Abstract
Translocation of tRNA and mRNA during protein synthesis is believed to be coupled to structural changes in the ribosome. The “ratchet model,” based on cryo-EM reconstructions of ribosome complexes, invokes relative movement of the 30S and 50S ribosomal subunits in this process; however, evidence that directly demonstrates a requirement for intersubunit movement during translocation is lacking. To address this problem, we created an intersubunit disulfide cross-link to restrict potential movement. The cross-linked ribosomes were unable to carry out polypeptide synthesis; this inhibition was completely reversed upon reduction of the disulfide bridge. In vitro assays showed that the cross-linked ribosomes were specifically blocked in elongation factor G-dependent translocation. These findings show that intersubunit movement is required for ribosomal translocation, accounting for the universal two-subunit architecture of ribosomes.
Keywords: ratchet, tRNA, hybrid state
Ribosomes, the ribonucleoprotein complexes responsible for protein synthesis, are always found to be composed of two subunits (1), called 30S and 50S in bacteria and archaea and 40S and 60S in eukarya. The underlying reason for this two-subunit architecture was addressed by Spirin (2) and Bretscher (3), who independently proposed the involvement of intersubunit movement in translocation of tRNAs and mRNA through the ribosome. Bretscher (3) depicted translocation as a two-step process involving an intermediate state in which the ends of the peptidyl-tRNA are bound to different sites on the two ribosomal subunits. Twenty years later, direct experimental evidence for hybrid states of tRNA binding as intermediates in translocation came from chemical probing studies (4). According to the hybrid-states model, translocation involves independent, sequential movement of the two extremities of tRNA. In the first step, after peptide bond formation, the acceptor ends of the A- and P-site tRNAs move into the 50S P and E sites, respectively, forming the A/P and P/E hybrid states. In the second step, whose catalysis requires elongation factor (EF)-G-dependent GTP hydrolysis, the anticodon ends of the tRNAs move from the 30S A and P sites into the P and E sites, coupled with movement of the mRNA. Implicit in this mechanism was the suggestion of relative movement of the 30S and 50S subunits, in which the classical and hybrid states correspond to two different conformational states of the ribosome (4, 5), recalling the proposals of Spirin (2) and Bretscher (3).
More recently, cryo-EM reconstructions have provided direct visualization of trapped tRNA-ribosome complexes, in which the ribosomal subunits have an altered rotational orientation compared with that of the classical state (6, 7). Vacant ribosomes, or ones containing peptidyl-tRNA bound to the P site, had similar subunit orientations and tRNA binding states to those observed crystallographically for classical-state ribosomes (8). In complexes containing the translocational EF-G bound with either a nonhydrolyzable GTP analog (with or without a deacylated tRNA bound to the P site) or with the translocation inhibitor fusidic acid (with deacylated tRNA bound to the P site), the 30S subunit, viewed from its solvent side, was rotated by ≈6° in a counterclockwise sense with respect to the 50S subunit around an axis perpendicular to the interface plane (6, 7) (Fig. 1). In the latter complexes, deacylated tRNA was observed bound in the P/E state, providing a direct link between the “ratcheted” state and the hybrid state. Although these findings make a compelling case, direct evidence showing a requirement for intersubunit movement in translocation is lacking.
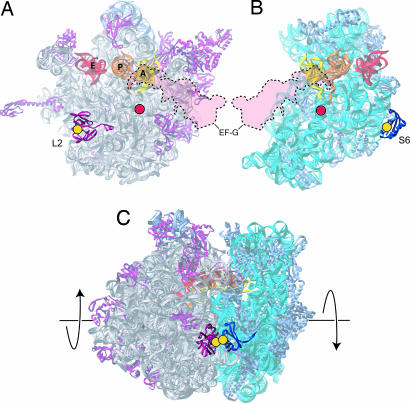
Fig. 1.
Position of the intersubunit cross-link. The observed axis of rotation from cryo-EM reconstructions (7) is indicated by a red dot, and the positions of cysteines introduced into proteins S6 (F81C) and L2 (I123C) are indicated by yellow dots in the structures of the 50S subunit interface (A), 30S subunit interface (B), and 70S ribosome (C) (8). The position of EF-G (7) is shown in transparent red with dotted outline, and the A-, P- and E-site tRNAs are in yellow, orange, and red, respectively.
To directly test this requirement, we created ribosomes with an intersubunit disulfide bond designed to prevent intersubunit movement. Based on the crystal structure of the 70S ribosome (8), single cysteines were introduced at positions in well folded globular domains of the proteins S6 and L2 that are juxtaposed across the subunit interface (Fig. 1). The predicted disulfide bond is distal to the observed axis of rotation and the binding sites of EF-G, tRNA, and mRNA (Fig. 1). The magnitude of a disulfide constraint can be estimated from the observed distances between β-carbons in the disulfide linkages of known protein crystal structures, which range from 3.4 to 4.6 Å (9). Thus, a given intersubunit disulfide should allow no more than 1.2 Å relative intersubunit motion (10). Based on the 6°–10° rotation reported from cryo-EM studies, the relative displacement of the two subunits at the S6-L2 region of the interface can be estimated to be on the order of 8–15 Å (6, 7).
Results
The mutant versions of the genes for S6 and L2 were introduced as precise chromosomal replacements in separate Escherichia coli strains, from which mutant 30S and 50S subunits, respectively, were isolated and used to construct S6C-L2C double-mutant 70S ribosomes. We first tested for formation of the intersubunit disulfide bridge by sucrose gradient sedimentation under subunit dissociation conditions (1 mM Mg2+) after dialysis of S6C-L2C 70S ribosomes against oxygen-saturated buffer (Fig. 2A). Oxidized double-mutant ribosomes sedimented at 70S in 1 mM Mg2+ and upon addition of reducing agent dissociated into 30S and 50S subunits. 70S ribosomes containing either S6C 30S or L2C 50S subunits failed to form 70S particles under the same oxidizing conditions (data not shown), showing that the intersubunit cross-link is specific to the S6C-L2C mutant pair. To identify the ribosomal proteins responsible for cross-link formation, proteins extracted from cross-linked 70S ribosomes were subjected to 2D gel electrophoresis (11), in which the first dimension was run under oxidizing conditions and the second under reducing conditions (Fig. 2B). In the first (oxidizing) dimension, a high-molecular-weight band appeared that was distinct from the positions of known ribosomal proteins; in the second (reducing) dimension, this band resolved into two off-diagonal lower-molecular-weight bands corresponding to proteins S6 and L2. The absence of a band corresponding to noncross-linked L2 in the first dimension indicates that cross-linking was virtually complete.
Fig. 2.
Formation of an intersubunit disulfide bridge. (A) Sedimentation analysis of S6-L2 mutant ribosomes under subunit dissociation conditions (1 mM Mg2+). Under oxidizing conditions the 30S and 50S subunits are covalently linked by disulfide formation to form 70S particles. The arrow indicates the direction of sedimentation. (B) Identification of the S6(F81C)–L2(I123C) cross-link. Proteins from oxidized mutant 70S ribosomes were analyzed by 2D SDS-gel electrophoresis run under oxidizing conditions in the first dimension and reducing conditions in the second dimension. Asterisk indicates the position of free L2 in the first-dimension gel. The off-diagonal spots confirm disulfide cross-linking of S6 and L2.
Next, the ability of the cross-linked ribosomes to carry out polypeptide synthesis was tested by using poly(U)-directed synthesis of poly(Phe), an assay that depends on all of the main steps of the elongation phase of protein synthesis. Because oxidizing conditions are required to maintain the cross-link, we first had to show that our in vitro translation system was unaffected by the absence of reducing agents (12). WT ribosomes showed no detectible differences in poly(Phe) synthesis between oxidizing and reducing conditions (Fig. 3A). In contrast, S6C-L2C ribosomes showed nearly complete loss of activity under oxidizing conditions. When the intersubunit cross-link was cleaved by addition of DTT, polypeptide synthesis was immediately restored to WT levels (Fig. 3A). Ribosomes containing either the S6C or L2C single mutations were active under both reducing and oxidizing conditions (data not shown).
Fig. 3.
Effects of intersubunit cross-linking on translational functions. (A) The S6-L2 cross-link blocks in vitro translation of a poly(U) mRNA; activity is restored upon reduction of the disulfide bridge by addition of DTT (arrow). (B) Intersubunit cross-linking does not prevent tRNA binding or peptide bond formation. P-site complexes (P-site) contained m32 mRNA and N-Ac-[3H]Phe-tRNAPhe. A-site complexes (A-site) contained m32 mRNA, tRNAfMet bound to the P site, and N-Ac-[3H]Phe-tRNAPhe in the A site. tRNA binding (to 1.5 pmol of ribosomes) was assayed by filter binding, and peptide bond formation was by formation of N-Ac-[3H]Phe-puromycin under oxidizing (ox) or reducing (red) conditions. N-Ac-Phe-tRNAPhe is reactive with puromycin when bound to the ribosomal P site and unreactive when in the ribosomal A site. (C) Intersubunit cross-linking does not prevent EF-Tu-dependent binding of aminoacyl-tRNA to the A site. [3H]Phe-tRNAPhe was added to ribosomes containing m32 mRNA and fMet-tRNAfMet either as a ternary complex with EF-Tu·GTP (+EF-Tu) or with GTP alone (−EF-Tu). Binding of [3H]Phe-tRNAPhe was normalized to that of WT ribosomes measured under reducing conditions.
We then asked which steps of elongation (mRNA binding, P-site tRNA binding, EF-Tu-dependent binding of aminoacyl-tRNA, peptidyl transfer, and EF-G-dependent translocation) were affected by intersubunit cross-link formation. Binding of N-Ac-[3H]Phe-tRNAPhe to the P site of S6C-L2C ribosomes in the presence of a defined mRNA was similar to that of WT under oxidizing and reducing conditions; furthermore, the bound peptidyl-tRNA was fully reactive with puromycin (Fig. 3B). Thus, the intersubunit cross-link does not prevent either P-site binding or the peptidyl transferase reaction. Nonenzymatic binding of peptidyl-tRNA to the A site was also tested, using an assay in which tRNAfMet was first bound to the P site, followed by binding N-Ac-[3H]Phe-tRNAPhe to the A site directed by a defined mRNA. The cross-linked ribosomes showed no A-site binding defects, and their lack of puromycin reactivity verified that N-Ac-[3H]Phe-tRNAPhe was indeed bound to the A site (Fig. 3B). EF-Tu-dependent binding of [3H]Phe-tRNAPhe to the A site of S6C-L2C cross-linked ribosomes was also found to be similar to that of WT ribosomes (Fig. 3C).
EF-G-dependent translocation was assayed by using two different approaches (Fig. 4). In the first approach, we monitored EF-G-dependent movement of peptidyl-tRNA from the A site to the P site by appearance of its puromycin reactivity (Fig. 4A; ref. 13). Deacylated tRNAfMet was bound to the P site and N-Ac-[3H]Phe-tRNAPhe to the A site in the presence of a defined mRNA. Reactivity of the bound N-Ac-[3H]Phe-tRNAPhe with puromycin was then tested before and after incubation with EF-G·GTP. With WT, S6C, and L2C ribosomes, EF-G-dependent translocation results in full puromycin reactivity under both oxidizing and reducing conditions. In contrast, S6C-L2C mutant ribosomes recover puromycin reactivity after incubation with EF-G·GTP under reducing conditions, but fail to translocate under oxidizing conditions (Fig. 4A). Using a second approach, we monitored translocation using toeprinting (14) in which extension by reverse transcriptase of a DNA oligonucleotide primer preannealed to the mRNA is used to determine the register of the mRNA on the ribosome (Fig. 4B). Ribosome complexes were assembled with a defined mRNA, deacylated tRNAfMet in the P site and N-Ac-Phe-tRNAphe in the A site. Translocation of S6C-L2C ribosomes with EF-G·GTP under reducing conditions resulted in movement of the mRNA by one codon, shortening the extended cDNA product by three nucleotides (corresponding to positions +16 and +19, respectively; Fig. 4B). Under oxidizing conditions, where the S6C-L2C intersubunit cross-link is formed, appearance of the +19 band was nearly abolished. The toeprinting experiments confirmed the ability of the cross-linked ribosomes to bind mRNA, and tRNA to the A and P sites, and their inability to support EF-G-dependent translocation (Fig. 4B). Thus, according to two independent assays, one of which monitors tRNA movement and the other mRNA movement, formation of the S6C-L2C intersubunit disulfide bridge specifically blocks EF-G-dependent translocation.
Fig. 4.
Intersubunit cross-linking blocks EF-G-dependent translocation. (A) Monitoring translocation of peptidyl-tRNA into the P site by the appearance of puromycin reactivity. Deacylated tRNAfMet and N-Ac-[3H]Phe-tRNAPhe were bound to the P and A sites, respectively, of m32-programmed ribosomes. Formation of N-Ac-[3H]Phe-puromycin was measured before (−EF-G) and after (+EF-G) translocation. WT, S6(F81C) and L2(I123C) single mutants, and S6(F81C)- L2(I123C) double mutant ribosomes were assayed under oxidizing (ox) and reducing (red) conditions. (B) Monitoring translocation by toe-printing. Complexes were assembled as in A except with m291 mRNA. Translocation of mRNA was followed by extending a primer bound to the 3′ side of the mRNA with reverse transcriptase. tRNA was first bound to the P site (P) giving the band at +16. The appearance of doublet bands (A) corresponds to A-site tRNA binding. The appearance of band (+19) in the EF-G (+) lane corresponds to translocation by one codon.
Discussion
These findings show that relative movement of the two ribosomal subunits is required for translocation. The importance of intersubunit movement for the translocation step of protein synthesis accounts for the universal two-subunit architecture of ribosomes, as originally suggested by Spirin (2) and Bretscher (3), and underscores the dynamic nature of the ribosome. Our evidence supports the rotational mechanism proposed by Frank and coworkers (6, 7) based on cryo-EM reconstructions, although it would also, in principle, support other models based on intersubunit movement. The role of EF-G in translocation is not yet fully understood. Experiments that used disulfide cross-link formation to restrict the conformational mobility of EF-G showed that it is the interdomain movement of EF-G, rather than GTP hydrolysis itself, that is required for translocation (13). EF-G movement could either drive intersubunit movement of the ribosome directly or alternatively trigger movement through an indirect mechanism. The ability of the ribosome to translocate mRNA and tRNA in the absence of EF-G and GTP (15–18) suggests that the underlying mechanism lies in the mRNA–tRNA–ribosome complex rather than in EF-G. In addition to intersubunit movement, there is increasing evidence that ribosome function involves many other examples of structural dynamics. Structural studies have already revealed different conformational states involving the head of the 30S subunit, the L1 arm and L11 regions of the 50S subunit, some of the intersubunit bridges, and other more detailed features (6, 7, 19–22). It will be a major challenge to obtain a full description of the structural dynamics of the ribosome and ultimately to explain the mechanism of protein synthesis in such terms.
Materials and Methods
Mutagenesis.
Strains containing chromosomal cysteine point mutations within the rpsF(S6) and rplB(L2) genes were made by selected recombination using the vector pKO3 as described (23). pK03 plasmids containing operons were constructed by PCR amplification of the S10 and S6 operons from E. coli K12 genomic DNA. QuikChange mutagenesis (Stratagene, La Jolla, CA) was used to create F81C in rpsF and I123C in rplB and to create unique AclI and SphI restriction sites in the S6 and L2 genes, respectively.
S6F81C QuikChange primer was: 5′-ctg gaa act acc ttc cgT tGc aac gat gcc gtt atc cg-3′; L2I123C QuikChange primer was: 5′-ct ggc gtt gat gct gca TGc aaa cca ggt aac aac c-3′; mutated sequences are capitalized.
Buffer A.
Buffer A is 50 mM Tris·HCl, pH 7.0/100 mM NH4Cl.
Cross-Link Formation.
E. coli K12 mutant strains (FS6C and IL2C) were grown at 37°C to an A550 of 0.4 and pelleted. Ribosomes were purified as described (24) except that 1 mM DTT replaced 2-mercaptoethanol in all buffers and addition of DNase was omitted. Subunits were collected from 10% to 30% sucrose gradients as described (25) except for dilution of ribosomes to 1 mM MgCl2 instead of dialysis and use of 1 mM DTT instead of 2-mercaptoethanol.
To create 70S S6-L2 cross-linked ribosomes mutant 30S (S6-F81C) and mutant 50S (L2-I123C) subunits were mixed at 1:1 stoichiometry in buffer A containing 20 mM MgCl2 and 1 mM DTT and incubated for 10 min at 37°C. Ribosomes were then dialyzed overnight at 4°C against oxygen-saturated buffer A containing 20 mM MgCl2. Buffer was saturated with oxygen by constantly bubbling air through the buffer during dialysis. Ribosomes were then diluted to 2 mM MgCl2 and centrifuged on 10–30% sucrose gradients in buffer A containing 1 mM MgCl2. Fractions corresponding to 70S ribosomes were collected. MgCl2 concentration was increased to 10 mM, and ribosomes were pelleted. 70S Ribosomes were resuspended to a concentration of 25 μM in buffer A containing 10 mM MgCl2, aliquoted, flash-frozen, and stored at −80°C.
WT oxidized ribosomes were purified from E. coli K12 as described above except that no reducing agent was added after the first salt wash (24). Subunits were purified from sucrose gradients as described (25) except that no reducing agent was used and during dialysis air was bubbled through the buffer. Subunits were collected and stored in buffer A containing 10 mM MgCl2. In all assays, WT 30S subunits were heat-activated at 42°C for 10 min before being associated into 70S ribosomes.
Sucrose Gradients.
Ten picomols of 70S (S6C-L2C) ribosomes in buffer A containing 1 mM MgCl2, with or without 30 mM DTT, was incubated at 25°C for 5 min then subjected to centrifugation on 10–30% sucrose gradients made with buffer A containing 1 mM MgCl2, with or without 1 mM DTT on an SW41 rotor (Beckman, Fullerton, CA) at 25,000 rpm for 15 h at 4°C.
2D Gel Electrophoresis.
Two hundred picomols of oxidized 70S (S6C-L2C) ribosomes was incubated in 60 μl of freshly made sample buffer [100 mM NH4Cl/500 mM iodoacetamide/50 mM Tris·HCl (pH 7.0) containing 4% SDS/12% glycerol] at 90°C for 4 min. 70S ribosomal proteins were resolved by using tricine-SDS/PAGE as described (26). Fifteen microliters (50 pmol) and 45 μl (150 pmol) of denatured 70S ribosomes were run in separate lanes on a 20-cm-long separating gel (16% polyacrylamide) with a 4-cm stacking gel (4% polyacrylamide). Samples were electrophoresed into the stacking gel at 1 W then run at 3 W overnight. The lane with 150 pmol was excised from the gel and soaked in 300 mM Tris·HCl (pH 8.5), 0.03% SDS, 30 mM 2-mercaptoethanol, and 20 mM DTT for 1 h. The reduced strip was then polymerized into the stacking gel of a second gel (27) and run a second time as above. Coomassie blue staining of the proteins in the 50-pmol lane and the 2D gel was performed as described (28).
tRNA, mRNA, and Enzymes.
Purified tRNAPhe was purchased from Sigma (St. Louis, MO) and tRNAfMet was from Subriden (Rollingbay, WA). tRNAphe was aminoacylated with [3H]Phe (Amersham Biosciences, Piscataway, NJ) and acetylated as described (24). tRNAfMet was aminoacylated with Met and formylated as described (29).
m32 mRNA was synthesized by Dharmacon (Lafayette, CO) (5′ ggc aag gag gua aaa aug uuu aaa ggu aaa ucu acu 3′).
m291 mRNA was made by in vitro T7 RNA polymerase transcription (30) (5′-guaaagugucauagcaccaacuguuaauuaaauuaaauuaaaaaggaaauaaaaauguuuguauacaaaucuacugcugaacucgcugcacaaauggcuaaacugaauggcaauaaagguuuuucuucugaagauaaag-3′).
EF-G containing a C-terminal His-6 tag was purified as described (30). EF-Tu containing a C-terminal His-6 tag was purified as described (31). S100 enzymes (ribosome-free supernatant) were purified as described (32) except that no reducing agent was used in buffers.
In Vitro Translation.
Poly(U)-directed in vitro translation was performed in polymix buffer (33) by adding 30 pmol of 70S ribosomes, 1 mM ATP, 0.5 mM GTP, 6 mM phosphoenolpyruvate, 1 μg/ml pyruvate kinase (Roche, Indianapolis, IN), 280 pmol tRNAPhe, 2.4 nmol [14C]-Phe (PerkinElmer, Wellesley, MA), 3.0 nmol Phe, S100 enzymes, and 0.5 A260 units poly(U) mRNA in total volume of 60 μl at 25°C. At 4 min the reaction was split and DTT was added to one tube to a concentration of 30 mM. Four microliters was spotted on a Whatman 1 filter (Maidstone, U.K.) for each time point. Filters were incubated on ice for 20 min in 10% trichoroacetic acid (TCA) (4 ml per filter) and then washed twice in 5% TCA at 90°C for 5 min, washed in 95% EtOH, dried, and counted in liquid scintillation mixture.
Filter Binding and Puromycin Reactivity.
Nonenzymatic filter binding was assayed in buffer A containing 20 mM MgCl2, with or without 20 mM DTT. P-site complexes were formed by incubating 6 pmol of 70S ribosomes, 12 pmol of m32 mRNA, and 8 pmol of N-Ac-[3H]Phe-tRNAPhe in 12 μl for 20 min at 37°C. A portion (1.5 pmol) was spotted on 0.45-μm HA nitrocellulose filters (Millipore, Billerica, MA) and washed three times with 5 ml of cold buffer A containing 20 mM MgCl2, with or without 1 mM DTT. Filters were dried and counted in liquid scintillation mixture. Another portion (1.5 pmol) was incubated with 1 mM puromycin for 10 min at 37°C. N-Ac-[3H]Phe-puromycin was extracted with ethyl acetate and counted in liquid scintillation mixture. A-site complexes were formed as above except with 10 pmol of tRNAfMet, followed by incubation with 8 pmol of N-Ac-[3H]Phe-tRNAPhe for 30 min at 37°C. Complexes were either extracted or filtered then counted as described above.
EF-Tu-dependent tRNA binding was assayed in buffer A containing 7.5 mM MgCl2, with or without 20 mM DTT. P-site complexes were formed by incubating 6 pmol of 70S ribosomes, 12 pmol of m32 mRNA, and 8 pmol of fMet-tRNAfMet in 6 μl of buffer A containing 20 mM MgCl2 for 20 min at 37°C then diluting MgCl2 concentration to 7.5 mM. To form ternary complex, 200 pmol of EF-Tu·GDP was incubated with 1 mM GTP, 3 mM phosphoenol pyruvate, and 1 μg/ml pyruvate kinase in 20 μl of 50 mM Tris·HCl (pH 7.0), 40 mM NH4Cl, and 10 mM MgCl2 for 10 min at 37°C. Fifteen picomols of [3H]Phe-tRNAPhe was incubated with 20 pmol of EF-Tu·GTP in 5 μl of the same buffer for 5 min at the same temperature. Ternary complex (concentration determined by [3H]Phe-tRNAPhe) or [3H]Phe-tRNA alone was added at 1:1 stoichiometry to 70S P-site ribosomes and incubated 5 min at 37°C. Nitrocellulose filter binding was assayed as described above and counts were normalized to that of WT ribosomes measured under reducing conditions.
EF-G-Dependent Translocation.
To monitor translocation using puromycin reactivity, A-site complexes were formed nonenzymatically as described above. All reactions used buffer A containing 20 mM MgCl2. Samples containing A-site complexes were split in half; one half was incubated with EFG·GTP (0.2 μm 70S, 0.3 μm EF-G, 0.3 mM GTP), and the other half was incubated with GTP (0.3 mM) alone. All samples were then split a second time and half were incubated with puromycin (1 mM). Samples were incubated 10 min at 37°C and then extracted or filtered and counted as described above. Results are reported as N-Ac-[3H]Phe-puromycin divided by ribosome bound N-Ac-[3H]Phe-tRNAPhe (counts bound to filter).
Toeprinting was done as described (18). Binding of tRNAs was done in buffer A containing 20 mM MgCl2, with or without 20 mM DTT using mRNA m291, tRNAfMet in the P site and N-Ac-Phe-tRNA bound to the A site. EF-G·GTP was added at 2:1 stoichiometry to 70S ribosomes and incubated for 5 min at 37°C. Because reverse transcriptase requires the presence of reducing agent, thiostrepton (1 μM) was added after incubation with EF-G·GTP to inhibit further translocation and 10 mM DTT was added during primer extension along with 2 units of RNase Inhibitor (SUPERase-IN; Ambion, Austin, TX).
Acknowledgments
We thank Iraj Ali, Laura Lancaster, Lee Hoang, Robyn Hickerson, Dmitri Ermolenko, and Clint Spiegel of the University of California, Santa Cruz for critical expertise and reagents; Carl Gorringe and Michael Pearson for contributing to Fig. 1; and members of H.F.N.'s laboratory for useful discussion and advice. These studies were supported by grants from the National Institutes of Health and National Science Foundation (to H.F.N.)
Abbreviation
- EF
elongation factor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tissiéres A, Watson JD. Nature. 1958;182:778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- 2.Spirin AS. Dokl Akad Nauk SSSR. 1968;179:1467–1470. [PubMed] [Google Scholar]
- 3.Bretscher MS. Nature. 1968;218:675–677. doi: 10.1038/218675a0. [DOI] [PubMed] [Google Scholar]
- 4.Moazed D, Noller HF. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 5.Noller HF, Moazed D, Stern S, Powers T, Allen PA, Robertson JM, Weiser B, Triman K. In: The Ribosome Structure, Function, and Evolution. Hill WE, Dahlberf A, Garrett RA, Moore PB, Schlessinger D, Warner JR, editors. Washington, DC: Am Soc Microbiol; 1990. pp. 73–92. [Google Scholar]
- 6.Frank J, Agrawal RK. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 7.Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 8.Yusupov M, Yusupova G, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan N, Sowdhamini R, Ramakrishnan C, Balaram P. Int J Pept Protein Res. 1990;36:147–155. doi: 10.1111/j.1399-3011.1990.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 10.Chervitz SA, Lin CM, Falke JJ. Biochemistry. 1995;34:9722–9733. doi: 10.1021/bi00030a010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer A, Traut RR. Proc Natl Acad Sci USA. 1974;71:3946–3950. doi: 10.1073/pnas.71.10.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traut RR, Haenni AL. Eur J Biochem. 1967;2:4–73. doi: 10.1111/j.1432-1033.1967.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 13.Peske F, Matassova NB, Savelsbergh A, Rodnina MV, Wintermeyer W. Mol Cell. 2000;6:501–505. doi: 10.1016/s1097-2765(00)00049-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartz D, McPheeters DS, Gold L. Genes Dev. 1989;3:1899–1912. doi: 10.1101/gad.3.12a.1899. [DOI] [PubMed] [Google Scholar]
- 15.Pestka S. J Biol Chem. 1969;244:1533–1539. [PubMed] [Google Scholar]
- 16.Gavrilova LP, Spirin AS. FEBS Lett. 1971;17:324–326. doi: 10.1016/0014-5793(71)80177-1. [DOI] [PubMed] [Google Scholar]
- 17.Southworth DR, Brunelle JL, Green R. J Mol Biol. 2002;324:611–623. doi: 10.1016/s0022-2836(02)01196-8. [DOI] [PubMed] [Google Scholar]
- 18.Fredrick K, Noller HF. Science. 2003;300:1159–1162. doi: 10.1126/science.1084571. [DOI] [PubMed] [Google Scholar]
- 19.Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 20.Harms J, Schluenzen F, Zarivach R, Bashan A, Gat S, Agmon I, Bartels H, Franceschi F, Yonath A. Cell. 2001;107:679–688. doi: 10.1016/s0092-8674(01)00546-3. [DOI] [PubMed] [Google Scholar]
- 21.Korostelev A, Trakhanov S, Laurberg M, Noller HF. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Ogle JM, Brodersen DE, Clemons WM, Tarry MJ, Carter AP, Ramakrishnan V. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- 23.Link AJ, Phillips D, Church GM. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moazed D, Noller HF. Cell. 1989;57:585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- 25.Moazed D, Van Stolk BJ, Douthwaite S, Noller HF. J Mol Biol. 1986;191:483–493. doi: 10.1016/0022-2836(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 26.Schägger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 27.Howard GA, Traut RR. FEBS Lett. 1973;29:177–180. doi: 10.1016/0014-5793(73)80555-1. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 29.Lancaster L, Noller HF. Mol Cell. 2005;20:623–632. doi: 10.1016/j.molcel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Fredrick K, Noller HF. Mol Cell. 2002;9:1125–1131. doi: 10.1016/s1097-2765(02)00523-3. [DOI] [PubMed] [Google Scholar]
- 31.Boon K, Vijgenboom E, Madsen LV, Talens A, Kraal B, Bosch L. Eur J Biochem. 1992;210:177–183. doi: 10.1111/j.1432-1033.1992.tb17406.x. [DOI] [PubMed] [Google Scholar]
- 32.Traub P, Mizushima S, Lowry CV, Nomura M. Methods Enzymol. 1971;20:391–407. [Google Scholar]
- 33.Jelenc PC, Kurland CG. Proc Natl Acad Sci USA. 1979;76:3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]