Abstract
We evolved muscarinic receptors in yeast to generate a family of G protein-coupled receptors (GPCRs) that are activated solely by a pharmacologically inert drug-like and bioavailable compound (clozapine-N-oxide). Subsequent screening in human cell lines facilitated the creation of a family of muscarinic acetylcholine GPCRs suitable for in vitro and in situ studies. We subsequently created lines of telomerase-immortalized human pulmonary artery smooth muscle cells stably expressing all five family members and found that each one faithfully recapitulated the signaling phenotype of the parent receptor. We also expressed a Gi-coupled designer receptor in hippocampal neurons (hM4D) and demonstrated its ability to induce membrane hyperpolarization and neuronal silencing. We have thus devised a facile approach for designing families of GPCRs with engineered ligand specificities. Such reverse-engineered GPCRs will prove to be powerful tools for selectively modulating signal-transduction pathways in vitro and in vivo.
Keywords: cell engineering, molecular evolution, receptorome
Because of the assorted cellular responses directed by them, their number, and the ease of which they are pharmacologically screened, the superfamily of G protein-coupled receptors (GPCRs) is one of the most therapeutically important targets in the proteome (1). However, the potential of this family is restricted by our ability to assess their function, which currently involves transgenic, knockout, and/or in vivo studies with selective drugs. Genetic studies are frequently limited to loss-of-function phenotypes, whereas nonselectiveness of a drug often interferes with interpretation of pharmacological studies. Knowledge of the roles of the individual family members is being bolstered by the ongoing creation of knockout mice for many GPCRs. Selective activation of individual GPCR subtypes in a defined tissue, in either a knockout or wild-type animal, is currently problematic but, if possible, would serve to complement present findings by providing novel insights into disease states resulting from overstimulation of certain signaling pathways.
One approach to this problem has been to rationally modify receptors to favor synthetic over natural substrate/ligand recognition, and subsequently, these mutant proteins have been used as bio-tools to study protein function in complex biological environments (2, 3). At the forefront of such modified GPCRs is Ro1, a Gi/o-coupled κ opioid receptor activated by a synthetic but not a native ligand, which has been conditionally expressed in transgenic mice to study cardiac function after its selective activation (4). Such mutant receptors, like Ro1, have been classified as receptors activated solely by synthetic ligands (RASSLs), because they are activated by synthetic ligands but not by their endogenous ligands (5). RASSLs, as in the case of Ro1, have been demonstrated to be valuable tools (4, 6); however, because the synthetic ligand frequently has high affinity and/or potency at the native receptor (5, 7, 8), this potentially limits their usefulness in vivo, at least in tissues with a wild-type receptor present. In this context, we sought to develop designer receptors exclusively activated by a designer drug (DREADD), or simply “designer” receptors, which represent receptors that are activated solely by a synthetic ligand(s) possessing minimal or no biologic activity.
In this study, we present a directed molecular evolution approach, which facilitated the creation of a family of muscarinic receptors that is potently activated by the pharmacologically inert compound clozapine-N-oxide (CNO) but not by its native ligand acetylcholine (ACh). We further demonstrate that such designer muscarinic receptors are active in a variety of native and artificial cellular contexts. We thus provide a validated and unbiased approach for generating GPCRs with defined ligand specificities and for proof of concept have created a family of muscarinic ACh receptor (mAChR) DREADDs having promise as unique biological tools to study either receptor-specific functions [e.g.,, human mAChR DREADD subtype 3 (hM3) vs. hM1] or general downstream signaling (e.g., Gq/11 vs. Gi/o) emanating from the activated GPCR.
Results
Directed Molecular Evolution of Rat M3 Receptor in Yeast.
We set out to develop an M3 DREADD that was responsive to a synthetic ligand of choice by using a directed molecular evolution approach. CNO was selected as the synthetic ligand because: (i) its parent compound, clozapine, has high affinity to M3 receptors and, therefore, we predicted few mutations would be required to permit CNO to be a potent agonist; (ii) CNO is highly bioavailable in rodents and humans (9, 10); and (iii) importantly, CNO is a pharmacologically inert molecule lacking appreciable (<1 μM) affinity for receptors [ref. 11 and supporting information (SI) Fig. 6]. For initial studies, a modified rat M3 receptor containing a sizable deletion in its third intracellular loop [(i3); rM3Δi3] was used (SI Fig. 7). The rat M3Δi3 receptor has been previously demonstrated to be functionally expressed in Saccharomyces cerevisiae genetically modified to enable ligand activation of heterologously expressed mammalian GPCRs to engage the pheromone signaling pathway to promote growth on selective medium (12).
By means of random mutagenesis, we created a large library of mutant rM3Δi3 receptors and screened them initially for activation by clozapine, which has high affinity but which is an exceedingly weak partial agonist at native M3 receptors. Ten independent clones, eight of which contained, among other mutations, a Y148X3.33 mutation, were isolated from this initial screen to identify rM3Δi3 receptor mutants activated by clozapine (see Material and Methods for details). Whereas yeast expressing wild-type rM3Δi3 receptor responded only to either ACh or carbachol (CCh), an ACh analog, in liquid growth assays, yeast expressing any of the 10 clones were efficaciously activated by clozapine within ≈10-fold range of 10 μM concentration used to screen the library (Fig. 1 and SI Table 1). Strikingly, all mutants were defective in their response (reduced potency) to CCh and/or ACh, with one clone (G6) unable to be activated by 10 mM ACh (Fig. 1 and SI Table 1). This loss in ACh potency was serendipitous, because diminished response to the native ligand is one requirement of a DREADD. Although most clones tested were not activated by up to 100 μM CNO, a single clone, G2, was found to be mildly responsive to CNO (Fig. 1 and SI Table 1). This was a promising finding, because we anticipated that clones selected to be activated by clozapine may be prone to CNO activation as well.
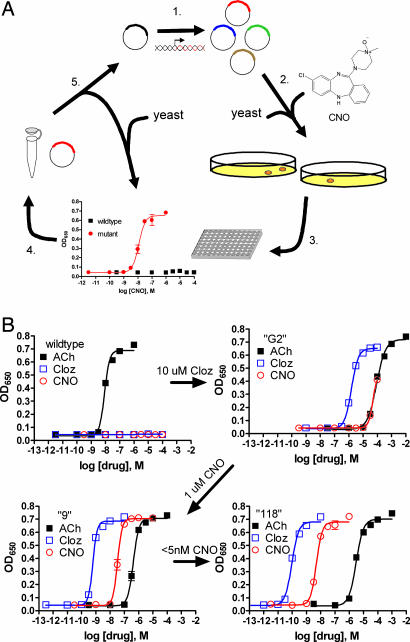
Fig. 1.
Pharmacological profiles of an rM3Δi3 receptor mutant selected during directed molecular evolution for CNO responsiveness. (A) Experimental design for directed evolution of mammalian GPCRs in yeast to create DREADDs. (1) Libraries of randomly mutated rM3Δi3 receptors were produced by mutagenic PCR; (2) yeast-expressing mutant receptors activated by synthetic ligands (e.g., CNO) were selected for by growth on nutrient deficient medium; (3) mutants were verified by secondary liquid growth assays in 96-well plates; (4) plasmid DNA was isolated from yeast; (5) clones were retransformed into yeast to pharmacologically profile mutants by liquid growth assays, and those with desirable properties were sequenced and remutagenized for subsequent rounds of selection to yield receptors with higher potency. (B) Optical density at 650 nm of liquid cultures of yeast transformed with either wild type, clone “G2” (first library, 10 μM clozapine screen), clone “9” (second library, 1 μM CNO screen), or clone “118” (third library, 5-nM CNO screen) rM3Δi3 receptors incubated with ACh (■), clozapine (□), or CNO (○). Data shown are mean ± SEM values of a representative experiment performed with two independent yeast transformants grown for each clone.
A subset of clones were selected to be remutated to manufacture a second-generation library to select for mutants activated by 1 μM CNO (SI Table 1 and SI Materials and Methods). The second-generation rM3Δi3 receptor mutants were efficaciously activated by CNO with a broad range of potencies (35–5,000 nM), which invariably was accompanied by a ≈100-fold increase in clozapine vs. CNO potency (Fig. 1 and SI Table 1). Again all mutants, with a single exception, had ≥1,000-fold reduction in ACh potency (SI Table 1).
A final library was constructed by using a subset of the second-generation CNO-responsive rM3Δi3 receptor mutants as the mutagenesis template and screened with ≈5 nM CNO to select for potently activated mutants (SI Table 1 and SI Materials and Methods). Two clones were isolated that were activated by CNO and clozapine with potencies of <10 nM and ≈0.1 nM, respectively (Fig. 1 and SI Table 1).
Engineering an hM3 Receptor to Be Potently and Efficaciously Activated by CNO in Human Cells.
Using the yeast-based screen, we were able to greatly enhance the pharmacological attributes of CNO at the M3 receptor. However, the ultimate goal was to create a full-length M3 receptor suitable for mammalian use. We next determined whether the mutants obtained by the yeast screen would be directly useful in human cells. In mammalian cells, activation of Gq/11-coupled receptors, like M3 receptor, can be measured by the accumulation of inositol monophosphate (IP1), a metabolite of inositol trisphosphate (IP3) produced by phospholipase C β catalyzed hydrolysis of phosphatidylinositol (PI).
CNO was essentially inactive at wild-type rM3Δi3 transiently expressed in HEK T cells, whereas selected mutant rM3Δi3 receptors displayed robust responses to CNO as well greatly diminished ACh potency (Fig. 2A and SI Table 2). Because many of the mutants with the highest CNO potencies had high levels of constitutive activity (Fig. 2A and SI Table 2), we next screened a focused library of hM3 receptor mutants in HEK T cells to generate a receptor that was potently activated by CNO with minimal constitutive activity.
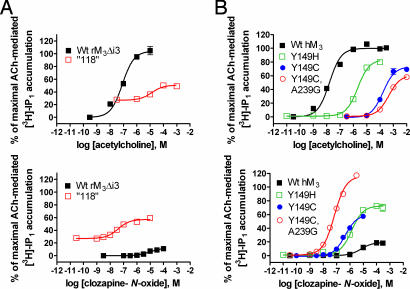
Fig. 2.
Focused screening of hM3 receptor mutants to optimize CNO stimulation of PI hydrolysis in HEK T cells. (A) Receptor-activated PI hydrolysis in HEK T cells transfected with a third-generation clone “118” (□) identified by the yeast screen and wild-type rM3Δi3 receptor (■) treated with ACh (Upper) or CNO (Lower). (B) Similarly, wild-type (■) human M3 receptors with the indicated single Y149H (□) and Y149C (●) or multiple mutations [Y149C, A239G (○); hM3 DREADD], found in yeast screen clones, were transiently expressed in HEK T cells to measure receptor activation after treatment with either ACh (Upper) or CNO (Lower). Data of accumulated radiolabeled inositol 1-phosphate ([3H]-IP1) are normalized to maximal ACh-mediated response in HEK T cells expressing either wild-type rM3Δi3 (A) or wild-type hM3 (B) receptors. Values shown are mean ± SEM from representative assays performed in duplicate.
We chose the Y149C3.33 mutant as an initial template because, (i) the rM3Δi3 Y1483.33 was the most consistently mutated residue to arise in the yeast screen, (ii) the Tyr3.33 residue is known to be important in ligand binding of muscarinic ligands (13–15), and (iii) preliminary experiments showed that mutation of Tyr-1493.33 in hM3 receptor to either Cys or His similarly increased CNO responsiveness without increasing basal activity, yet the Y149C mutant had ≈100-fold better reduction in ACh potency compared with Y149H when expressed in HEK T cells (Fig. 2 and SI Table 2). Ultimately, we found the double mutant Y149C3.33/A239G5.46 to be the best possible combination of mutations to generate a hM3 DREADD (hM3D) (Fig. 2 and SI Table 2).
Characterization of hM3 DREADD in Immortalized Human Pulmonary Artery Smooth Muscle Cells (hPASMC).
Although smooth muscle cells endogenously express M3 receptors (16), we found a particular human hPASMC line, devoid of endogenous muscarinic receptors, which would allowed us to investigate hM3D receptor function in a null but native environment. We were able to immortalize hPASMCs, as determined by propagating cells well past senescence, by the stable introduction of the gene encoding the human catalytic subunit of telomerase, hTERT (SI Fig. 8). When wild-type hM3 receptors were stably expressed in immortalized hPASMCs, they faithfully elicited PI hydrolysis, Ca2+ mobilization and ERK-1/2 phosphorylation (see below and SI Materials and Methods for assay description) after ACh treatment (Fig. 3 and SI Tables 3–5). Significantly, the hM3D receptor stably expressed in immortalized hPASMCs was potently (≈20–30 nM) and efficaciously activated by CNO (Fig. 3 A and B and SI Tables 3 and 4). The hM3D was found to have a severe reduction (>40,000-fold) in ACh potency compared with the wild-type receptor (Fig. 3 and SI Tables 3 and 4).
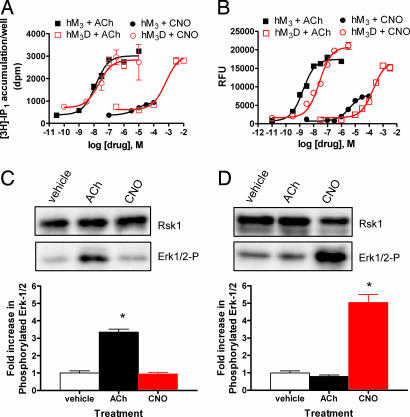
Fig. 3.
Functional characterization of HA-epitope tagged wild-type and DREADD hM3 receptors in immortalized hPASMC. (A) Drug-induced PI hydrolysis in immortalized hPASMCs stably expressing wild-type (hM3) or DREADD (hM3D) receptors. Shown are mean ± SEM values of a representative experiment performed in duplicate comparing [3H]-IP1 accumulation after ACh or CNO treatment of hM3 (■ or ●, respectively) or hM3D (□ and ○, respectively) cells. (B) Calcium mobilization resulting from delivery of ACh or CNO to hM3 (■ or ●, respectively) or hM3D (□ or ○, respectively) expressing immortalized hPASMCs. A representative experiment, performed in quadruplicate, with mean values of Ca2+ mobilization in relative fluorescent units (RFU), is shown. (C and D) A representative experiment determining change in ERK-1/2 phosphorylation compared with p90 ribosomal S6 kinase loading control after incubating 1 μM of the indicated drugs for 5 min with immortalized hPASMCs expressing either wild-type (C) or DREADD hM3 (D) receptors by immunoblot (Upper) with quantification of ERK-1/2 phosphorylation (Lower) from three independent experiments with significant differences (∗, P < 0.001) between drug and vehicle treatment as determined by one-way ANOVA is shown.
Because some interactions between drug and receptor result in the onset of some but not all GPCR-related functions (17), we wanted to test whether hM3D receptors could stimulate distinct downstream pathways after CNO treatment. A common signaling event radiating from activated GPCRs is the downstream phosphorylation of the MAPK proteins, ERK-1/2, resulting from the association of the MAPK-β-arrestin complex with the activated receptor (1). Treatment with 1 μM ACh but not CNO resulted in an enhanced level of ERK-1/2 phosphorylation in hPASMCs expressing hM3 receptors (Fig. 3C). Conversely, hPASMCs expressing hM3D receptors responded only to CNO under the same conditions (Fig. 3D). Together, these experiments demonstrate that the hM3D receptor, created through the mutation of Y149C3.33/A239G5.46, fits the definition of a DREADD receptor.
Creation of a Family of Designer Muscarinic Receptors.
Examination of protein alignments of mammalian mAChR family members revealed that Y3.33 and A5.46 are strictly conserved (data not shown). We also showed mutation of Y6.51, a similarly strictly conserved residue, enhances the agonistic properties of clozapine-like drugs in two distinct family members (SI Table 2 and ref. 18). Taken together, we hypothesized that introduction of Y3.33C and A5.46G mutations in the other mAChR family members would allow CNO to activate these as well.
We individually expressed human M1, 2, 4, and 5 DREADDs in immortalized hPASMCs to test whether CNO could similarly activate these receptors. Like the hM3 receptor, we found that substitution of Tyr3.33/Ala5.46 into the other mAChR family members successfully transformed these receptors into CNO-activated DREADDs (Fig. 4A and SI Tables 3 and 4). Receptor activation remained sensitive, albeit with reduced potency, to the nonselective mACh receptor antagonists, atropine and (±)-quinuclidinyl benzilate, as monitored by Ca2+ mobilization (SI Table 6). We found treatment of immortalized hPASMCs with pertussis toxin, an irreversible Gi/o inhibitor, selectively abolished the Ca2+ response in those cells expressing hM2 and hM4 compared with hM1, 3, and 5 receptors (Fig. 4B and data not shown). Additionally, treatment of CNO selectively reduces forskolin-stimulated cAMP formation in hPASMCs expressing hM2D receptors with a potency similar to that seen by Ca2+ mobilization assays (SI Materials and Methods and SI Tables 4 and 7). Therefore, hM2 and hM4 receptors retain their coupling properties when expressed in hPASMCs and presumably activate phospholipase C β by Gβγ subunits (19).
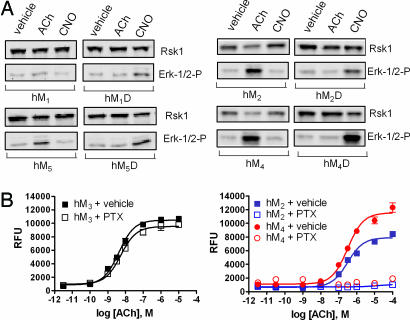
Fig. 4.
Transformation of CNO response in mACh receptor family members. (A) Representative Western blots detecting either phosphorylated ERK-1/2 or, as a loading control, p90 ribosomal S6 kinase 1, after a 5-min application of a 1 μM final concentration of the indicated drugs to immortalized hPASMCs expressing wild-type or DREADD hM1, hM2, hM4, and hM5 receptors, as indicated. (B) Ca2+ mobilization response in immortalized hPASMCs expressing either the Gq/11-coupled hM3 (black symbols; Left) or the Gi/o-coupled receptors hM2 and hM4 (blue and red symbols, respectively; Right) is shown. Cells were treated with ACh after overnight incubation with either vehicle control (solid symbols) or pertussis toxin or pertussis toxin (open symbols) on receptor-mediated Ca2+ release. Values shown are mean ± SEM from a representative experiment performed in triplicate.
CNO Silences Hippocampal Neurons Expressing hM4D Receptors.
M4 receptors coupled to Gi/o and Gi/o-coupled responses are increasingly used for neuronal silencing; we next determined whether the hM4D could induce a G protein inward-rectifying potassium channel (GIRK) response in hippocampal neurons. Additionally, because mAChR family members are present, to a varying extent, in the brain and have been implicated in many processes, mACh DREADDs, expressed individually in isolated neuronal regions would be ideal tools to evaluate their roles in this multifarious background. Therefore, we investigated hM4D receptor functionality in hippocampal neurons in which Gi/o coupled receptors are known to induce neuronal silencing by activation of GIRK (20).
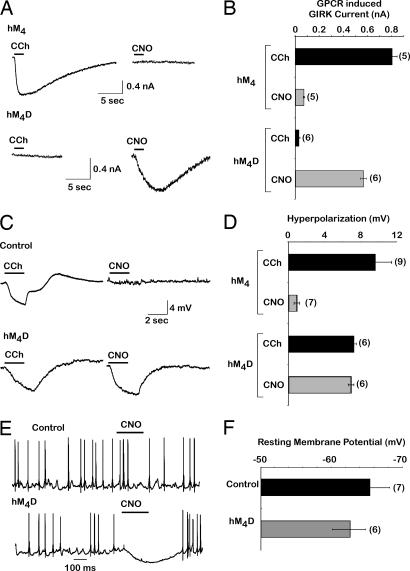
We first verified that the hM4D receptor could modify GIRK currents in HEK cells transiently expressing GIRK1/2 subunits in combination with hM4D receptor. As a control, we found that 10 μM CCh could induce currents in HEK cells transfected with GIRK1/2 subunits and wild-type hM4 receptors, whereas as little to no current was found in cells treated with the same concentration of CNO (Fig. 5 A and B Upper). As anticipated, CNO compared with CCh, which had no effect, selectively activated GIRK channels in HEK cells that hM4D receptors were coexpressed (Fig. 5 A and B Lower).
Fig. 5.
Characterization of hM4 DREADD on GIRK channel activation, membrane hyperpolarization, and neuronal silencing. (A) Sample traces of receptor-induced GIRK currents in HEK293 cells cotransfected with GIRK1/2 channel subunits and either hM4 (Upper) or hM4D (Lower) receptors and treated with either 10 μM CCh or CNO at a holding potential of −60 mV as described in SI Materials and Methods. (B) Comparison of induced GIRK channel currents at −60 mV when coexpressed with either hM4 or hM4D receptors. (C) Sample traces of CCh- and CNO-induced voltage changes in cultured hippocampal neurons infected with either hM4 (Upper) or hM4D (Lower). (D) Summary of the hM4- and hM4D-induced voltage changes by CCh and CNO. (E) Sample traces of hippocampal neurons spontaneously firing action potentials. In the presence of hM4D receptors (lower trace), application of CNO induces hyperpolarization and neuronal silencing. (F) Comparison between the resting membrane potential of hM4D receptor-expressing and control hippocampal neurons, indicating that the expression of hM4D receptors does not change the resting membrane potential without activation of the receptor. Number of cells tested is indicated in parentheses with mean ± SEM shown.
We next examined whether this behavior was recapitulated in cultured hippocampal neurons. When wild-type hM4 receptors were transiently expressed in hippocampal neurons, we found that CCh, but not CNO, induced hyperpolarization (Fig. 5 C and D Upper). Significantly, CNO treatment of culture hippocampal neurons transiently expressing hM4D receptor also resulted in neuronal hyperpolarization (Fig. 5 C and D Lower). Expression of hM4D receptors in these neurons did not prevent CCh from modulating hyperpolarization through endogenous receptors (Fig. 5C and data not shown), suggesting that expression of hM4D does not impede native receptor function. Additionally, CNO can selectively prevent action potential firing in cultured hippocampal neurons expressing hM4D receptors (Fig. 5E) without significantly altering resting membrane potential in untreated infected neurons (Fig. 5F). These experiments clearly establish the unique ability of CNO to exclusively activate a cellular response in cells exogenously expressing hM4D receptors and demonstrate their utility as a tool for in vivo neuronal silencing.
Discussion
Here we demonstrate that directed molecular evolution can be used to engineer a GPCR to be potently and efficaciously activated by a synthetic ligand that is pharmacologically inert. We extend this approach to show that an entire family of GPCRs, in this case the human muscarinic receptor family, can be created to be activated by an inert ligand. We also show that the signal transduction pathways and novel pharmacologies are faithfully recapitulated in a variety of cellular contexts including smooth muscle cells and hippocampal neurons. We suggest that at least one of these designer receptors, hM4D, will prove useful for neuronal silencing in vitro and in vivo. Using this general approach, it should be possible to eventually evolve GPCRs to bind any arbitrary drug-like small molecule by essentially designing the “lock” (GPCR) to fit the “key” (the ligand).
There are a number of technical considerations that should be borne in mind when embarking on a campaign to reverse engineer a GPCR. We found, in general, that most of the key “transforming” residues were found in the initial screen and, compared with those mutants found in later screens, more of these mutants had unchanged basal activity when expressed in human cells. Therefore, it is advisable to screen a large initial library, which is likely to have the highest impact in unearthing important and easily identifiable residues, as well as counter screen promising mutants in mammalian cells early on to reduce the risk of evolving constitutively active receptors. A distinct advantage to directed evolution by using randomized libraries is that is unbiased. However, the screen can be significantly enhanced with prior knowledge of residue importance. Not surprisingly, four residues (Y3.33, T5.42, W6.48, and Y6.51) involved in binding to ACh and other mAChR ligands (13, 21) were mutated in our screen, illustrating that special attention should be given to these residues or local helical regions. Intriguingly, this lends evidence to the notion that clozapine-related ligands, such as clozapine, CNO, and olanzapine (data not shown), which readily activate the hM3D receptor, may either bind distinctly within the ACh orthosteric site or, as previously suggested, are allosteric ligands (18). Similarly, it is potentially worthwhile to perform saturating mutagenesis at residue “hot spots” to optimize functionality, because we found multiple substitutions at one residue (e.g., Y148C/H/N3.33) enhanced rM3Δi3 receptor activation by clozapine/CNO in yeast and considering most single-nucleotide mutations within a codon will not change the translated amino acid to all of the other possible amino acids. For example, in our initial library, if all possible single-nucleotide mutations were introduced within the Y148 codon, only 7 of 19 possible amino acid substitutions would have been achieved. In this way, subsequent libraries would be seeded with an optimized receptor while avoiding propagation of unimportant secondary mutations.
The first reported receptor that can be considered a DREADD is a β2-adrenergic receptor rationally mutated at the highly conserved residue D113S3.32. This mutant had a severe reduction in both potency and efficacy to catecholamine agonists but instead was fully activated, albeit with low potency (>40 μM), by compounds foreign to the wild-type receptor (22). More recently, rational mutation of F435A6.55 in the histamine H1 receptor (23) and D100A3.32 of the 5-HT4 receptor (8) [the first Gq/11 and Gs-coupled receptors activated solely by synthetic ligands (RASSLs), respectively] enhanced the potency for synthetic ligands and reduced affinity and potency for their native ligands, although not nearly to same extent as the mACh DREADDs described here.
Here, guided by mutations arising from directed molecular evolution in yeast, we discovered Y3.33C and A5.46G mutations to be sufficient to generate a human hM3 DREADD that responded potently, efficaciously, and selectively to CNO vs. ACh by PI hydrolysis, Ca2+ mobilization, and ERK-1/2 phosphorylation in a physiologically relevant background. Fortuitously, mutations at these same two conserved residues effectively switched the responsiveness of all other human mAChR family members so that CNO selectively activated them in two independent cellular settings by using a variety of signaling readouts. Interestingly, this dramatic enhancement of functional activation is accompanied by a minimal (<5-fold) increase in CNO affinity, which remains to be explored (not shown).
Knockout of individual or combinations of mACh receptors in mice has given enormous insight into function and possible pathophysiologies resulting to disruption of these receptors (16). However, these studies provide limited insight into what role either acute or chronic receptor overstimulation plays in disease states. For example, studies have shown that M1 and M4 knockout mice have increased locomotor activity and modified dopamine levels and/or release (24–26); however, it is difficult to decipher whether and which receptors exert a tonic check for movement or whether overstimulation of either receptor may participate in Parkinson's disease. The unique ability of CNO to induce neuronal silencing in neurons that express hM4 DREADD will facilitate studies aimed at investigating the role of defined neuronal populations in a large number of physiological and pathological processes.
Materials and Methods
Plasmid Construction and Materials.
p416GPD was purchased from American Type Culture Collection. p416GPD-rM3Δi3R and pMP290 were previously described (12). pcDNA3.1(+) containing inserts of each human hM1–5 receptor was obtained from (University of Missouri Rolla cDNA Resource Center, Rolla, MO). Single codon mutations were sequentially introduced into pcDNA3.1(+) constructs containing the human muscarinic receptors by Quikchange Site-directed mutagenesis kit (Stratagene, La Jolla, CA). hM1 and hM3 receptors were subcloned into the BamHI and XbaI sites of a modified pcDNA3.1(+), pcDNA3.1(+)-EcoRI, which contains an additional EcoRI site 3′ of the XbaI site. Retroviral vectors pBabepuro (27) and pHA-Babepuro were generated by the addition of a Kozak sequence and sequence encoding an HA epitope tag with an initiating methionine (MYPYDVPDYA) into the BamHI site of pBabepuro. pcDNA3.1(+)-EcoRI-hM1 and pcDNA3.1(+)-EcoRI-hM3 wild-type and mutant receptors were digested with BamHI and EcoRI and inserted into pBabepuro and, in the case of hM3, also pHA-Babepuro digested with the same enzymes. pcDNA3.1(+)-hM2 was digested with EcoRI and XhoI and inserted into EcoRI and SalI sites of pBabepuro. pcDNA3.1(+)-hM4 and pcDNA3.1(+)-hM5 were digested with BamHI and XhoI and inserted into BamHI and SalI sites of pBabepuro. GIRK1/2 subunits were cloned into pcDNA3.1. Sindbis virus vector SinRep(nsP2S726) and helper DH-BB were kindly provided by P. Osten (Max Planck Institute for Medical Research, Heidelberg, Germany) (28). Human M4 was cloned into the PmlI site of SinRep(nsP2S726) after digesting of pcDNA3.1(+)-hM4 with PmeI. pBabehygro-flag-hTERT was previously described (29). Sequence identity was determined by automated sequencing (Cleveland Genomics, Cleveland, OH).
Mutant libraries were generated by mutagenic PCR by using GeneMorph II Random Mutagenesis kit (Stratagene) following the manufacturer's protocol by using 30 cycles and ≈250 ng of p416GPD-rM3Δi3R DNA template and primers 5′-ACACCAAGAACTTAGTTTCGACGG and 5′-GGCGTGAATGTAAGCGTGAC, which resulted in an empirical mutational rate of ≈3.5 mutations per kilobase. Mutagenic products were digested with XhoI and XbaI and cloned into the same sites in p416GPD and then transformed into XL10 Gold bacteria (Stratagene) to amplify plasmid library. First-generation library (3 × 104 independent clones, with 6 × 104 colonies screened) used wild-type p416GPD-rM3Δi3R as template. Second-generation library (7 × 104 independent clones, with 2.5 × 104 colonies screened) was amplified from equimolar amounts of plasmid clones “B4,” “B11,” “G2,” “G6,” and “G15,” chosen for their diversity in mutations and alteration in drug response. Similarly, plasmid clones “3,” “7,” “8,” “9,” and “16” were used to produce the third-generation library (6 × 105 independent clones, with 8 × 104 colonies screened).
L-Quinuclidinyl[phenyl-4-3H]benzilate (36.5 Ci/mmol) was purchased from GE Healthcare Biosciences (Piscataway, NJ). All other chemicals [(±)-quinuclidinyl benzilate, CNO, ACh, CCh, and atropine, 3-amino-1,2,4-triazole (3-AT)] and amino acids were purchased from Sigma–Aldrich (St. Louis, MO). Synthetic yeast media was purchased from Q-BIOgene (Irvine, CA) and BD Biosciences (San Diego, CA).
Yeast Library Screen and Growth Assays.
Plasmid library DNA was transformed into S. cerevisiae strain MPY578q5 (12) by a high-efficiency procedure (30) and selected for growth at 30°C for 3–4 days in the presence 10 μM clozapine, 1 μM CNO, or ≈5 nM CNO for first-, second-, and third-generation libraries, respectively, on agar plates containing yeast assay buffer [synthetic complete (SC) media (pH 6.9) lacking uracil and histidine and supplemented with 20 mM 3-AT]. Colonies were then submitted to a secondary liquid-assay screen in which they were grown overnight in SC media without uracil, pelleted, washed with water, then diluted in yeast assay buffer to a final concentration of OD650 ≈0.003. Diluted yeast (150 μl) was added to a 96-well plate containing 50 μl of yeast assay buffer supplemented with varying drug concentrations and grown with mild agitation at 25°C for 3 days, at which time the plate was read at OD650 on a microplate reader by using SOFTmax Pro 4.3.1 software (Molecular Devices, Sunnyvale, CA). Plasmids were isolated from responsive colonies as described (31) and retransformed into MPY578q5 and/or sequenced. Pharmacological profiling of clones was performed by liquid assay by using two to three independent transformants and typically repeated two to six times.
Viral Production, Cell Line Establishment, and Cell Culture.
Human embryonic kidney strain, HEK T, was purchased from American Type Culture Collection (#CRL-11268; Manassas, VA), hPASMCs were purchased from ScienCell (San Diego, CA), and HEK strain 293TS cells were kindly provided by C. M. Counter (Duke University, Durham, NC). Amphotropic retrovirus was produced by cotransfecting a 6-cm plate of 293TS grown in DMEM/10% FBS with 1 μg of pBabehygro-flag-hTERT with 1 μg of the amphotropic packaging plasmid pCL-10A1 (Imgenex, San Diego, CA) by using FuGene6 (Roche, Indianapolis, IN) transfection reagent. Virus-containing medium was collected between 24 and 60 h after transfection, filtered with a sterile 0.45-μm filter, supplemented with a final concentration of 4 μg/ml polybrene (Sigma), and incubated with hPASMCs plated on 0.2% porcine gelatin-coated plates for 6–12 h to infect. After hTERT infection, hPASMCs were then media-changed into Smooth Muscle Cell Medium (ScienCell) and 36–48 h after infection, cells were selected and subsequently grown in Smooth Muscle Cell Medium and 50 μg/ml hygromycin B. The first confluent plate under selection was designated as population doubling 0. hPASMCs were grown on 0.2% gelatin-coated plates in hygromycin B-supplemented Smooth Muscle Cell Medium for immortalization study. Polyclonal hPASMCs stably expressing human mACh receptors were created by retroviral infection, as described above by using pBabepuro or pHA-Babepuro constructs, and grown on 0.2% gelatin-coated plates in DMEM/10% FBS supplemented with 1 μg/ml puromycin. Sindbis virus was produced from electroporated BHK cells grown in MEM/5% FBS according to published procedures (32). All cells were grown at 37°C in 5% CO2 atmosphere.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health [Grants RO1MH57635, RO1MH61887, and KO2MH01366 (to B.L.R.) and Grants NS0447752 and NS42623 (to S.H.)] and the National Institute of Mental Health Psychoactive Drug Screening Program (B.L.R.). B.N.A. was supported by an Individual National Research Service Aware (F32-GM074554).
Abbreviations
- GPCR
G protein-coupled receptor
- DREADD
designer receptors exclusively activated by a designer drug
- CNO
clozapine-N-oxide
- ACh
acetylcholine
- CCh
carbachol
- mAChR
muscarinic ACh receptor
- hM1–5
human mAChR subtypes 1–5
- hM1–5D
human mAChR DREADD subtypes 1–5
- rM3Δi3
rat M3 receptor containing a third intracellular loop deletion
- hPASMC
human pulmonary artery smooth muscle cell
- PI
phosphatidylinositol
- GIRK
G protein inward-rectifying potassium channel.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 4777.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700293104/DC1.
References
- 1.Armbruster BN, Roth BL. J Biol Chem. 2004;280:5129–5132. doi: 10.1074/jbc.R400030200. [DOI] [PubMed] [Google Scholar]
- 2.Bishop A, Buzko O, Heyeck-Dumas S, Jung I, Kraybill B, Liu Y, Shah K, Ulrich S, Witucki L, Yang F, et al. Annu Rev Biophys Biomol Struct. 2000;29:577–606. doi: 10.1146/annurev.biophys.29.1.577. [DOI] [PubMed] [Google Scholar]
- 3.Scearce-Levie K, Coward P, Redfern CH, Conklin BR. Trends Pharmacol Sci. 2001;22:414–420. doi: 10.1016/s0165-6147(00)01743-0. [DOI] [PubMed] [Google Scholar]
- 4.Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR. Nat Biotechnol. 1999;17:165–169. doi: 10.1038/6165. [DOI] [PubMed] [Google Scholar]
- 5.Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Proc Natl Acad Sci USA. 1998;95:352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 7.Pauwels PJ, Colpaert FC. Br J Pharmacol. 2000;130:1505–1512. doi: 10.1038/sj.bjp.0703455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claeysen S, Joubert L, Sebben M, Bockaert J, Dumuis A. J Biol Chem. 2003;278:699–702. doi: 10.1074/jbc.C200588200. [DOI] [PubMed] [Google Scholar]
- 9.Bender D, Holschbach M, Stocklin G. Nucl Med Biol. 1994;21:921–925. doi: 10.1016/0969-8051(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW, Jann MW. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:723–739. doi: 10.1016/s0278-5846(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 11.Weiner DM, Meltzer HY, Veinbergs I, Donohue EM, Spalding TA, Smith TT, Mohell N, Harvey SC, Lameh J, Nash N, et al. Psychopharmacology (Berl) 2004;177:207–216. doi: 10.1007/s00213-004-1940-5. [DOI] [PubMed] [Google Scholar]
- 12.Erlenbach I, Kostenis E, Schmidt C, Hamdan FF, Pausch MH, Wess J. J Neurochem. 2001;77:1327–1337. doi: 10.1046/j.1471-4159.2001.00344.x. [DOI] [PubMed] [Google Scholar]
- 13.Wess J, Maggio R, Palmer JR, Vogel Z. J Biol Chem. 1992;267:19313–19319. [PubMed] [Google Scholar]
- 14.Lu ZL, Hulme EC. J Biol Chem. 1999;274:7309–7315. doi: 10.1074/jbc.274.11.7309. [DOI] [PubMed] [Google Scholar]
- 15.Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, Li B, Wess J. J Biol Chem. 2005;280:34849–34858. doi: 10.1074/jbc.M506711200. [DOI] [PubMed] [Google Scholar]
- 16.Wess J. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 17.Kenakin T. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 18.Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ. Proc Natl Acad Sci USA. 2003;100:13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer WD, Brown HA, Sternweis PC. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Proc Natl Acad Sci USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wess J, Nanavati S, Vogel Z, Maggio R. EMBO J. 1993;12:331–338. doi: 10.1002/j.1460-2075.1993.tb05661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strader CD, Gaffney T, Sugg EE, Candelore MR, Keys R, Patchett AA, Dixon RA. J Biol Chem. 1991;266:5–8. [PubMed] [Google Scholar]
- 23.Bruysters M, Jongejan A, Akdemir A, Bakker RA, Leurs R. J Biol Chem. 2005;280:34741–34746. doi: 10.1074/jbc.M504165200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyakawa T, Yamada M, Duttaroy A, Wess J. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Proc Natl Acad Sci USA. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgenstern JP, Land H. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Dittgen T, Nimmerjahn A, Waters J, Pawlak V, Helmchen F, Schlesinger S, Seeburg PH, Osten P. J Neurosci Methods. 2004;133:81–90. doi: 10.1016/j.jneumeth.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Armbruster BN, Banik SS, Guo C, Smith AC, Counter CM. Mol Cell Biol. 2001;21:7775–7786. doi: 10.1128/MCB.21.22.7775-7786.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gietz RD, Woods RA. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 31.Robzyk K, Kassir Y. Nucleic Acids Res. 1992;20:3790. doi: 10.1093/nar/20.14.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.