m2 quadrat frames (n = 45) distributed regularly along each of three parallel 50-m transects. Allometric relationships developed in our study area by species (39) were used to estimate ANPP of each plot for each year.
To characterize soil properties in each stand, we collected 20 samples of the upper 15 cm of mineral soil at random locations in each plot during summer 2002. Soil samples were also obtained from adjacent stands that did not burn during summer 2000. Samples were homogenized and composited by plot, air dried, and sent to the Soil and Plant Analysis Lab at the University of Wisconsin (Madison, WI) for determination of general soil characteristics. Total C was determined by dry combustion by using the Tekmar-Dohrman 183 TOC Boat Sampler DC-190 (Tekmar-Dohrman, Mason, OH). A microKjeldahl procedure was used for total N determination (75). Acid extractable P was analyzed colorimetrically by using the Truog method, and potassium (K), calcium (Ca), and magnesium (Mg) were measured by atomic absorption after extraction with H2SO4.
N Pools and in Situ N Mineralization.
The resin core incubation method (76, 77) was used to estimate initial pools of NO3− and NH4+ and annual rates of net N mineralization in each stand at Glade and Moran from July 2001 to July 2004 (i.e., three 1-yr incubations). Ten resin cores were placed along each of two parallel east–west transects located in the center of each stand (n = 20 per stand). Transects were 8 m apart, and cores were separated by 2 m along each transect. Incubations used open poly(vinyl chloride) (PVC) tubes, 5 cm in diameter and 15 cm deep, buried such that the top of the tube was level with the soil surface, with a resin bag at the bottom (77, 78). The resin cores were open to water flow, thereby allowing the products of mineralization to leach from the soil column into the resin bags. At each core location, cores were placed in a consistent relative position each year (facing north, east, and south in 2001, 2002, and 2003, respectively) and were within 0.25 m of one another.
Resin bags contained 20 g wet mass of mixed bed exchange resin (J. T. Baker, Phillipsburg, NJ; no. 4631) in commercial nylon stocking material. Initial soil samples were collected to the same depth (15 cm) adjacent to each resin core by using a clean PVC tube and processed within 24 h. To determine the initial ammonium and nitrate pools at the onset of incubation, the soil was homogenized, and a 20-g subsample was extracted in 75 ml of 2 M KCL by shaking the sample for 1 h. Extracts were filtered after a brief settling time (between 1 and 3 h) with 0.7-μm sample-rinsed filter paper, and frozen for subsequent analysis. At the end of the 1 yr incubation, the resin bags and the soil within the PVC tube above the bag were collected and returned to the lab and a new set of cores was installed. The soil from the tube was homogenized and weighed, and a 20-g subsample was extracted in 75 ml of 2 M KCL. Resin bags were extracted in 50 ml of 2 M KCL. Extracts were frozen and subsequently analyzed colorimetrically for nitrate and ammonium by using a flow-injected autoanalyzer (Lachat Instruments, Milwaukee, WI). Soil moisture content was determined for the pre- and postincubation soils by oven-drying at 105°C for 24 h. Net N mineralization was calculated as the postincubation NH4+ plus NO3− in the soil and resin bag minus the quantity in the preincubation soil and expressed as mg N·kgsoil−1·yr−1.
Statistical Analyses.
All measured variables were averaged by plot and year, and subsequent analyses were conducted on the plot means (n = 10) for each year. Data were tested for normality before analysis and transformed if needed. ANOVA was used to determine whether inorganic N pools, N mineralization rates, ANPP, and aboveground cover varied by site, fire severity, and year. We used correlation analysis to explore the relationship between ANPP and N mineralization rates among the 10 plots. All statistical analyses were performed by using SAS (79).
Variation in N Pools and Mineralization Rates with Time Since Fire.
To evaluate our second question, we augmented our study (1) by collecting new information in the East Fire of 2003 and comparable mature forest, and (2) by incorporating into our analysis previous data for mature forests (>250 yr) and from stands burned during the 1988 Yellowstone fires (SI Fig. 7). In the East Fire (n = 3 plots) and comparable unburned forest (n = 3 plots north of the West Thumb of Yellowstone Lake), 20 resin cores were deployed following the same protocols described above and incubated from September 2003 to September 2004. Biotic cover in the burned plots was 0% at the time cores were deployed. Because of access limitations, we were unable to record percent abiotic cover within these plots and we report here only the values for the N pools and N mineralization rates.
Annual incubations of resin cores and the same laboratory methods were also implemented for incubations in 10-yr-old stands in the 1988 fires (n = 3; see ref. 72) and mature forest (n = 2; see ref. 72), and in 15-yr-old stands burned in the 1988 fires (n = 3; K.L.M., E.A.H.S., D.B.T., W.H.R., T. Balser, and M.G.T., unpublished data). Therefore, our extended analysis included N measurements made during the 1st, 2nd, 3rd, 4th, 10th, and 15th years after stand-replacing fire as well as mature forest.
Pool dilution studies of ammonium production, consumption and net were conducted as follows. In the Glade and Moran fires, 15N isotope pool dilution was performed in 2002 (n = 2 of the 10 plots that were established in 2001), 2 yr postfire. In the 1988 fires, pool dilution was done in 2003 (n = 14 plots). Smithwick et al. (25) reported pool dilution results for a range of older stands. Briefly, gross rates of NH4+ production and consumption were calculated by using 15N isotope dilution (68) using 24-h soil incubations and the equations of Kirkham and Bartholomew (80). We followed the methods described by Smithwick et al. (25).
We used one-way ANOVA to evaluate differences in N pools, net N mineralization rates, and NH4+ production and consumption with time since fire. Significant differences among means were identified by using Tukey's test.
Supplementary Material
Acknowledgments
This manuscript was improved by constructive suggestions from F. S. (Terry) Chapin, Martin Simard, and Peter M. Vitousek. We thank the numerous people who served on our Yellowstone field crews during the summers of 2001–2004, and especially Nicole DeCrappeo, Donna Kashian, and Aaron Thiel, who managed our summer field laboratory. Dr. Hank Harlow and the staff of the University of Wyoming-National Park Service Research Center provided valuable logistical support for the field studies. Dr. Teri Balser provided laboratory facilities for many of the analyses. We thank Bill Feeny and Michael Turner for preparation of graphics. M.G.T. especially thanks Dr. F. S. (Terry) Chapin for numerous valuable discussions about fire and nitrogen dynamics that helped shape this study. This study was funded by a grant from the Andrew W. Mellon Foundation.
Abbreviations
- ANPP
aboveground net primary production
- GYE
Greater Yellowstone Ecosystem
- GTNP
Grand Teton National Park
- SOM
soil organic matter
- YNP
Yellowstone National Park.
Footnotes
The authors declare no conflict of interest.
See Profile on page 4779.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700180104/DC1.
References
- 1.Whitlock C, Shafer SL, Marlon J. Forest Ecol Manag. 2003;178:5–21. [Google Scholar]
- 2.Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. Science. 2006;313:940–943. doi: 10.1126/science.1128834. [DOI] [PubMed] [Google Scholar]
- 3.Allen CD, Savage M, Falk DA, Suckling KF, Swetnam TW, Schulke T, Stacey PB, Morgan P, Hoffman M, Klingel JT. Ecol Appl. 2002;12:1418–1433. [Google Scholar]
- 4.Stephens SL, Ruth LW. Ecol Appl. 2005;15:532–542. [Google Scholar]
- 5.Johnson EA. Fire and Vegetation Dynamics. New York: Cambridge Univ Press; 1992. [Google Scholar]
- 6.Turner MG, Romme WH. Landscape Ecol. 1994;9:59–77. [Google Scholar]
- 7.Schoennagel TL, Veblen TT, Romme WH. BioScience. 2004;54:661–676. [Google Scholar]
- 8.Raison RJ. Plant Soil. 1979;51:73–108. [Google Scholar]
- 9.Whelan RJ. The Ecology of Fire. Cambridge, UK: Cambridge Univ Press; 1995. [Google Scholar]
- 10.Bormann FH, Likens GE. Pattern and Process in a Forested Ecosystem. New York: Springer; 1979. [Google Scholar]
- 11.Attiwill PM, Adams MA. New Phytol. 1993;124:561–582. doi: 10.1111/j.1469-8137.1993.tb03847.x. [DOI] [PubMed] [Google Scholar]
- 12.Chapin FS, III, Matson PA, Mooney HA. Principles of Terrestrial Ecosystem Ecology. New York: Springer; 2002. [Google Scholar]
- 13.Gresswall RE. Trans Am Fish Soc. 1999;128:193–221. [Google Scholar]
- 14.Feller MC. J Am Wat Res Assoc. 2005;41:785–811. [Google Scholar]
- 15.Vitousek PM, Gosz JR, Grier CC, Melillo JM, Reiners WA, Todd RL. Science. 1979;204:469–474. doi: 10.1126/science.204.4392.469. [DOI] [PubMed] [Google Scholar]
- 16.Boerner REJ. BioScience. 1982;32:187–192. [Google Scholar]
- 17.Ice GG, Neary DG, Adams PW. J Forest. 2004;102(6):16–20. [Google Scholar]
- 18.Johnson EA, Wowchuck DR. Can J Forest Res. 1993;23:1213–1222. [Google Scholar]
- 19.Bessie WC, Johnson EA. Ecology. 1995;76:747–762. [Google Scholar]
- 20.Flannigan MD, Wotton BM. In: Forest Fires. Johnson EA, Miyanishi K, editors. New York: Academic; 2001. pp. 351–373. [Google Scholar]
- 21.Turner MG, Hargrove WH, Gardner RH, Romme WH. J Veg Sci. 1994;5:731–742. [Google Scholar]
- 22.Turner MG, Romme WH, Gardner RH, Hargrove WW. Ecol Monogr. 1997;67:411–433. [Google Scholar]
- 23.Turner MG, Romme WH, Tinker DB. Front Ecol Environ. 2003;1:351–358. [Google Scholar]
- 24.Kashian DM, Turner MG, Romme WH. Ecosystems. 2005;8:48–61. [Google Scholar]
- 25.Smithwick EAH, Turner MG, Metzger KL, Balser TC. Soil Biol Biochem. 2005;37:1546–1559. [Google Scholar]
- 26.MacKenzie MD, DeLuca TH, Sala A. Forest Ecol Manag. 2004;203:331–343. [Google Scholar]
- 27.Treseder KK, Mack MC, Cross A. Ecol Appl. 2004;14:1826–1838. [Google Scholar]
- 28.Wan S, Hui D, Luo Y. Ecol Appl. 2001;11:1349–1365. [Google Scholar]
- 29.Certini G. Oecologia. 2005;143:1–10. doi: 10.1007/s00442-004-1788-8. [DOI] [PubMed] [Google Scholar]
- 30.Smithwick EAH, Turner MG, Mack MC, Chapin FS., III Ecosystems. 2005;8:163–181. [Google Scholar]
- 31.Harden JW, Mack M, Veldhuis H, Gower ST. J Geophys Res. 2003;108(D3):8223. [Google Scholar]
- 32.Vitousek PM, Matson PA. Ecology. 1985;66:1360–1376. [Google Scholar]
- 33.Covington WW, Sacket SS. Forest Ecol Manag. 1992;54:175–191. [Google Scholar]
- 34.Kaye JP, Harte SC. Trends Ecol Evol. 1997;12:139–143. doi: 10.1016/s0169-5347(97)01001-x. [DOI] [PubMed] [Google Scholar]
- 35.DeLuca TH, MacKenzie MG, Gundale MJ, Holben WE. Soil Sci Soc Am J. 2006;70:448–453. [Google Scholar]
- 36.Grogan P, Bruns TD, Chapin FS., III Oecologia. 2000;122:537–544. [Google Scholar]
- 37.Turner MG, Romme WH, Gardner RH. Int J Wildl Fire. 1999;9:21–36. [Google Scholar]
- 38.Chapman SK, Langley JA, Hart SC, Koch GW. New Phytol. 2006;169:27–34. doi: 10.1111/j.1469-8137.2005.01571.x. [DOI] [PubMed] [Google Scholar]
- 39.Turner MG, Tinker DB, Romme WH, Kashian DM, Litton CM. Ecosystems. 2004;7:751–775. [Google Scholar]
- 40.Prescott CE, Corbin JP, Parkinson D. Can J Forest Res. 1988;19:309–317. [Google Scholar]
- 41.Hart SC, DeLuca TH, Newman GS, MacKenzie MD, Boyle SI. Forest Ecol Manag. 2005;220:166–184. [Google Scholar]
- 42.Vitousek PM, Melillo JM. Forest Sci. 1979;25:605–619. [Google Scholar]
- 43.Romme WH. Ecol Monog. 1982;52:199–221. [Google Scholar]
- 44.Romme WH, Despain DG. BioScience. 1989;39:695–699. [Google Scholar]
- 45.Millspaugh SH, Whitlock C, Bartlein PJ. In: After the Fires: The Ecology of Change in Yellowstone National Park. Wallace LL, editor. New Haven, CT: Yale Univ Press; 2004. pp. 10–28. [Google Scholar]
- 46.Stark JM, Hart SC. Nature. 1997;385:61–64. [Google Scholar]
- 47.Martin CW, Harr RD. Can J Forest Res. 1989;19:35–43. [Google Scholar]
- 48.Yermakov Z, Rothstein DE. Oecologia. 2006;149:690–700. doi: 10.1007/s00442-006-0474-4. [DOI] [PubMed] [Google Scholar]
- 49.Hart SC, Binkley D, Perra A. Soil Biol Biochem. 1997;29:1111–1123. [Google Scholar]
- 50.Killham K. Plant Soil. 1990;128:31–44. [Google Scholar]
- 51.Ste-Marie C, Paré D. Soil Biol Biochem. 1999;31:1579–1589. [Google Scholar]
- 52.Haynes RJ. In: Mineral Nitrogen in the Plant-Soil System. Haynes RJ, editor. New York: Academic; 1996. pp. 127–165. [Google Scholar]
- 53.Myrold DD. In: Principles and Applications of Soil Microbiology. Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA, editors. Upper Saddle River, NJ: Prentice–Hall; 1998. pp. 259–294. [Google Scholar]
- 54.Booth MS, Stark JM, Rastetter E. Ecol Monog. 2005;75:139–157. [Google Scholar]
- 55.Stark JM, Firestone MK. Appl Environ Microbiol. 1995;61:218–221. doi: 10.1128/aem.61.1.218-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw MR, Harte J. Global Change Biol. 2001;7:193–210. [Google Scholar]
- 57.Binkley D, Stottlemyer R, Suarez F, Cortina J. Ecoscience. 1994;1:64–70. [Google Scholar]
- 58.Andersen MK, Jensen LS. Soil Biol Biochem. 2001;33:511–521. [Google Scholar]
- 59.MacKenzie MD, DeLuca TH. Plant Soil. 2006;287:257–266. [Google Scholar]
- 60.Griffiths RP, Swanson AK. Can J For Res. 2001;31:1871–1879. [Google Scholar]
- 61.Smithwick EAH, Mack MC, Turner MG, Chapin FS, III, Zhu J, Balser TC. Biogeochemistry. 2005;76:517–537. [Google Scholar]
- 62.Reich PB, Grigal DF, Aber JD, Gower ST. Ecology. 1997;78:335–347. [Google Scholar]
- 63.Metzger KL, Romme WH, Turner MG. Forest Ecol Manag. 2006;227:22–30. [Google Scholar]
- 64.Neff JC, Chapin FS, Vitousek PM. Front Ecol Environ. 2003;1:205–211. [Google Scholar]
- 65.Levitt LL. Madison, WI: Univ of Wisconsin; 2006. MS thesis. [Google Scholar]
- 66.Scott NA, Binkley D. Oecologia. 1997;111:151–159. doi: 10.1007/s004420050219. [DOI] [PubMed] [Google Scholar]
- 67.Tateno M, Chapin FS., III Am Nat. 1997;149:723–744. [Google Scholar]
- 68.Hart S, Nason GE, Myrold DD, Perry DA. Ecology. 1994;75:880–891. [Google Scholar]
- 69.Chapin FS, III, Moilanen L, Kielland K. Nature. 1993;361:150–153. [Google Scholar]
- 70.Schimel JP, Chapin FS., III Ecology. 1996;77:2142–2147. [Google Scholar]
- 71.Schimel JP, Bennett J. Ecology. 2004;85:591–602. [Google Scholar]
- 72.Romme WH, Turner MG. In: After the Fires: The Ecology of Change in Yellowstone National Park. Wallace LL, editor. New Haven, CT: Yale Univ Press; 2004. pp. 318–361. [Google Scholar]
- 73.Lathrop RG., Jr Int J Wildl Fire. 1994;4:169–175. [Google Scholar]
- 74.Likens G, Bormann FH, Johnson N, Fisher D, Pierce R. Ecol Monog. 1970;40:23–47. [Google Scholar]
- 75.Jackson ML. Soil Chemical Analysis. Englewood Cliffs, NJ: Prentice–Hall; 1958. [Google Scholar]
- 76.Binkley D, Hart SC. Adv Soil Sci. 1989;10:57–112. [Google Scholar]
- 77.Binkley D, Bell R, Sollins P. Can J Forest Res. 1992;22:858–863. [Google Scholar]
- 78.DiStefano J, Gholz H. Commun Soil Sci Plant Anal. 1986;17:989–998. [Google Scholar]
- 79.SAS Institute. SAS. Cary, NC: SAS Institute; 2003. Version 9.1.3. [Google Scholar]
- 80.Kirkham D, Bartholomew WV. Soil Soc Sci Amer Proc. 1954;18:33–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.