Abstract
Although HIV is the necessary and sufficient causative agent of AIDS, genetic and environmental factors markedly influence the pace of disease progression. Clinical and experimental evidence suggests that human herpesvirus 6A (HHV-6A), a cytopathic T-lymphotropic DNA virus, fosters the progression to AIDS in synergy with HIV-1. In this study, we investigated the effect of coinfection with HHV-6A on the progression of simian immunodeficiency virus (SIV) disease in pig-tailed macaques (Macaca nemestrina). Inoculation of HHV-6A resulted in a rapid appearance of plasma viremia associated with transient clinical manifestations and followed by antibody seroconversion, indicating that this primate species is susceptible to HHV-6A infection. Whereas animals infected with HHV-6A alone did not show any long-term clinical and immunological sequelae, a progressive loss of CD4+ T cells was observed in all of the macaques inoculated with SIV. However, progression to full-blown AIDS was dramatically accelerated by coinfection with HHV-6A. Rapid disease development in dually infected animals was heralded by an early depletion of both CD4+ and CD8+ T cells. These results provide in vivo evidence that HHV-6A may act as a promoting factor in AIDS progression.
Keywords: simian immunodeficiency virus, animal models, herpesviruses
Human herpesvirus 6 (HHV-6) is a β-herpesvirus that was discovered at the dawn of the AIDS era (1). Two viral variants have been recognized, designated HHV-6A and HHV-6B, which exhibit different genetic, immunologic, biological, and epidemiological features (2). Although HHV-6B is virtually ubiquitous in the human population and is the etiologic agent of roseola infantum (3), the epidemiology and disease associations of HHV-6A are less well defined. Several lines of experimental and clinical evidence implicate HHV-6A as a promoting factor in the progression to AIDS (4). HHV-6A shares with HIV-1 a primary tropism for CD4+ T lymphocytes (5) and kills these cells in synergy with HIV-1 (6). Moreover, HHV-6A can enhance the virulence and/or pathogenicity of HIV-1 by several mechanisms, including activation of the HIV-1 LTR (6–8), induction of CD4 expression and HIV-1 susceptibility in otherwise HIV-refractory cells such as CD8+ T cells (9) and NK cells (10), induction of HIV-enhancing cytokines (11), and facilitation of the switch to CXCR4 usage (12). The clinical evidence includes the frequent isolation of HHV-6 from HIV-1-infected patients (1, 13–15), its frequent reactivation in patients with progressive HIV-1 disease (16), its widespread dissemination in terminal AIDS patients (17, 18), its sustained replication in lymphoid tissue from HIV-infected subjects (19) associated with an increased HIV-1 load (20), as well as the correlation between an early acquisition of HHV-6 in infancy and an accelerated progression of HIV-1 disease (21). Strikingly, unlike typical opportunistic infections, HHV-6 reactivation/reinfection tends to occur at a relatively early stage during the progression of HIV-1 disease (16), as attested by still elevated numbers of circulating CD4+ T cells (22, 23) and preserved lymphoid tissue architecture (18, 19), corroborating the hypothesis that HHV-6 might contribute to the process of CD4+ T cell destruction that leads to the immunodeficiency.
Despite the bulk of data hitherto accumulated, conclusive evidence of the role played by HHV-6A in the progression of HIV-1 disease is still lacking. In this study, we investigated the effects of HHV-6A on AIDS progression by experimentally coinfecting pig-tailed macaques (Macaca nemestrina) with HHV-6A and simian immunodeficiency virus (SIV). A dramatic acceleration of the immunological and clinical progression of SIV disease was observed in animals coinfected with HHV-6A.
Results
Study Design.
To investigate the effects of HHV-6A on AIDS progression, we experimentally coinfected pig-tailed macaques, whose T cells are highly susceptible to HHV-6A infection in vitro (24), with HHV-6A (strain GS) and a pathogenic SIV strain (smE660) (25). Three groups of young adult animals, each comprising four randomly assigned animals, were infected by i.v. inoculation with either SIV alone (group 1; animals 299, 301, 303, and 307), HHV-6A alone (group 2; animals 309, 310, 311, and 312) or both SIV and HHV-6A (group 3; animals 313, 315, 316, and 317). Dually infected animals were first inoculated with SIV and then superinfected with HHV-6A 14 days later. None of the animals had detectable antibodies to HHV-6A before inoculation, suggesting that they were not naturally infected with HHV-6-related monkey herpesviruses, as documented in drill monkeys and chimpanzees (26). The animals were followed for up to 32 months after inoculation, after which all surviving animals were euthanized.
Primary HHV-6A Infection.
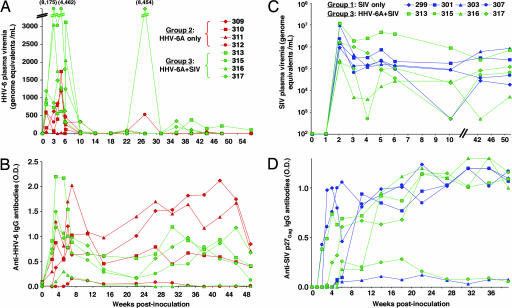
All macaques inoculated with HHV-6A (groups 2 and 3) showed evidence of primary HHV-6A infection. As seen in Fig. 1A, HHV-6A plasma viremia became detectable by quantitative calibrated real-time PCR at week 1 after inoculation, peaked between weeks 1 and 5, and then declined to disappear in all animals by week 14. All of the animals had anti-HHV-6A IgG seroconversion, which occurred after a mean of 3.0 ± 1.4 weeks in group 2 and 2.2 ± 0.5 weeks in group 3 (Fig. 1B), even though in two (312 and 316), the antibody reactivity was low and transient. The establishment of a systemic infection and its productive nature were confirmed by the detection of viral RNA transcripts by in situ hybridization in lymph-node tissues obtained within 4 weeks of inoculation (Table 1). Primary HHV-6A infection was associated with clinical manifestations of mild to moderate intensity, such as fever, nasal discharge, splenomegaly, and generalized lymphadenopathy; in one singly infected animal (309), an abdominal skin rash appeared at week 2. Altogether, these findings indicated that M. nemestrina is a susceptible animal model for HHV-6A infection.
Fig. 1.
Virological markers in pig-tailed macaques singly or dually infected with SIVsmE660 and HHV-6AGS. (A and B) Course of HHV-6A plasma viremia (A) and anti-HHV-6A IgG antibodies (B) during the acute phase of infection and the first year of follow-up in pig-tailed macaques singly infected with HHV-6A (group 2, red symbols) or coinfected with HHV-6A and SIV (group 3, green symbols). Time 0 corresponds to the time of HHV-6A inoculation; (C and D) Course of SIV plasma viremia (C) and anti-SIV p27Gag IgG antibodies (D) during the acute phase of infection and the first year of follow-up in pig-tailed macaques singly infected with SIV (group 1, blue symbols) or coinfected with HHV-6A and SIV (group 3, green symbols). Time 0 corresponds to the time of SIV inoculation. Coinfected monkeys were inoculated with HHV-6A at day 14 after SIV inoculation.
Table 1.
Expression of SIV and HHV-6A RNA in lymph nodes from singly and dually infected pig-tailed macaques as detected by in situ hybridization
| Animals | First biopsy (4 weeks after inoculation) |
Second biopsy (6 months after inoculation) |
||||
|---|---|---|---|---|---|---|
| SIV-RNA | SIV-RNA+ cells/cm2 | SIV RNA on FDC | HHV-6A RNA | SIV-RNA | SIV RNA on FDC | |
| Group 1: SIV only | ||||||
| 299 | ± | 370 | + | n.a. | ± | ± |
| 301 | ++ | 1,078 | ++ | n.a. | + | ++ |
| 303 | ++ | 1,033 | ± | n.a. | − | − |
| 307 | ++ | 808 | +++ | n.a. | + | − |
| Mean ± SD | 822.2 ± 323.8 | |||||
| Group 2: HHV-6A only | ||||||
| 309 | n.a. | n.a. | n.a. | + | n.a. | n.a. |
| 310 | n.a. | n.a. | n.a. | + | n.a. | n.a. |
| 311 | n.a. | n.a. | n.a. | ± | n.a. | n.a. |
| 312 | n.a. | n.a. | n.a. | − | n.a. | n.a. |
| Group 3: SIV + HHV-6A | ||||||
| 313 | +++ | 2,059 | +++ | ++ | ++ | ++ |
| 315 | + | 639 | +++ | ++ | + | ± |
| 316 | ± | 10 | ± | ++ | − | − |
| 317 | +++ | 11,135 | ++ | ± | + | − |
| Mean ± SD | 3,460.8 ± 5,187.5 | |||||
The frequency of productively infected cells (SIV RNA) was graded as follows: −, undetectable; ±, rare; +, infrequent; ++, abundant; +++, very abundant. Deposition of SIV RNA on the surface of follicular dendritic cells (FDC) was graded as follows: −, undetectable; ±, minimal; +, low; ++, abundant; +++, very abundant. The amount of HHV-6A RNA transcripts was graded as follows: −, undetectable; ±, minimal; +, low; ++, abundant.
Primary SIV Infection.
All of the animals inoculated with SIV (groups 1 and 3) showed evidence of primary SIV infection. Both SIV plasma viremia (Fig. 1C) and SIV p27Gag antigenemia [supporting information (SI) Fig. 4] became detectable and peaked at week 2 after inoculation. No significant differences were observed between groups 1 and 3 in the mean peak levels of antigenemia (1.51 ± 0.81 vs. 1.74 ± 2.0 ng/ml) and plasma viremia (6.56 ± 6.62 vs. 6.54 ± 6.75 log10 genome equivalents/ml). Antigenemia was transient, becoming undetectable at week 4 in all animals except one (313, HHV-6A-coinfected); by contrast, SIV viremia remained persistently positive in all animals. All animals showed anti-p27Gag antibody seroconversion, which occurred after a mean of 3.7 ± 1.5 weeks in group 1 vs. 4.5 ± 1.9 weeks in group 3 (Fig. 1D), with an inverse correlation between the time to antibody seroconversion and the time to full-blown AIDS (Pearson coefficient, −0.725; P = 0.042). Of note, the levels of anti-p27Gag reactivity were persistently low in two animals (303 and 315), both of which experienced a rapid progression to AIDS. The establishment of systemic SIV infection was confirmed by in situ hybridization in lymph nodes (Table 1) and by the repeated isolation of SIV from peripheral blood mononuclear cells (data not shown). Clinical signs observed during primary SIV infection included fever, nasal discharge, generalized lymphadenopathy and splenomegaly; the fever was generally higher and of longer duration in animals coinfected with SIV and HHV-6A. Immunologically, a transient loss of circulating CD4+ T cells was detected in two singly infected and three coinfected animals.
Natural History of SIV Disease in Singly and Dually Infected Macaques.
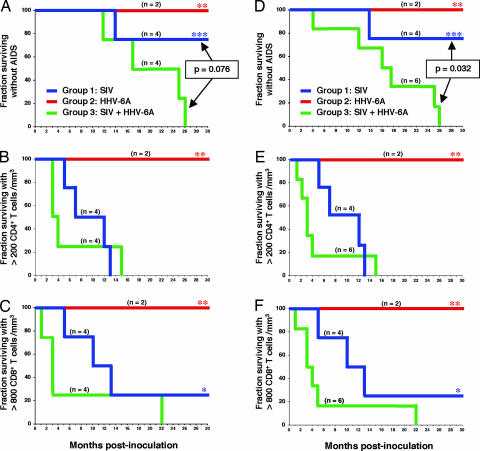
After the acute phase, the animals were monitored at monthly intervals for multiple clinical, virologic, and immunologic parameters. No long-term clinical and hematological alterations were seen in animals singly infected with HHV-6A, despite the occasional detection of low levels of plasma viremia (<100 genome equivalents/ml). In particular, their CD4+ and CD8+ T cell counts remained stably within the normal range. By contrast, a progressive loss of circulating CD4+ T cells was seen in all SIV-infected animals (Fig. 2B), associated with a marked reduction of lymphocyte proliferation indices (data not shown). However, the progression toward full-blown AIDS was dramatically accelerated in macaques coinfected with SIV and HHV-6A (Fig. 2A). During the 32 months of the study, AIDS-defining clinical conditions developed in all 4 coinfected macaques, but in only one of 4 singly infected with SIV (SI Table 2). Survival analysis showed that the difference in AIDS progression between the two groups of SIV-infected animals was close to statistical significance (P = 0.076), although it failed to reach it, most likely because of the low number of animals included in each group.
Fig. 2.
Clinical and immunological disease progression in pig-tailed macaques singly or dually infected with SIVsmE660 and HHV-6AGS. Shown are Kaplan–Meier curves for pig-tailed macaques singly infected with SIV (group 1, blue lines) or HHV-6A (group 2, red lines) or coinfected with SIV and HHV-6A (group 3, green lines). (A and D) AIDS-free survival. Survival analysis was performed nonparametrically by means of Kaplan–Meier curves. Log-rank tests were used to investigate differences in survival between groups. (B and E) Depletion of peripheral blood CD4+ T cells. (C and F) Depletion of peripheral blood CD8+ T cells. A–C shows Kaplan–Meier curves calculated for the original three groups of animals (group 3, n = 4). D–F shows Kaplan–Meier curves calculated after inclusion in group 3 of two additional animals (310 and 312), which were accidentally superinfected with SIV at month 13 and 21, respectively, from the initial HHV-6A inoculation (group 3, n = 6). For these two animals, survival analysis was performed starting from the time of SIV superinfection. The asterisks denote data that were censored because of drop-out or study termination. In group 2, only two animals (309 and 311) regularly completed the follow-up, because the remaining two animals were reclassified as SIV/HHV-6A coinfected during the course of the study.
The accelerated AIDS progression in HHV-6A-coinfected macaques was heralded by a faster depletion of circulating CD4+ T cells (Fig. 2B), with a mean loss of 45.3 ± 18.3 cells per mm3/week in HHV-6A-coinfected macaques vs. 23.9 ± 12.5 in singly infected macaques over the first 14 weeks of infection. Strikingly, coinfected animals also exhibited a faster depletion of circulating CD8+ T lymphocytes (Fig. 2C), with a mean loss of 35.7 ± 22.2 cells per mm3/week (vs. 11.7 ± 28.4) over the first 32 weeks of infection. Using an exponential mixed-effect model, there was a significant difference both in CD4+ and in CD8+ T cell decline between the two groups (P < 0.01). Of note, the mean CD4+ and CD8+ T cell counts did not adequately reflect the dramatic immunologic progression seen in three coinfected animals (315, 316, and 317) because the fourth animal in this group (313) consistently showed outlier values: for example, the time to reach CD4+ T cell counts <200/mm3 was 63 weeks in animal 313 vs. a mean of 14.8 ± 2.9 weeks in the remaining three animals; the time to reach CD8+ T cell counts <800/mm3 was 94 weeks vs. a mean of 9.8 ± 5.9 weeks.
Unlike the immunologic parameters, the levels of SIV plasma viremia and antigenemia during follow-up were not significantly different between singly and dually infected animals. Conversely, disease progression in dually infected animals was accompanied by frequent episodes of HHV-6A reactivation (Fig. 1A). Using a linear mixed effect model, the levels of HHV-6A viremia were significantly higher in SIV-coinfected than in singly infected animals (P < 0.01), suggesting that SIV infection exerted boosting effects on HHV-6A replication. Dually infected animals also showed a significantly faster decrease in anti-HHV-6A antibody reactivity over time (P = 0.01).
SIV Superinfection of Two HHV-6A-Infected Macaques.
An opportunity to further evaluate the effect of HHV-6A coinfection on the natural course of SIV disease was offered by the accidental SIV superinfection of two animals that were initially infected with HHV-6A alone (310 and 312) during the course of the study. At months 13 and 21, respectively, of HHV-6A inoculation, the animals escaped from their cage and were involved in fighting with SIV coinfected animals. As a result, both animals became superinfected with SIV, as shown by a rapid appearance of p27Gag antigenemia (SI Fig. 4). At that time, neither animal presented any signs of clinical or immunological deterioration, with CD4+ and CD8+ T cell counts stably within the normal range. Despite the uncontrolled conditions of SIV superinfection and the different timing and route of infection, these animals fitted the definition of HHV-6A/SIV coinfection. After SIV acquisition, both animals exhibited a very rapid decline of CD4+ and CD8+ T cells (Fig. 2 E and F), developing AIDS-related conditions after 69 (310) and 15 (312) weeks of SIV superinfection. Upon inclusion of these two additional macaques into the survival analysis, the difference in AIDS-free time between singly and dually infected animals became statistically significant (P = 0.032) (Fig. 2C).
Histopathology and Viral Replication in Lymphoid Tissue.
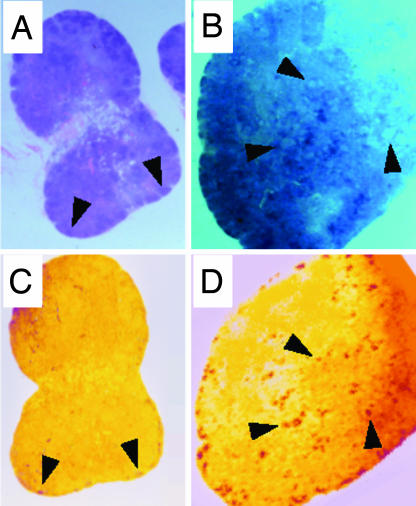
Two lymph-node biopsies were obtained from each animal, one during the acute phase of infection and one 6 months after inoculation. In the first set of biopsies, all of the animals showed evidence of follicular hyperplasia. However, in animals singly infected with SIV (Fig. 3A) or HHV-6A (data not shown), the nodal architecture was conserved, whereas in dually infected animals it was largely effaced by a florid follicular hyperplasia with confluent germinal centers (Fig. 3B). Coinfected lymph nodes showed higher levels of SIV RNA deposited on the surface of follicular dendritic cells (Fig. 3D) compared with those singly infected with SIV (Fig. 3C); likewise, there was a higher number of SIV RNA-expressing cells, albeit not statistically significant (Table 1). HHV-6A mRNA expression was documented primarily in the extrafollicular area, with a higher intensity in lymph nodes coinfected with SIV than in those singly infected with HHV-6A (Table 1). Thus, HHV-6A and SIV were simultaneously replicating in coinfected lymph nodes. In biopsies obtained 6 months after inoculation, both the frequency of SIV-infected cells and the levels of SIV-RNA deposition on follicular dendritic cells were lower than in the first biopsies (Table 1); animals with the most rapid disease progression showed an early involution of the nodal architecture with germinal center atrophy and effacement (data not shown).
Fig. 3.
Histopathology and in situ hybridization in lymph node tissues from macaques singly or dually infected with SIVsmE660 and HHV-6AGS. Tissues from two representative macaques are shown. Animal 303, singly infected with SIV (A and C); 317, coinfected with HHV-6A and SIV (B and D). A and B show Hematoxylin-eosin staining. C and D show In situ hybridization for SIV RNA of successive sections from the same lymph nodes as above. The arrows indicate enlarged lymphoid follicles with reactive germinal centers (A and B) and specific SIV RNA hybridization signal in correspondence to reactive germinal centers (C and D). In tissue from animal 303, the overall architecture is conserved, with lymphoid follicles barely discernible in the subcapsular area; low levels of SIV RNA are visible throughout the parenchyma, with little, if any, specific signal within reactive germinal centers. In tissue from animal 317, the histological architecture is largely effaced by a striking, florid follicular hyperplasia with clear-appearing, confluent reactive germinal centers; an intense SIV RNA signal is visible in correspondence to both subcapsular and deeper-located hyperplastic follicles.
Discussion
A major hindrance to elucidating the role played by HHV-6A in AIDS has been the lack of reliable animal model systems (4). In the present study, we identified M. nemestrina as a suitable model for the study of HHV-6A infection, as indicated by a rapid appearance of plasma viremia after inoculation, accompanied by clinical manifestations and followed by antibody seroconversion; moreover, as typically seen in human subjects, HHV-6A was found to persist in vivo after primary infection. Taking advantage of this new model, we obtained the first conclusive in vivo evidence that HHV-6A accelerates the progression of SIV disease toward full-blown AIDS. Although macaques coinfected with HHV-6A showed a significantly faster depletion of CD4+ T cells compared with macaques singly infected with SIV, the difference in AIDS-free survival between the two groups failed to reach statistical significance (P = 0.076), most likely because of the relatively low number of animals, which is a common limitation of studies with nonhuman primates. However, additional ground to our conclusions was provided by the accidental SIV superinfection of two animals originally infected with HHV-6A alone, both of which experienced a very rapid disease progression after acquiring SIV. A note of caution in interpreting these results is mandatory because of the different conditions in which SIV superinfection occurred (inoculation route, inoculum size, timing, and temporal sequence with respect to HHV-6A infection), even though such conditions may in fact have played against an optimal synergy between the two viruses. Regardless, when these two additional animals were included in the analysis, the difference in AIDS-free survival between singly and dually infected animals reached statistical significance (P = 0.032).
Various opportunistic agents have been suggested to accelerate the course of HIV-1 disease. However, the case of HHV-6A is unique because of the peculiar biological characteristics of this herpesvirus, which shares with HIV/SIV a primary tropism for CD4+ T cells (5, 6). Thus, although other agents may foster the development of AIDS by inducing typical opportunistic diseases, as documented in macaques coinfected with SIV and rhesus cytomegalovirus (27) or Mycobacterium bovis (28), HHV-6A has the potential to directly affect the basic pathogenetic mechanism of the immunodeficiency.
Although the precise mechanism whereby HHV-6A triggered a faster progression of SIV disease in our macaques is still at present unknown, some indications emerged from our study. Using in situ hybridization, we documented a simultaneous replication of HHV-6A and SIV in coinfected lymphoid tissue, indicating that a direct interaction between the two viruses could occur. A trend toward higher levels of SIV RNA expression was evident in HHV-6A-coinfected lymph nodes compared with those singly infected with SIV, but the overall levels of SIV viremia and antigenemia did not clearly distinguish the two groups of animals. By contrast, in agreement with the frequency of HHV-6 reactivation/reinfection seen in HIV-1-infected patients (1, 13–19, 23), an evident amplification of HHV-6A replication was seen in SIV-coinfected monkeys, most likely favored by the immunologic dysregulation induced by SIV infection. Studies have demonstrated that HHV-6A can synergize with HIV/SIV by multiple mechanisms (6–12, 24): one of the most peculiar is its ability to expand the repertoire of HIV/SIV-susceptible cells by inducing de novo expression of the primary HIV/SIV receptor, CD4, in productively infected CD8+ T cells (9) or other cytotoxic effectors (10). Indeed, our HHV-6A-coinfected macaques experienced a rapid depletion of both CD4+ and CD8+ T cells. This early loss of CD8+ T cells, which play a critical role in antiviral defenses, presumably facilitated the in vivo spread of both SIV and HHV-6A, thereby fostering the progression of immunodeficiency.
Another intriguing hypothesis to interpret the present results stems from our recent observation that SIV isolates obtained from HHV-6A-coinfected macaques after one year of infection had acquired resistance to regulated on activation normal T cell expressed and secreted (RANTES) (A.B., J. C. Grivel, A. Lisco, P.D.M., R.C.G., L. B. Margolis, and P.L., unpublished work). RANTES is a CCR5-binding chemokine that blocks the entry of SIV into cells (29). We reported that HHV-6A is a potent RANTES inducer in lymphoid tissue (12), a property that may have contributed to the initial containment of SIV replication in dually infected macaques. In fact, no evident amplification of SIV was observed in dually infected animals compared with those singly infected with SIV. However, in HHV-6A-coinfected macaques SIV subsequently evolved toward RANTES resistance, most likely under the selective pressure of elevated RANTES levels. Resistance to RANTES is increasingly recognized as a key virulence factor in HIV-1 infection (30–32), which may allow the virus to replicate in the high-RANTES milieu present within inflamed lymphoid tissues (33), particularly on coinfection with other microbes (34). Thus, one of the possible mechanisms whereby HHV-6A may foster the progression to AIDS is by facilitating an early acquisition of RANTES resistance. Additional studies in patients and nonhuman primates will be important to confirm the relevance of these mechanisms to HIV/SIV disease progression. A definitive elucidation of the role of HHV-6A in AIDS will have implications not only for our understanding of AIDS pathogenesis, but also for the implementation of novel therapeutic and preventive measures for the control of HIV infection.
Methods
Animals.
Twelve juvenile pig-tailed macaques (M. nemestrina) were included in the study; their age at the time of enrollment was 6–7 years. All were negative for SIV, STLV, herpes B virus, filovirus, SRV-1 and -2, measles virues, and HHV-6A. The animals were housed in individual cages at the animal facility of Advanced Bioscience Laboratories (Rockville, MD). The study protocol was approved by the National Cancer Institute Animal Care and Use Committee. Transportation and housing conditions, as well as all procedures including euthanasia were in conformity to the Animal Welfare Act and the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training. After quarantine, the animals were tested for baseline hematological and immunological parameters before inoculation with SIV and/or HHV-6A.
Virus Strains and Inoculation Procedure.
SIVsmE660 is an uncloned virus that was obtained at the time of AIDS-related death from the spleen of a rhesus macaque infected with SIVE543, which in turn was derived from strain sm/F236 (25). SIVsmE660 replicates in macaque and human CD4+ T lymphocytes and macrophages. The virus stock was expanded in vitro in CEMx174 cells. In vivo titration in macaques showed an infectivity of 104.5 half-maximal macaque infectious doses (MID50)/ml (25). HHV-6AGS, isolated from an HIV-1-infected patient with a lymphoproliferative disorder (1), was expanded in vitro in activated HHV-6-negative human cord blood mononuclear cells. The viral stock was derived from culture supernatants clarified by centrifugation at 2,000 × g and filtered through a 0.4 μM device; its in vitro infectivity was ≈106 half maximal cell culture infection doses (CCID50)/ml. As a mock HHV-6A inoculum, we used uninfected culture supernatants from the same cells used for producing the HHV-6A stock prepared according to the same protocol.
Each animal was randomly assigned to one study group and inoculated intravenously. The SIV inoculum contained 500 MID50 diluted in 2 ml of sterile PBS. The HHV-6A inoculum contained 3 × 106 CCID50 diluted in 3 ml of RPMI medium 1640. At time 0, animals in groups 1 and 3 received SIV, and animals in group 2 received HHV-6A; 14 days later, animals in group 3 received HHV-6A, whereas animals in group 1 were injected with the mock HHV-6 inoculum.
Virologic and Serologic Assays.
SIV antigenemia was measured by using a commercial p27 antigen-capture assay (Abbott Laboratories, Abbott Park, IL). Plasmatic levels of SIV RNA (SIV viremia) were measured by NASBA as described (35). Plasmatic levels of HHV-6A DNA (HHV-6A viremia) were measured by using a quantitative calibrated real-time PCR (TaqMan), using specific primers and probe as reported in ref. 36, with the addition of an exogenous calibrator molecule to normalize for the DNA-recovery rate after extraction as well as for the efficiency of the amplification reaction (37).
Antibodies to SIV were assayed by ELISA, using a commercial kit (Beckman Coulter, Fullerton, CA). Sera were tested at the standard dilution of 1:40. Specific OD values were calculated by subtracting from the average of triplicate wells incubated with monkey serum the average of quadruplicate negative control wells (incubated with all other reagents except monkey serum) plus two times the standard deviation (SD) value.
Antibodies to HHV-6A were assayed by using an in-house ELISA, modified from a method reported in ref. 38. Briefly, 30 × 106 HSB-2 cells infected with HHV-6AGS were extensively washed with PBS and lysed by treatment for 20 min at 4°C with 0.5% Triton X-100 in PBS, pH 7.4; uninfected homologous cells were also processed in parallel as a control. After clarification by centrifugation for 10 min at 9,000 × g, 100 μl of the HHV-6A antigen or control cell lysate (each 100 μg/ml) was used to coat 96-well microtiter plates (Nunc, Naperville, IL) in 60 mM sodium carbonate buffer (pH 9.6) for 24 h at 4°C. The wells were then washed, and macaque sera were incubated in triplicate HHV-6A or control wells at the standard dilution of 1:40 for 2 h at room temperature. After repeated washings, peroxidase-conjugated goat anti-human IgG (Sigma, St. Louis, MO), crossreactive with macaque IgG, was added at 1:250 dilution for 2 h at room temperature. After additional washings, the appropriate substrate was added for 30 min, after which the reaction was halted. Specific OD values were calculated by subtracting from the average of triplicate wells containing HHV-6A antigen the average OD values (plus two times the SD value) from triplicate negative-control wells incubated with the same serum.
In Situ Hybridization.
In situ hybridization was performed by Dr. Cecil Fox (Molecular Histology Laboratories, Gaithersburg, MD) as described in refs. 39–41. Tissues were hybridized with antisense 35S-labeled RNA probes; sense probes were used in parallel as negative controls. For SIV, both virus-expressing cells and virion-associated viral RNA trapped on the surface of follicular dendritic cells were evaluated. Determination of the number of SIV-expressing cells and the amount of follicular dendritic cells-deposited SIV RNA was achieved by image analysis of lymph-node sections, using a phosphorimager (Fuji Medical Systems, Burbank, CA), as described (40). In situ hybridization for HHV-6A RNA was performed by using as a probe in vitro RNA transcripts from molecular clone pZVH14, which encodes, among others, the large tegument protein (U31) (42).
Statistical Analysis.
Statistical analysis was performed by using the S-statistical software, Version 2.1.1. Correlation analyses were performed with Pearson and Spearman correlation coefficients to account for possible nonnormality in the data. Survival analysis was performed nonparametrically by means of Kaplan–Meier curves. Log–rank tests were used to investigate possible differences in survival time between groups. Generalized mixed effect models were used to assess differences in CD4 and CD8 slopes as well as levels of viremia between groups. Because we focused on modeling the trend components of quantitative outcomes and their evolution over time, the response variables were addressed as time series adding a random component to the fixed effects of the variable “group.” The random component accounts for unobservable heterogeneity among subjects, which is a critical issue in longitudinal studies with a high source of random variation. A linear mixed-effect model was used to compare the levels of HHV-6A and SIV viremia in different groups. An exponential mixed effect model was used to fit the decline of circulating CD4+ and CD8+ T cells over time, which clearly showed an exponential trend; statistical evidence in favor of the exponential model was obtained by Akaike information criteria. The significance level for the estimation results was α = 0.05.
Supplementary Material
Acknowledgments
We thank V. Hirsch (National Institute of Allergy and Infectious Diseases, Bethesda, MD) for providing the SIVsmE660 viral stock and for technical advice; J. L. Southers, R. Woodward, and J. Treece for veterinarian assistance; G. Franchini for technical advice; P. Farci for reading the manuscript; A. Ambrosi for statistical advice; A. Nonis for support in data analysis; P. Secchiero, G. Locatelli, S. Silva, T. Rutigliano, and G. Fagà for performing PCR assays; H. Miao for SIV ELISA; and S. Orndorff for administrative management. The initial part of this work was conducted when three of the authors (P.L., R.W.C., R.C.G.) were at the Laboratory of Tumor Cell Biology, National Cancer Institute, Bethesda, MD. This work was supported by the National Cancer Institute Intramural Research Program; the European Union Biomed-2 Program (Brussels) Grant BMH4CT961301 (to P.L.); and the Istituto Superiore di Sanità Italian AIDS Program (Rome) Grants 40B.57, 50C.17, 50D.17, and 50F.23 (to P.L.).
Abbreviations
- HHV-6
Human herpesvirus 6
- RANTES
regulated on activation normal T cell expressed and secreted
- SIV
simian immunodeficiency virus.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700929104/DC1.
References
- 1.Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzen-negger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, Gallo RC. Science. 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 2.Ablashi DV, Balachandran N, Josephs SF, Hung CL, Krueger GRF, Kramarski B, Salahuddin SZ, Gallo RC. Virology. 1991;184:545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- 3.Yamanishi K, Okuna T, Shiraki K. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 4.Lusso P, Gallo RC. Immunol Today. 1995;16:67–71. doi: 10.1016/0167-5699(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 5.Lusso P, Markham PD, Tschachler E, DiMarzo Veronese F, Salahuddin SZ, Ablashi DV, Pahwa S, Gallo RC. J Exp Med. 1988;167:1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lusso P, Ensoli B, Markham PD, Ablashi DV, Salahuddin SZ, Tschachler E, Wong-Staal F, Gallo RC. Nature. 1989;337:368–370. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- 7.Ensoli B, Lusso P, Schachter F, Josephs SF, Rappaport J, Negro F, Gallo RC, Wong-Staal F. EMBO J. 1989;8:3019–3028. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvat RT, Wood C, Balachandran N. J Virol. 1989;63:970–973. doi: 10.1128/jvi.63.2.970-973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lusso P, De Maria A, Malnati M, Lori F, De Rocco SE, Baseler M, Gallo RC. Nature. 1991;349:533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- 10.Lusso P, Malnati M, Garzino-Demo A, Crowley RW, Long EO, Gallo RC. Nature. 1993;362:458–462. doi: 10.1038/362458a0. [DOI] [PubMed] [Google Scholar]
- 11.Flamand L, Gosselin J, D'Addario M, Hiscott J, Ablashi DV, Gallo RC, Menezes J. J Virol. 1991;65:5105–5110. doi: 10.1128/jvi.65.9.5105-5110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grivel JC, Ito Y, Fagà G, Santoro F, Shaheen F, Malnati MS, Fitzgerald W, Lusso P, Margolis L. Nat Med. 2001;7:1232–1235. doi: 10.1038/nm1101-1232. [DOI] [PubMed] [Google Scholar]
- 13.Downing RG, Sewankambo N, Serwadda D, Honess R, Crawford D, Jarrett R, Griffin BE. Lancet. 1987;2:390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- 14.Tedder RS, Briggs M, Cameron CH, Honess R, Robertson D, Whittle H. Lancet. 1987;2:390–391. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- 15.Lopez C, Pellett P, Stewart J, Goldsmith C, Sanderlin K, Black J, Warfield D, Feorino P. J Infect Dis. 1988;157:1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- 16.Secchiero P, Carrigan DR, Asano Y, Benedetti L, Crowley RW, Komaroff AL, Gallo RC, Lusso P. J Infect Dis. 1995;171:273–280. doi: 10.1093/infdis/171.2.273. [DOI] [PubMed] [Google Scholar]
- 17.Corbellino M, Lusso P, Gallo RC, Parravicini C, Galli M, Moroni M. Lancet. 1993;342:1242. doi: 10.1016/0140-6736(93)92226-j. [DOI] [PubMed] [Google Scholar]
- 18.Knox KK, Carrigan DR. Lancet. 1994;343:577–578. doi: 10.1016/s0140-6736(94)91524-5. [DOI] [PubMed] [Google Scholar]
- 19.Knox KK, Carrigan DR. J AIDS. 1996;11:370–378. doi: 10.1097/00042560-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Emery VC, Atkins MC, Bowen EF, Clark DA, Johnson MA, Kidd IM, McLaughlin JE, Phillips AN, Strappe PM, Griffiths PD. J Med Virol. 1999;57:278–282. doi: 10.1002/(sici)1096-9071(199903)57:3<278::aid-jmv11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Kositanont U, Wasi C, Wanprapar N, Bowonkiratikachorn P, Chokephaibulkit K, Chearskul K, Chimabutra U, Sutthent R, Foongladda S, Inagi R, et al. J Infect Dis. 1999;180:50–55. doi: 10.1086/314826. [DOI] [PubMed] [Google Scholar]
- 22.Fairfax MR, Schacker T, Cone RW, Collier AC, Corey L. J Infect Dis. 1994;169:1342–1345. doi: 10.1093/infdis/169.6.1342. [DOI] [PubMed] [Google Scholar]
- 23.Fabio G, Knight SN, Kidd IM, Noibi SM, Johnson MA, Emery VC, Griffiths PD, Clark DA. J Clin Microbiol. 1997;35:2657–2659. doi: 10.1128/jcm.35.10.2657-2659.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusso P, Secchiero P, Crowley RW. AIDS Res Hum Retroviruses. 1994;10:181–187. doi: 10.1089/aid.1994.10.181. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch VM, Johnson PR. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 26.Lacoste V, Verschoor EJ, Nerrienet E, Gessain A. J Gen Virol. 2005;86:2135–2140. doi: 10.1099/vir.0.81034-0. [DOI] [PubMed] [Google Scholar]
- 27.Sequar G, Britt WJ, Lakeman FD, Lockridge KM, Tarara RP, Canfield DR, Zhou SS, Gardner MB, Barry PA. J Virol. 2002;76:7661–7671. doi: 10.1128/JVI.76.15.7661-7671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou D, Shen Y, Chalifoux L, Lee-Parritz D, Simon M, Sehgal PK, Zheng L, Halloran M, Chen ZW. J Immunol. 1999;162:2204–2216. [PubMed] [Google Scholar]
- 29.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 30.Scarlatti G, Tresoldi E, Björndal Å., Fredriksson R, Colognesi C, Deng HK, Malnati MS, Plebani A, Siccardi AG, Littman DR, et al. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 31.Koning FA, Kwa D, Boeser-Nunnink B, Dekker J, Vingerhoed J, Hiemstra H, Schuitemaker H. J Infect Dis. 2003;188:864–872. doi: 10.1086/377105. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson I, Antonsson L, Shi Y, Karlsson A, Albert J, Leitner T, Olde B, Owman C, Fenyo EM. AIDS. 2003;17:2561–2569. doi: 10.1097/01.aids.0000096853.36052.36. [DOI] [PubMed] [Google Scholar]
- 33.Trumpfheller C, Tenner-Racz K, Racz P, Fleischer B, Frosch S. Clin Exp Immunol. 1998;112:92–99. doi: 10.1046/j.1365-2249.1998.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis L. Nat Biotechnol. 2003;21:15. doi: 10.1038/nbt0103-15. [DOI] [PubMed] [Google Scholar]
- 35.Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, Markham PD, Pal R. J Virol Methods. 2000;86:61–70. doi: 10.1016/s0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 36.Locatelli G, Santoro F, Veglia F, Gobbi A, Lusso P, Malnati MS. J Clin Microbiol. 2000;38:4042–4048. doi: 10.1128/jcm.38.11.4042-4048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broccolo F, Locatelli G, Sarmati L, Piergiovanni S, Veglia F, Andreoni M, Buttò S, Ensoli B, Lusso P, Malnati MS. J Clin Microbiol. 2002;40:4652–4658. doi: 10.1128/JCM.40.12.4652-4658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou S, Scott KM. J Clin Microbiol. 1990;28:851–854. doi: 10.1128/jcm.28.5.851-854.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox CH, Cottler-Fox M. In: Current Protocols in Immunology. Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. New York: Wiley; 1993. pp. 12.8.1–12.8.21. [Google Scholar]
- 40.Martin LN, Murphey-Corb M, Mack P, Baskin GB, Pantaleo G, Vaccarezza M, Fox CH, Fauci AS. J Infect Dis. 1997;176:374–383. doi: 10.1086/514054. [DOI] [PubMed] [Google Scholar]
- 41.Rizzardi GP, De Boer RJ, Hoover S, Tambussi G, Chapuis A, Halkic N, Bart PA, Miller V, Staszewski S, Notermans DW, et al. J Clin Invest. 2000;105:777–782. doi: 10.1172/JCI9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Josephs SF, Ablashi DV, Salahuddin SZ, Jagodzinski LL, Wong-Staal F, Gallo RC. J Virol. 1991;65:5597–5604. doi: 10.1128/jvi.65.10.5597-5604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.