Abstract
The key step of the protonmotive Q-cycle mechanism of the cytochrome bc1 complex is the bifurcated oxidation of ubiquinol at the Qp site. It was postulated that the iron–sulfur protein (ISP) accepts the first electron from ubiquinol to generate ubisemiquinone anion to reduce bL. Because of the difficulty of following the reduction of ISP optically, direct evidence for the early involvement of ISP in ubiquinol oxidation is not available. Using the ultra-fast microfluidic mixer and the freeze-quenching device, coupled with EPR, we have been able to determine the presteady-state kinetics of ISP and cytochrome bL reduction by ubiquinol. The first-phase reduction of ISP starts as early as 100 μs with a t1/2 of 250 μs. A similar reduction kinetic is also observed for cytochrome bL, indicating a simultaneous reduction of both ISP and bL. These results are consistent with the fact that no ubisemiquinone was detected at the Qp site during oxidation of ubiquinol. Under the same conditions, by using stopped flow, the reduction rates of cytochromes bH and c1 were 403 s−1 (t1/2 1.7 ms) and 164 s−1 (t1/2 4.2 ms), respectively.
Keywords: EPR, rapid freeze-quenching, stopped-flow, ubiquinol-cytochrome c reductase, electron transfer
The cytochrome bc1 complex (also known as ubiquinol-cytochrome c reductase or complex III) is an essential segment of the electron transfer chain of mitochondria and many respiratory and photosynthetic bacteria (1). It catalyzes electron transfer from ubiquinol to cytochrome c with concomitant translocation of protons across the membrane to generate a proton gradient and membrane potential for ATP synthesis. The complex from bovine heart mitochondria is composed of 11 protein subunits: three core subunits, subunits III, IV, and V, which house b-type cytochromes, c-type cytochrome, and iron-sulfur center, respectively, and eight supernumerary subunits that contain no redox prosthetic groups. Cytochrome bc1 complexes from other sources contain the three major subunits with one to seven supernumerary subunits (2). In either case, the complex contains four redox prosthetic groups: cytochrome bL (b566) and bH (b562), cytochrome c1, and a high potential iron–sulfur cluster (ISC; 2Fe-2S Rieske center).
The 3D structure of the cytochrome bc1 complexes from beef (3, 4), chicken (5), yeast (6), and Rhodobacter capusulata (7) were recently determined. The complex is crystallized in an intertwined dimer form. The iron–sulfur proteins (ISPs) in the two bc1 monomers are intertwined with the head domain in one monomer interacting with cytochrome b and cytochrome c1 of the other monomer. The intertwined dimer observed in the crystalline complex also exists in solution, which was confirmed (8) in the R. sphaeroides bc1 complex through the formation of a four-subunit (two ISPs and two cytochrome bs) adduct by two intersubunit disulfide bonds between two engineered cysteine pairs: one pair links the ISP head domain to cytochrome b, and the other pair links the ISP tail domain to cytochrome b of another monomer. Two apparently noncommunicating cavities in the dimeric complex are presented: each connecting the Qp pocket of one monomer to the Qn pocket of the other. The distance between the Fe atoms of the two hemes bL is only 21 Å, which is approximately the same as the distance between heme bL and bH in one monomer (Fig. 1). The short distance between the two hemes bL and the presence of several aromatic amino acid residues at the interface of the two cytochrome b proteins have caused investigators (9–12) to speculate about the existence of electron transfer and equilibration between the two hemes bL. The involvement of an aromatic residue in such an electron transfer has recently been confirmed in the bacterial complex (12).
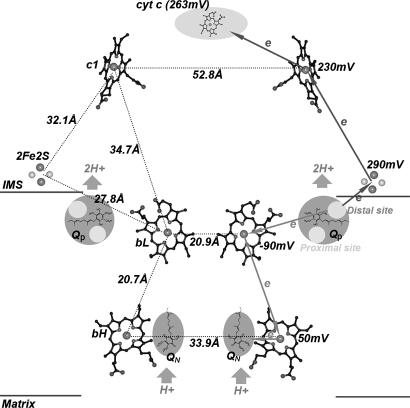
Fig. 1.
The relative location of the essential redox groups of the bovine heart mitochondrial cytochrome bc1 complex (59). The bL, bH, and c1 hemes are shown as ball-and-stick models, whereas the 2Fe2S clusters are shown as ball models. The quinone oxidation pockets are near the intermembrane space (IMS) side of the membrane, and the quinol reduction pockets are near the matrix side of the membrane. Cytochrome c is shown as a shaded oval as labeled. Distances between redox centers are given on the left, and the redox potential for each center is given on the right. The high potential ET path is depicted by arrowed lines pointing upward, and the low potential ET path is shown by arrowed lines pointing downward. Circles in the upper and lower parts within the Qp pockets are distal and proximal quinone bindings, respectively.
On the basis of functional data (13, 14) and structural information (3–7), the “protonmotive Q cycle” is the most favored mechanism for electron and proton transfer in the cytochrome bc1 complex (15, 16). The key step of the Q-cycle mechanism (Fig. 2) is the bifurcation of electrons from ubiquinol at the Qp site. It was postulated that the first electron of ubiquinol is transferred to the “high-potential chain,” consisting of the ISP and cytochrome c1. Then the second electron of ubiquinol is passed through the “low-potential chain” consisting of cytochromes bL and bH to reduce ubiquinone or ubisemiquinone bound at the Qn site. Although crystallographic data clearly indicates the presence of two separated quinone binding domains: one for ubiquinol oxidation (Qp) and the other for quinone reduction (Qn), only the binding of ubiquinone (17–19) and the presence of ubisemiquinone radical (20–22) at the Qn site were demonstrated. The binding of ubiquinol or ubiquinone at the Qp site has not been detected even though the binding of various Qp site inhibitors (18, 23, 24) is well known.
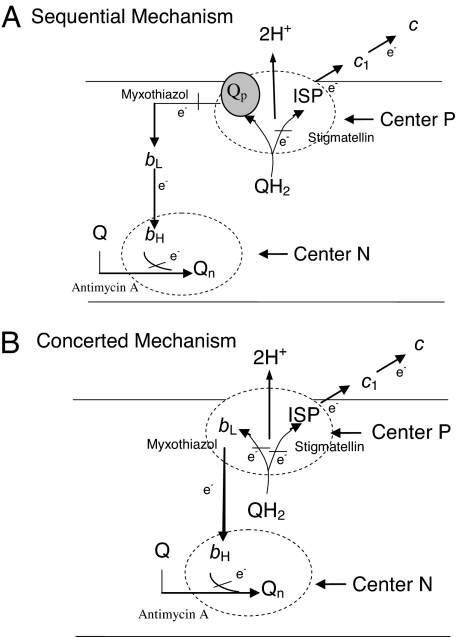
Fig. 2.
The proton motive Q-cycle mechanism. (A) Sequential bifurcated oxidation of quinol at the Qp site. Ubiquinol bound in cytochrome b is first oxidized by ISP to generate an ubisemiquinone radical to reduce cytochrome bL. (B) Concerted bifurcated oxidation of ubiquinol at the Qp site. Bifurcated oxidation of ubiquinol at the Qp site by ISP and cytochrome bL takes place simultaneously with no generation of ubisemiquinone radical at the Qp site.
The mechanism of the bifurcated oxidation at the Qp site is still a matter of controversy. Evidences supporting “sequential oxidation” (25–28) and the “concerted oxidation” (29–33) of the cytochrome b-bound ubiquinol are both available. In the sequential oxidation, the first electron of the ubiquinol is transferred to the ISC, and the resulting ubisemiquinone is used to reduce cytochrome bL. The absence of detectable ubisemiquinone radical at the Qp site (33) questions the validity of the sequential oxidation mechanism, even though several plausible explanations for the absence of Q-radical have been offered (27, 34). Even though the rate constant for the presteady-state reduction of the cytochrome b by ubiquinol is much larger than that of cytochrome c1, many investigators (35–38) assume that the first electron goes to the ISC, the thermodynamically favorable acceptor. The requirement for ISP (39) during the reduction of cytochrome c1 by ubiquinol in the cytochrome bc1 complex supports this assumption. The observed smaller rate constant of cytochrome c1 reduction, compared with the cytochrome b reduction rate constant, was attributed to the slow electron transfer between the ISC and heme c1. In fact, there is no evidence, so far, showing that the ISC receives an electron from ubiquinol before other redox components, because of the difficulty in detecting the redox change of the ISC in a fast time scale.
Taking advantage of the unique EPR signatures of the ISC (40), cytochromes bL, bH, and c1 (41–43), and ubisemiquinone radical (21, 22), we recently investigated the presteady-state kinetics of ISP and cytochrome bL reduction by ubiquinol at the Qp site by using an ultra-fast freeze-quenching technique (44) coupled with EPR spectroscopy. Our results are consistent with the concerted oxidation mechanism (29–32) of the ubiquinol at the Qp site, although we could not fully exclude the sequential mechanism because of our limited time resolution of ≈50 μs. To further confirm this mechanism, measurements with a better time resolution are required.
Results and Discussion
EPR Characteristics of the Redox Components of Cytochrome bc1 Complex.
The EPR spectra of cytochromes b, c1, and the ISC in the cytochrome bc1 complex have been well characterized. In the oxidized complex, cytochromes bL (b566), bH (b562), and c1 have peaks at g = 3.78, g = 3.45, and g = 3.35, respectively (41–43). The g = 3.78 peak of cytochrome bL is sharp and asymmetric and difficult to power saturate even at 7 K. Peaks of cytochromes bH and c1 overlap to show a peak at g = 3.4 (Fig. 3A). The roughly symmetric peak (g = 3.45) for cytochrome bH can be detected by using ascorbate-reduced complex. The EPR spectrum (g = 3.35) of cytochrome c1 can be obtained by subtracting the ascorbate-reduced spectrum from the fully oxidized one. Because EPR spectra of cytochromes bH and c1 have similar power saturation behavior, they cannot be resolved by power manipulation (41–43).
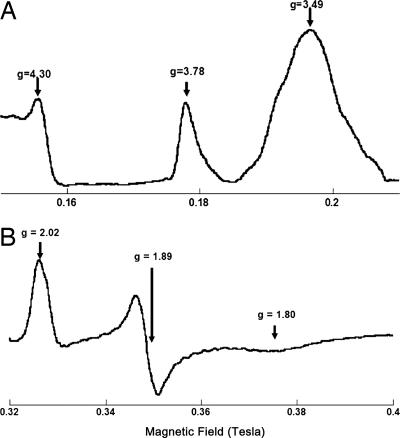
Fig. 3.
EPR spectra of oxidized cytochromes b and c1 (A) and the reduced Rieske ISP (B). The spectra were taken at 6 K, with the following instrument settings: microwave frequency, 9.45 GHz; modulation amplitude, 6.30 G; modulation frequency, 100 KHz; time constant, 0.655 s.
The EPR spectrum of the reduced ISC of the Rieske's protein (Fig. 3B) shows three peaks, gz = 2.02, gy = 1.89, and gx = 1.80 (40). While the shape and g value of gx is affected by the redox states of other components (42, 43) and the presence of ubiquinone (45), the intensity of the signal of gy (g = 1.89) is directly proportional to the degree of reduction of ISC. Thus, the rate of ISC reduction can be followed by the gy = 1.89 signal.
The EPR signature of ubisemiquinone radical is a single sharp peak at g = 2.004, which is easily power saturated at low temperature. Because ubisemiquinone at the Qn site is well characterized (20–24), it should not be difficult to differentiate it from any ubisemiquinone radical that is generated at the Qp site. Ubisemiquinone at the Qn site is antimycin A-sensitive and has a reduction kinetic similar to that of cytochrome bH (37). Ubisemiquinone at the Qp site, if any, would be expected to have a kinetic similar to that of ISC or cytochrome bL.
Presteady Reduction Rates of Cytochrome bH and c1 by Ubiquinol Determined by Conventional Stopped-Flow Method.
The electron transfer rates between the ubiquinol and heme bH or heme c1 in the cytochrome bc1 complex can be determined by mixing the fully oxidized cytochrome bc1 complex in 50 mM Tris·HCl (pH 8.0) buffer, containing 0.33 M sucrose and 0.01% dodecyl maltoside (DM), with an equal volume of various concentrations of ubiquinol at room temperature in a stopped-flow system (SX.18MV; Applied Photophysics, Leatherhead, UK). The final concentration of cytochrome bc1 complex is 50 μM based on cytochrome c1, and the concentrations of ubiquinol are 84, 167, 333, and 500 μM. The substrate, ubiquinol, is prepared in the same buffer containing 0.2% DM and 0.2% octyl glucoside (OG) at double concentrations. Reductions of cytochromes bH and c1 are monitored by the increase of absorbance at 559 and 550 nm, respectively. Table 1 shows the observed rate constants for bH and c1 reduction obtained with various concentrations of ubiquinol.
Table 1.
Ubiquinol concentration-dependent reduction rate constants of cytochromes bH and c1
| QH2 concentration, μM | Reduction rate constants, s−1 |
|
|---|---|---|
| bH | c1 | |
| 84 | 224 | 87 |
| 167 | 300 | 100 |
| 333 | 407 | 167 |
| 500 | 432 | 187 |
The cytochrome bc1 complex was diluted in 50 mM phosphate buffer, pH 7.4, containing 1 mM EDTA and 0.01% DM to a concentration of 100 μM based on cytochrome c1. Ubiquinol was diluted in 0.2% DM and 0.2% OG to various concentrations to give a final concentration of 1.7-, 3.34-, 6.66-, and 10-fold greater than that of cytochrome c1. Reductions of cytochrome b and cytochrome c1 were monitored by the increase of absorption at 559 nm and 550 nm, respectively, by using an Applied Photophysics stopped-flow reaction analyzer SX.18MV.
Although the reduction rate constants of both cytochromes increase as the concentration of substrate increases, the degrees of increase level off as the concentration of ubiquinol approaches 500 μM. An ≈50% increase in substrate, from 333 to 500 μM, results in an increase in the rate constants for cytochromes bH and c1 reduction by only 6% and 12%, respectively. Because of the high hydrophobicity of ubiquinol, it is difficult to reach a final concentration >500 μM in an aqueous solution with a limited amount of detergent present. All of the subsequent rate measurements were carried out with a substrate concentration of 333 μM. Under this condition the reduction rate constants of heme bH and heme c1 are 403 and 164 s−1 with t1/2 of 1.7 and 4.2 ms, respectively.
When the reduction of cytochromes b and c1 in the stopped–flow experiment is followed with photodiode array scanning, the reduction of bH, instead of bL, can be clearly identified. The reaction tracings show the early reduction of heme bH, starting before 1 ms, followed by the reduction of heme c1 after 3 ms. The reduction of bL is completed within the dead time of stopped-flow apparatus.
Presteady-State Reduction Rates of Cytochrome bL and ISC by Ubiquinol Determined by Ultra-Fast Microfluidic Mixing and the Freeze-Quenching Method.
The large reduction rate constant of cytochrome bL renders the conventional stopped-flow experiment inadequate for presteady-state reduction kinetics. Also, the close absorption peaks of cytochrome bL and bH complicate spectroscopic rate analysis. Ultra-fast microfluidic mixing and the freeze-quenching method (44) coupled with EPR detection offers an excellent way to determine the reduction kinetics of cytochrome bL and ISC. The ultra-fast microfluidic mixer has a dead time of 50 μs as described (44), hence it is valuable for detecting large reduction rate constants. The well resolved EPR signals of bL and bH make it easy to follow bL reduction without complication from bH.
The cytochrome bc1 complex was diluted in the same storage buffer to a cytochrome c1 concentration of 100 μM and mixed with an equal volume of 666 μM QH2 in 0.2% DM and 0.2% OG in the ultra-fast microfluidic mixer (44). The reaction mixture was freeze-quenched at liquid nitrogen temperature at various time points, from 66 μs to 5 ms, packed into EPR tubes, and stored at liquid nitrogen temperature before EPR analysis.
The redox state of ISP and cytochromes b and c1 were determined by EPR (42, 45) in the same sample, using a Bruker (Billeria, MA) EMX spectrometer at a temperature ≈6 K. The instrument settings were as follows: microwave frequency, 9.45 GHz; time constant, 0.655 s; and modulation frequency, 100 KHz. The modulation amplitude was 19.57 and 6.30 G for cytochromes and ISP, respectively. A microwave power of 2.15 mW was used, except for the power saturation study. During the EPR experiments it was noticed that air trapped inside the sample caused a distortion in the base line, especially in the region of magnetic field (0.15 to 0.21 Tesla) where EPR signals of b/c1 appear. To achieve high signal sensitivity, air trapped in the sample was removed by evacuation. The freeze-quenched samples in EPR tubes were first dipped into a mixture of isopentane and liquid nitrogen. The temperature of the samples was maintained at approximately −160°C. The EPR tubes were then subjected to vacuum for 30 s before being subjected to EPR measurements. Fig. 4 shows the EPR spectra of the cytochromes b and c1 (Fig. 4 A and B) and the reduced Rieske ISP (Fig. 4 C and D) at various time points after mixing with ubiquinol. The reduction of ISC was calculated from the intensity of peak to valley at g = 1.89 (gy). The reduction of bL was calculated from the integrated peak area at g = 3.78. Because the freeze-quenching reaction mixtures used for EPR measurements were in powder form and packed into EPR tubes in liquid nitrogen, the packing factor varied from sample to sample. To ascertain the concentration of the sample in the EPR tube at each time point, an internal spin label standard, proxyl, was added to the bc1 sample before the rapid mixing experiment. The amounts of reduction for each redox center at various time point were normalized by using the spin label signal at g = 1.98. The g = 1.98 peak of proxyl was chosen over the two other stronger signals because it is not overlapping with the gz signal of ISP.
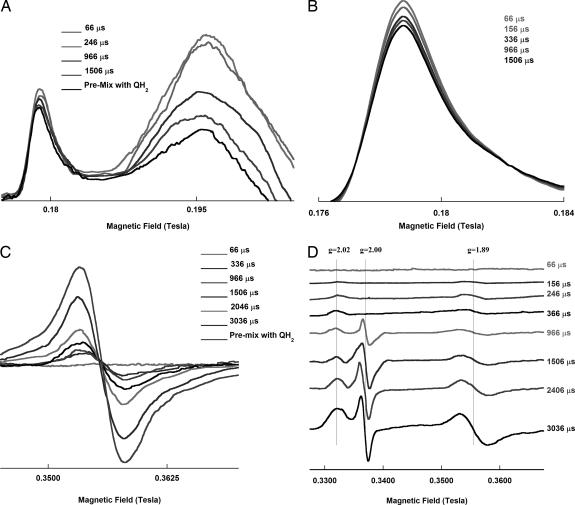
Fig. 4.
EPR spectra of cytochromes b and c1 and the reduced Rieske ISP at various time points after mixing with ubiquinol. The EPR spectra of cytochromes bL and bH/c1 (A and B) and ISC (C and D) in the cytochrome bc1 complex reacting with ubiquinol are shown at different time points as indicated. B and C are the EPR spectra of cytochrome bL and ISC at gy = 1.89, respectively. D shows the EPR spectra of ISC with g = 2.00 region. Samples were prepared as described in Experimental Procedures. The EPR spectra were taken at 6 K, with the following instrument settings: microwave frequency, 9.45 GHz; microwave power, 2.15 mW; and modulation frequency, 100 KHz; time constant, 0.655 s. The modulation amplitude used was 19.57 and 6.30 G for cytochromes and ISP, respectively. All curves are in the same order as the reaction times given.
As shown in Fig. 4D, there is no detectable semiquinone radical in the samples with a reaction time <360 μs. Semiquinone that appears after 366 μs is the radical at the On site, because it is sensitive to antimycin treatment and its formation rate is comparable to that of cytochrome bH. Correlation between semiquinone formation and cytochrome bH reduction has been reported (21).
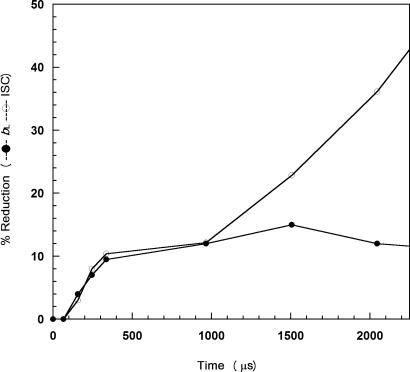
Fig. 5 shows the reduction of ISC and heme bL as a function of time. Note that the zero time point is based on a control sample prepared under the same conditions in the absence of QH2. Reduction of ISC appears to be biphasic. The first phase starts at ≈100 μs and is complete within 1 ms. The reduction of heme bL proceeds with similar kinetics but reaches a maximum at ≈400 μs. On the basis of these kinetic traces, the rate constants for electron transfer from ubiquinol to ISC and bL at the Qp site is 2,770 s −1, which is larger than the rate constants obtained by various indirect methods (36, 46, 47).
Fig. 5.
The degree of reduction of ISC and heme bL against time. ISC (○) and heme bL (●) reduction percentage by QH2, calculated from EPR spectra, at time points between 0 and 1 ms and 0 to 2.25 ms. The percentage reduction of bL was calculated based on percentage decrease in the integrated area of EPR peak at 3.78 using the peak area of the fully oxidized complex as 100%. The percentage reduction of ISC was based on the signal intensity of gy in the sample that was premixed with QH2 as 100%.
One possibility for the biphasic reduction of ISP is the dimeric nature of the cytochrome bc1 complex. When both monomers are in the oxidized form, the rate of reduction of ISP would be higher than that in the partially reduced complex. The slower reduction of ISP in the second phase cannot be resulted from the oxidation of ISP by cytochrome c1 because little cytochrome c1 is reduced in a reaction time < 2 ms. The t1/2 for c1 reduction was determined by conventional stopped flow to be 4.7 ms. Probably cytochrome bL is also reduced biphasically. However, if the rate of the second (slower) phase of bL reduction is comparable to that of bH reduction then no increase in bL reduction would be observed, and the kinetic would appear monophasic. In other words, the observed rate of bL reduction is a “net” rate that includes both the reduction of bL by ubiquinol and reoxidation by bH. This proposal is consistent with the hypothesis of the half-of-the-sites activity of the dimeric bc1 complex (49).
Using a light flash to initiate the electron-transfer cycle in the bacterial cytochrome bc1 complex, a 1,650 s−1 rate constant was reported for electron transfer from ubiquinol to ISP at the Qp site of the complex, in a chromatophores preparation from R. capsulatus (46). A rate constant of 1,200 s−1 was reported when using a binuclear ruthenium complex to rapidly photooxidize cytochrome c1 in the purified R. sphaeroides bc1 complex (47). Using the same technique of rapid photoreduce/photooxidize cytochrome c1 with ruthenium dimer (Ru2D), oxidant-induced reduction of cytochrome bH was observed with a rate constant of 250 s−1 in the presence of antimycin A with bovine bc1 complex and 1,000 s−1 with R. sphaeroides bc1 complex (48). The rate constant of the reduction of heme bH by ubiquinol was found to be 270 s−1 with a photo-releasable caged ubiquinol substrate with mitochondrial cytochrome bc1 (36).
The rate constants found in this study are significantly larger than those observed previously. The similar rate constants for electron transfer from ubiquinol to heme bL and from ubiquinol to ISC indicate that either when the first electron of ubiquinol is transferred to the thermodynamically favored ISC the second electron is immediately transferred to heme bL, or the two electrons of ubiquinol are transferred to ISC and bL simultaneously from a transient ternary complex of ISP–QH2–cytochrome b. Either case is consistent with the Q-cycle mechanism with no ubisemiquinone radical formation at thenQp site. The simultaneous reduction of ISC and cytochrome bL is also consistent with the high-resolution structural data of bovine bc1 complex.
No Ubisemiquinone Radical Is Detected at the Qp Site.
If a ubisemiquinone radical is present during the oxidation of ubiquinol at the Qp site, it should be easily detected by EPR. However, there is no g = 2.00 signal in the EPR spectra of rapid mixed freeze-quenched samples, which shows reduction of ISP (see Fig. 4D) and cytochrome bL within 500 μs of reaction time. To avoid the error of overlooking the signal of ubisemiquinone radical because of its power saturation behavior, the EPR measurements in g = 2.00 region were carried out at various powers. The absence of ubisemiquinone radical is consistent with the observation that the amounts of ISP reduction are equal to those of cytochrome bL reduction in the early events of ubiquinol oxidation.
Whether or not a ubisemiquinone radical is generated at the Qp site during the oxidation of ubiquinol has been a subject of debate. It was reported that under special experimental conditions a transient antimycin A-insensitive ubisemiquinone radical was observed (50) at the Qp site. These data were later refuted (33) because the radical was found to be insensitive to Qp site inhibitors such as myxothiazol, (E)-β-methoxyacrylate-stilbene, or stigmatellin.
To support the idea that the first electron of ubiquinol goes to ISP and thus generates a highly reducing ubisemiquinone to reduce cytochrome bL during the oxidation of ubiquinol at the Qp site, several plausible explanations have been offered for the failure to detect a semiquinone radical at the Qp site. One suggestion is that the radical is antiferromagnetically coupled with the reduced ISC and the radical is only separated from ISP when the second electron is transferred to bL (34). Another suggestion is that the ubisemiquinone radical formed at the Qp site is too transient and the concentration is too low to be detected by EPR (27). The transient nature of the ubisemiquinone radical is easily understood if one pictures that the electron donor of ISP is the ubiquinol–bL complex and not the ubiquinol alone. Once the first electron of ubiquinol in the complex is transferred to ISC, the second electron is immediately transferred to bL, thus no ubisemiquinone radical would accumulate. It is important to note that a ubisemiquinone radical signal, g = 2.00, starts to accumulate when the reaction time is >500 μs. This radical is sensitive to antimycin treatment and has a formation kinetic similar to that of the reduction of cytochrome bH, hence it is the ubisemiquinone radical of the Qn site.
Structural Basis for Concerted Bifurcated Ubiquinol Oxidation at the Qp Site.
Although the information concerning the precise ubiquinol binding site in the Qp pocket is still lacking, recent structural analysis of several Qp site inhibitor-loaded crystals suggests that when ubiquinol enters the Qp pocket it arrests the head domain of ISP to the b-position with the formation of a transient cytochrome b–QH2–ISP complex. This ternary complex is formed with the aid of hydrogen bond formation between Ser-151 and Lys-283 of cytochrome b and the backbone carbonyl atoms of ISP, and the formation of a hydrogen bond between the hydroxyl group at C-1 position of the substrate and the Nε of the His-161 of ISP, a situation similar to the binding of stigmatellin. The C-4 hydroxyl group of the substrate forms a hydrogen bond with either Tyr-131 or Glu-271, a situation similar to the binding of 5-n-undecyl-6-hydroxy-4,7-dioxobenzothiazole (UHDBT) (51). If QH2 binds in the similar position as UHDBT, the distance between the ISC and the substrate would be shorter than the distance between heme bL and the substrate in the transient cytochrome b–QH2–ISP complex. Nonetheless, in the actual cytochrome b–QH2–ISP complex, the two distances could be very similar because the presence of a long alky side chain in ubiquinol may pull the ISP closer to bL than what is observed in the b-position. This proposal is supported by the tighter binding of hexahydrodibenzothiophene, than UHDBT, in the yeast complex. Moreover, a water molecule might be present between ubiquinol and Glu-271 or Tyr-131, facilitating its electron transfer to bL. Thus a comparable electron transfer rate from ubiquinol to ISC and to heme bL could be achieved. The involvement of a water molecule in electron transfer has been observed in other systems (52). The specific role of Glu-271 in substrate binding has been questioned (53). These factors may account for the observed comparable electron transfer rates from ubiquinol to ISC and heme bL.
Experimental Procedures
Materials.
DM and OG were purchased from Anatrace (Maumee, OH). 2,3-Dimethoxy-5-methyl-6-decyl-1,4-benzoquinone bromine (Q0C10Br) was synthesized in our laboratory at Oklahoma State University (54). Stigmatellin, myxothiazol, and antimycin A were purchased from Sigma (St. Louis, MO). Other chemicals were of the highest purity commercially available.
Enzyme Preparations and Assays.
Bovine heart mitochondrial cytochrome bc1 complex was prepared as reported (55). The purified complex was dissolved in 50 mM Tris·HCl buffer, pH 8.0, containing 0.33 M sucrose and 0.01% DM to a cytochrome c1 concentration of 100 μM. In some cases, 3-maleimido-proxyl (proxyl), a spin label, was added to a final concentration of 50 μM (from a 50 mM stock solution in ethanol) as an internal standard. The mixtures were frozen at −80°C until use. The purified bc1 complex contained 9 nmol of cytochrome b and 5.5 nmol of cytochrome c1 per milligram of protein. The concentrations of cytochromes b and c1 were determined spectrophotometrically by using millimolar extinction coefficients of ΔE562–575 nm = 28.5 cm−1·mM−1 and ΔE552–540 nm = 17.5 cm−1·mM −1 for cytochromes b and c1, respectively.
For activity assay, the cytochrome bc1 complex was diluted with 50 mM phosphate buffer, pH 7.4, containing 1 mM EDTA and 0.01% DM to a protein concentration of 0.1 mg/ml. Diluted enzyme solution (5 μl) was added to 990 μl of an assay mixture containing 50 mM phosphate buffer, pH 7.4, containing 1 mM EDTA and 100 μM cytochrome c in the presence or absence of inhibitor. Activity was determined by measuring the reduction of cytochrome c after the addition of 5 μl of 5 mM Q0C10BrH2 (QH2). A millimolar extinction coefficient of 18.5 cm−1·mM−1 was used to calculate the activity. The bc1 complex used in these experiments had a specific activity of ≈20 μmol of cytochrome c reduced per minute per nanomole of cytochrome b.
Stopped-Flow Experiments.
For the determination of electron transfer rates between the ubiquinol and heme b or heme c1, the cytochrome bc1 complex was mixed with equal volumes of various concentrations of ubiquinol at room temperature in an Applied Photophysics stopped-flow reaction analyzer SX.18MV. The cytochrome c1 concentration of bc1 complex was 100 μM. Ubiquinol was diluted in 0.2% DM and 0.2% OG to various concentrations. Reductions of cytochromes b and c1 were monitored by the increase of absorption at 559 and 550 nm, respectively, and a photodiode array scan between 600 and 500 nm. A set of observed rate constants was measured as a function of the concentration of quinol, 1.7-, 3.34-, 6.66-, and 10-fold greater than that of the concentration of cytochrome c1. When an inhibitor was used, the cytochrome bc1 complex was treated with 5-fold molar excess of the inhibitor over heme c1 for 15 min before the experiment.
Freeze-Quenching Experiments.
The cytochrome bc1 complex, with concentration of cytochrome c1 at 100 μM, was mixed at a 1:1 ratio with 667 μM QH2 in 0.2% DM and 0.2% OG inside the ultrafast microfluidic mixer (44) with a modification in the design of the mixing chamber (T.E., Jorge Durand, and S.-R.Y., unpublished work). The reaction mixture was freeze-quenched at various time points from 66 μs to ≈3 ms at liquid nitrogen temperature and packed into EPR tubes. All EPR tubes were stored at liquid nitrogen temperature before measurements.
EPR Experiments.
The redox states of the ISP and cytochromes b and c1 were determined by EPR (43), using a Bruker EMX Spectrometer at a temperature of ≈6 K. The instrument settings were as follows: microwave frequency, 9.45 GHz; microwave power, 2.15 mW; time constant, 0.655 s; and modulation frequency, 100 KHz. The modulation amplitudes used were 19.57 and 6.30 G for cytochrome and ISP, respectively. Previous experiments indicated that air trapped inside the sample causes a distorted base line, especially in the heme b/c1 region of the EPR spectrum. To achieve high signal sensitivity, the air remaining within the sample was eliminated by first dipping the EPR tubes containing the freeze-quenched samples into a mixture of isopentane and liquid nitrogen. This solution was at a temperature of approximately −160°C. Then the EPR tubes were subjected to vacuum for 30 s before being put into the EPR spectrometer. The amount of reduction of ISP was calculated from the intensity of peak to valley around g = 1.89 (gy). Reduction of bL was calculated from the integrated peak area at g = 3.78. As described in Enzyme Preparations and Assays, spin label proxyl was added to enzyme solution as an internal standard. The data collected at each reaction time were normalized by comparison to the spin label intensity at g = 1.98. For detection of ubisemiquinone radical, freeze-quenching experiments were carried out with cytochrome bc1 complex containing no proxyl internal standard.
Other Biochemical and Biophysical Techniques.
Protein concentration was determined by the method of Lowry et al. (56). Cytochrome b (57) and cytochrome c1 (58) contents were determined according to reported methods.
Acknowledgments
We thank Dr. Roger Koeppe for critical review of this manuscript. This work was supported by National Institutes of Health Grants GM30721 (to C.-A.Y.) and HL065465 (to S.-R.Y.) and Agricultural Experiment Station Projects 1819 and 2372, Oklahoma State University.
Abbreviations
- ISP
iron–sulfur protein
- ISC
iron–sulfur cluster
- DM
dodecyl maltoside
- OG
octyl glucoside.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Trumpower BL, Gennis RB. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 2.Berry E, Guergova-Kuras MH, Huang L-S, Crofts AR. Annu Rev Biochem. 2000;69:1007–1077. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 3.Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 4.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZL, Huang L-S, Shulmeister VM, Chi Y-I, Kim KK, Huang L-W, Crofts AR, Berry EA, Kim S-H. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 6.Hunte C, Koepke J, Lange C, Robmanith T, Michel H. Structure (London) 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 7.Berry EA, Huang L-S, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. Photosynth Res. 2004;81:251–275. doi: 10.1023/B:PRES.0000036888.18223.0e. [DOI] [PubMed] [Google Scholar]
- 8.Xiao K, Chandrasekaran A, Yu L, Yu CA. J Biol Chem. 2001;276:46125–46131. doi: 10.1074/jbc.M107436200. [DOI] [PubMed] [Google Scholar]
- 9.Soriano GM, Ponamarev MV, Carell CJ, Xia D, Smith J, Cramer WA. J Bionenerg Biomembr. 1999;31:201–213. doi: 10.1023/a:1005463527752. [DOI] [PubMed] [Google Scholar]
- 10.Covian R, Gutierrez-Cirlos EB, Trumpower BL. J Biol Chem. 2004;279:15040–15049. doi: 10.1074/jbc.M400193200. [DOI] [PubMed] [Google Scholar]
- 11.Osyczka A, Moser CC, Daldal F, Dutton PL. Nature. 2004;427:607–612. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 12.Gong X, Yu L, Xia D, Yu C-A. J Biol Chem. 2005;280:9251–9257. doi: 10.1074/jbc.M409994200. [DOI] [PubMed] [Google Scholar]
- 13.Erecinska M, Chance B, Wilson DF, Dutton PL. Proc Natl Acad Sci USA. 1972;69:50–56. doi: 10.1073/pnas.69.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexandre A, Lehninger AL. J Biol Chem. 1979;254:11555–11560. [PubMed] [Google Scholar]
- 15.Mitchell P. J Theor Biol. 1976;62:327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 16.Brandt U, Trumpower B. Crit Rev Biochem Mol Biol. 1994;29:165–197. doi: 10.3109/10409239409086800. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Wen X, Esser L, Quinn B, Yu L, Yu CA, Xia D. Biochemistry. 2003;42:9067–9080. doi: 10.1021/bi0341814. [DOI] [PubMed] [Google Scholar]
- 18.Kolling DRJ, Samoilova RI, Holland JT, Berry EA, Dikanov SA, Crofts AR. J Biol Chem. 2003;278:39747–39754. doi: 10.1074/jbc.M305913200. [DOI] [PubMed] [Google Scholar]
- 19.Cooley JW, Ohnishi T, Daldal F. Biochemistry. 2005;44:10520–10532. doi: 10.1021/bi050571+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CA, Nakaoka S, Yu L, King TE. Biochem Biophys Res Commun. 1978;82:1070–1078. doi: 10.1016/0006-291x(78)90296-6. [DOI] [PubMed] [Google Scholar]
- 21.Yu CA, Nagaoka S, Yu L, King TE. Arch Biochem Biophys. 1980;204:59–70. doi: 10.1016/0003-9861(80)90007-7. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi T, Trumpower BL. J Biol Chem. 1980;255:3278–3284. [PubMed] [Google Scholar]
- 23.Esser L, Quinn B, Li F-F, Zhang M, Elberry M, Yu L, Yu CA, Xia D. J Mol Biol. 2004;341:281–302. doi: 10.1016/j.jmb.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Xia D, Yu CA, Xia J-Z, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Proc Natl Acad Sci USA. 1998;95:8026–8033. doi: 10.1073/pnas.95.14.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link TA. FEBS Lett. 1997;412:257–264. doi: 10.1016/s0014-5793(97)00772-2. [DOI] [PubMed] [Google Scholar]
- 26.Brandt U, Von Jagow G. Eur J Biochem. 1991;196:163–170. doi: 10.1111/j.1432-1033.1991.tb15690.x. [DOI] [PubMed] [Google Scholar]
- 27.Crofts AR. Annu Rev Physiol. 2004;66:689–733. doi: 10.1146/annurev.physiol.66.032102.150251. [DOI] [PubMed] [Google Scholar]
- 28.Hong SJ, Uggulava N, Guergova-Kuras M, Crofts AR. J Biol Chem. 1999;274:33931–33944. doi: 10.1074/jbc.274.48.33931. [DOI] [PubMed] [Google Scholar]
- 29.Yu CA, Wen X, Xiao K, Xia D, Yu L. Biochim Biophys Acta. 2002;1555:65–70. doi: 10.1016/s0005-2728(02)00256-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Xia D, Yu CH, Xia J-Z, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Proc Natl Acad Sci USA. 1998;95:8026–8033. doi: 10.1073/pnas.95.14.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trumpower BL. Biochim Biophys Acta. 2002;1555:166–173. doi: 10.1016/s0005-2728(02)00273-6. [DOI] [PubMed] [Google Scholar]
- 32.Hunte C, Palsdottir H, Trumpower BL. FEBS Lett. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- 33.Junemann S, Heathcote P, Rich PR. J Biol Chem. 1998;273:21603–21607. doi: 10.1074/jbc.273.34.21603. [DOI] [PubMed] [Google Scholar]
- 34.Link TA. FEBS Lett. 1997;412:257–264. doi: 10.1016/s0014-5793(97)00772-2. [DOI] [PubMed] [Google Scholar]
- 35.De Vries S, Albracht SPJ, Berden JA, Slater EC. J Biol Chem. 1982;256:11996–11998. [PubMed] [Google Scholar]
- 36.Hansen KC, Schulz BE, Wang G, Chan SI. Biochim Biophys Acta. 2000;1456:121–137. doi: 10.1016/s0005-2728(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 37.King TE, Yu CA, Yu L, Chiang YL. In: Electron Transfer Chains and Oxidative Phosphorylation. Quayliariello E, Papa S, Palmieri F, Slater EC, Siliprandi N, editors. New York: Elsevier; 1975. pp. 105–118. [Google Scholar]
- 38.Tsai A-L, Olson JS, Palmer G. J Biol Chem. 1983;258:2122–2125. [PubMed] [Google Scholar]
- 39.Trumpower BL, Edwards CA. J Biol Chem. 1979;254:8697–8706. [PubMed] [Google Scholar]
- 40.Orme-Johnson NR, Hansen RE, Beinert H. Biochem Biophys Res Commun. 1974;45:871–878. doi: 10.1016/0006-291x(71)90419-0. [DOI] [PubMed] [Google Scholar]
- 41.Salerno JC. J Biol Chem. 1984;259:2331–23366. [PubMed] [Google Scholar]
- 42.De Vries S, Albracht SPJ, Leeuwerk FJ. Biochim Biophys Acta. 1979;546:316–333. doi: 10.1016/0005-2728(79)90049-5. [DOI] [PubMed] [Google Scholar]
- 43.McCurley JP, Miki T, Yu L, Yu CA. Biochim Biophys Acta. 1990;1020:176–186. doi: 10.1016/0005-2728(90)90049-a. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y, Gary J, Gerfen GJ, Rousseau DL, Yeh S-R. Anal Chem. 2003;75:5381–5386. doi: 10.1021/ac0346205. [DOI] [PubMed] [Google Scholar]
- 45.Ding H, Robertson DE, Daldal F, Dutton PL. Biochemistry. 1992;31:3144–3158. doi: 10.1021/bi00127a015. [DOI] [PubMed] [Google Scholar]
- 46.Crofts AR, Wang Z. Photosynth Res. 1989;22:69–87. doi: 10.1007/BF00114768. [DOI] [PubMed] [Google Scholar]
- 47.Sadoski RC, Engstrom G, Tian H, Zhang L, Yu L, Yu CA, Durham B, Millett F. Biochemistry. 2000;39:4231–4236. doi: 10.1021/bi000003o. [DOI] [PubMed] [Google Scholar]
- 48.Engstrom G, Xiao K-H, Yu CA, Yu L, Durham W, Millett F. J Biol Chem. 2002;277:31072–31078. doi: 10.1074/jbc.M202594200. [DOI] [PubMed] [Google Scholar]
- 49.Covian R, Trumpower BL. J Biol Chem. 2006;281:30925–30932. doi: 10.1074/jbc.M604694200. [DOI] [PubMed] [Google Scholar]
- 50.De Vries S, Albracht SPJ, Berden JA, Slater EC. J Biol Chem. 1982;256:11996–11998. [PubMed] [Google Scholar]
- 51.Palsdottir H, Lojero GC, Trumpower BL, Hunte C. J Biol Chem. 2003;278:31303–31311. doi: 10.1074/jbc.M302195200. [DOI] [PubMed] [Google Scholar]
- 52.Lin J, Balabin IA, Beratan DN. Science. 2005;310:1311–1313. doi: 10.1126/science.1118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osyczka A, Zhang H, Mathe C, Rich P, Moser CC, Dutton PL. Biochemistry. 2006;45:10492–10503. doi: 10.1021/bi060013a. [DOI] [PubMed] [Google Scholar]
- 54.Yu CA, Yu L. Biochemistry. 1982;21:4096–4101. doi: 10.1021/bi00260a028. [DOI] [PubMed] [Google Scholar]
- 55.Yu CA, Yu L. Biochim Biophys Acta. 1980;591:409–420. doi: 10.1016/0005-2728(80)90172-3. [DOI] [PubMed] [Google Scholar]
- 56.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 57.Berden JA, Slater EC. Biochim Biophys Acta. 1970;216:237–249. doi: 10.1016/0005-2728(70)90215-x. [DOI] [PubMed] [Google Scholar]
- 58.Yu CA, Yu L, King TE. J Biol Chem. 1972;247:1012–1019. [PubMed] [Google Scholar]
- 59.Esser L, Gong X, Yang S-Q, Yu L, Yu CA, Xia D. Proc Natl Aced Sci USA. 2006;35:13045–13050. doi: 10.1073/pnas.0601149103. [DOI] [PMC free article] [PubMed] [Google Scholar]