Abstract
In hippocampal pyramidal cells, dopamine acts at D1 receptors to reduce peak Na+ currents by activation of phosphorylation by PKA anchored via an A kinase-anchoring protein (AKAP15). However, the mechanism by which AKAP15 anchors PKA to neuronal Na+ channels is not known. By using a strategy of coimmunoprecipitation from transfected tsA-201 cells, we have found that AKAP15 directly interacts with Nav1.2a channels via the intracellular loop between domains I and II. This loop contains key functional phosphorylation sites. Mutagenesis indicated that this interaction occurs through a modified leucine zipper motif near the N terminus of the loop. Whole-cell patch clamp recordings of acutely dissociated hippocampal pyramidal cells revealed that the D1 dopamine receptor agonist SKF 81297 reduces peak Na+ current amplitude by 20.5%, as reported previously. Disruption of the leucine zipper interaction between Nav1.2a and AKAP15 through the inclusion of a small competing peptide in the patch pipette inhibited the SKF 81297-induced reduction in peak Na+ current, whereas a control peptide with mutations in amino acids important for the leucine zipper interaction did not. Our results define the molecular mechanism by which G protein-coupled signaling pathways can rapidly and efficiently modulate neuronal excitability through local protein phosphorylation of Na+ channels by specifically anchored PKA.
Keywords: sodium channel
Voltage-dependent Na+ channels play a primary role in neuronal excitability (1) and are a critical target for neuromodulation (2). The Na+ channel α subunit is phosphorylated by PKA in vitro (3, 4) and in intact cells (5), and this reduces peak Na+ current in expression systems (6–9) and in neurons (6, 8). Recent research has established that neuromodulation of the Na+ channel by PKA, as well as PKC, is caused by voltage-dependent enhancement of intrinsic slow inactivation (10, 11).
The hippocampus receives rich dopaminergic innervation from the mesocorfico limbic system (12). Activation of D1-like dopamine receptors, which stimulates adenylate cyclase activity (13), reduces peak Na+ current amplitude in hippocampal pyramidal cells without altering the voltage dependence of activation or fast inactivation (8). This effect is caused by PKA phosphorylation of a family of key sites in the intracellular loop connecting domains I and II of the channel (8, 9, 14). Na+ channels bind PKA via interaction with A kinase anchoring protein 15 (AKAP15) (15). Effective neuromodulation by PKA requires anchoring of the kinase to the channel by AKAP15 (16). AKAP15 interacts with LI–II of the neuronal Na+ channel (14), which is the region of the channel that contains most of the functionally significant phosphorylation sites (8, 9, 14, 17–19). However, the site and mechanism through which this interaction occurs are unknown.
AKAPs are functionally related proteins having a targeting domain that directs them to a specific subcellular compartment or substrate and a kinase-anchoring domain with an amphipathic α-helix that binds the regulatory subunit of PKA (20–24). AKAP15 is an 81-residue protein containing an N-terminal lipid anchor, an amphipathic helix that binds PKA, and a C-terminal domain with potential interaction sites [ref. 25; see also Fraser et al., who named this protein AKAP18 (26)]. AKAP15 anchors PKA to skeletal muscle Cav1.1 channels and cardiac Cav1.2 channels through a modified leucine zipper interaction (27, 28). Here we demonstrate that AKAP15 interacts with the neuronal Na+ channel (Nav1.2a) in the N-terminal region of LI–II through a modified leucine zipper motif, suggesting a conserved mechanism for AKAP interaction with ion channels. Dopaminergic modulation of hippocampal Na+ currents is inhibited by competing peptides that disrupt this interaction but not by control peptides with mutations in the leucine zipper-like motif. Our results define the mechanism by which G protein-coupled signaling pathways can rapidly and efficiently modulate neuronal excitability through local protein phosphorylation of sodium channels by specifically anchored PKA.
Results
AKAP15 Directly Interacts with the N-Terminal Domain of LI–II of Nav1.2.
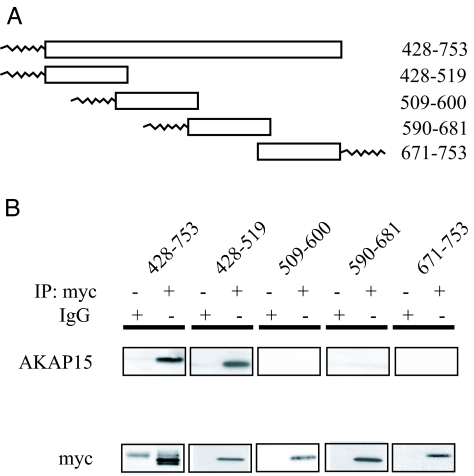
Previous work has established that AKAP15 interacts with brain Na+ channels in the intracellular loop between domains I and II (LI–II) (14). To further characterize this interaction, myc-tagged, lipid-anchored LI–II constructs were cotransfected with AKAP15 into tsA-201 cells, and coimmunoprecipitation experiments were performed (Fig. 1). A myc Ab was used to pull down the LI–II constructs from cellular lysates, and an AKAP15 Ab was used to probe immunoblots to detect coimmunoprecipitation. The complete loop (p428–753) coimmunoprecipitated AKAP15, as expected (Fig. 1B). The N-terminal portion of LI–II (p428–519) coimmunoprecipitated AKAP15 as well, indicating that AKAP15 interacts with Nav1.2 within this 92-aa portion of the channel (Fig. 1B). Fusion proteins p509–600, p590–681, and p671–753 did not coimmunoprecipitate AKAP15, despite robust expression and immunoprecipitation of these constructs (Fig. 1B).
Fig. 1.
Interaction of LI–II of Nav1.2a with AKAP15. (A) Schematic diagram of myc-tagged LI–II constructs with N-terminal or C-terminal lipid anchors. Numbers refer to amino acids. (B) (Upper) Blots show coimmunoprecipitation of AKAP15 detected by using anti-AKAP15 (25) with the whole LI–II loop (p428–753) and p428–519 immunoprecipitated by using an anti-myc Ab or nonimmune IgG, as indicated. (Lower) Blots reprobed with anti-myc Ab to confirm immunoprecipitation (IP) of constructs. The faint band in the bottom left blot (55 kDa) is the heavy chain of the Ab used for immunoprecipitation.
AKAP15 Binds to Nav1.2 via a Modified Leucine Zipper Interaction.
Modified leucine zipper interactions are known to mediate the interaction between AKAPs and ion channels (29, 30), including Cav1.1 and Cav1.2 (27, 28). To determine whether LI–II of Nav1.2 might contain a leucine zipper motif, we performed secondary structure prediction analysis (31), which indicated that the N-terminal region of LI–II is likely to be α-helical and has a high probability of forming a coiled coil.
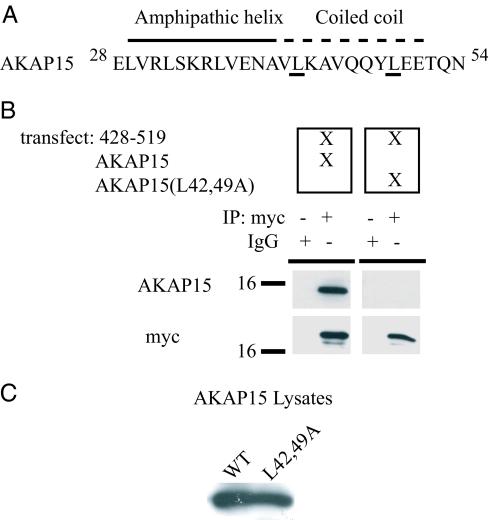
Leucine zippers are characterized by heptad repeats of hydrophobic amino acids (32, 33). To test whether Nav1.2 and AKAP15 interacted through a modified leucine zipper motif in vivo, we cotransfected p428–519 and a mutant AKAP15 (AKAP15 L42,49A) into tsA201 cells. This mutant has a heptad repeat of leucines at positions 42 and 49 that have been changed to alanine, substitutions that are known to diminish the ability of leucine zipper motif to mediate protein–protein interactions without disrupting the overall structure of the protein (34). Whereas WT AKAP15 coimmunoprecipitates with p428–519, AKAP15 (L42,49A) does not, despite robust expression of the mutant (Fig. 2).
Fig. 2.
Interaction of AKAP15 with the N terminus of LI–II of Nav1.2a via a modified leucine zipper motif. (A) The amphipathic helix and modified leucine zipper motif of AKAP15. Underlined leucines at positions 42 and 49 were mutated to alanine. (B) (Upper) Blots show coimmunoprecipitation of WT AKAP15 or AKAP15(L42,49A) with myc-tagged p428–519, as indicated. (Lower) Blots reprobed with anti-myc Ab to confirm adequate immunoprecipitation (IP) of p428–519. (C) Lysates from transfected cells probed with AKAP15 Ab demonstrating approximately equal expression of WT and mutant (L42,49A) AKAP15.
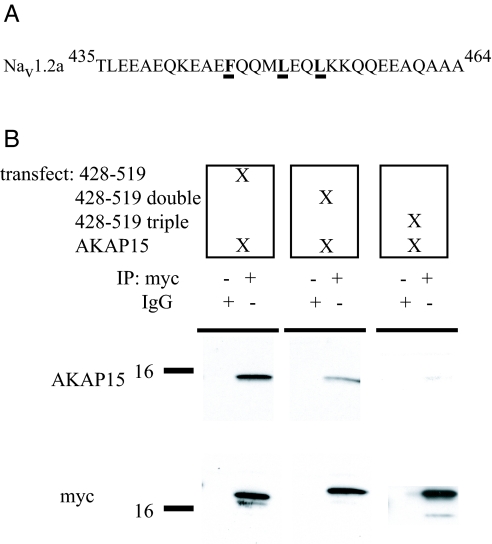
To determine the complementary binding site on Nav1.2, we made alanine mutations of candidate sites within the predicted coiled-coil region of LI–II (Fig. 3A). When the p428–519 (F446A/L453A) double mutant was cotransfected into tsA-201 cells along with WT AKAP15, coimmunoprecipitation of AKAP15 was significantly reduced compared with WT p428–519, despite robust immunoprecipitation and expression of the mutant channel fragment (Fig. 3B). The p428–519 (F446A/L450A/L453A) triple mutation caused further reduction of the coimmunoprecipitation of AKAP15 (Fig. 3B). Taken together, these data provide evidence that AKAP15 and Nav1.2 specifically interact through a modified leucine zipper motif.
Fig. 3.
Binding of AKAP15 to the WT or mutant N terminus (amino acids 428–519) of LI–II of Nav1.2a (A) The modified leucine zipper motif of Nav1.2a. (B) (Upper) Blots show coimmunoprecipitation of AKAP15 with myc-tagged WT and mutant p428–519 or IgG, from transfected tsA-201 cells, as indicated. (Lower) Blots reprobed with anti-myc Ab to confirm similar immunoprecipitation (IP) of p428–519, the double mutant, and the triple mutant.
AKAP15 Binding to Neuronal Na+ Channels via a Modified Leucine Zipper Interaction Is Required for Dopaminergic Modulation of Hippocampal Na+ Currents.
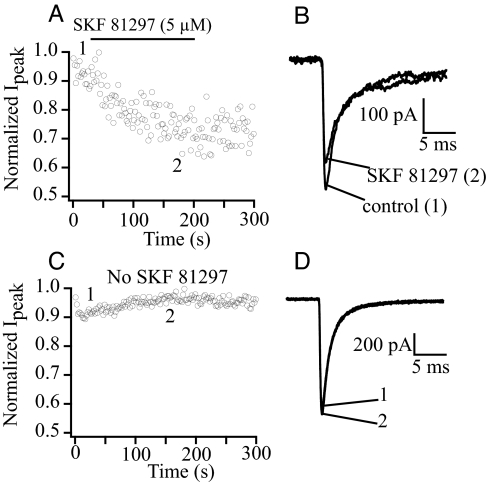
Previous studies have shown that dopamine agonists cause PKA-dependent regulation of brain Na+ channels in hippocampal pyramidal neurons (6, 8, 35). To test whether this neuromodulation requires specific anchoring of PKA to the modified leucine zipper motif in LI–II of the Nav1.2 channel, we performed whole-cell voltage clamp experiments on acutely dissociated pyramidal cells. Na+ currents were recorded by using solutions that inhibited other voltage-dependent currents (see Materials and Methods) (36). Cells were held at a potential of −70 mV, and a 40-ms depolarizing step to −20 mV was applied once every 2 s. Under these conditions, sodium currents remained stable for the duration of the experiment. Application of 5 μM SKF 81297, a specific D1 dopamine receptor agonist, reduced peak Na+ current by 20.5 ± 4.1% (Fig. 4 A and B; n = 6), whereas no change in Na+ current was observed during perfusion with control solution (Fig. 4 C and D). These results are in good agreement with data reported previously for the modulation of Na+ current by dopamine agonists in hippocampal pyramidal cells (8).
Fig. 4.
Modulation of hippocampal Na+ current by a dopaminergic agonist. (A) Normalized peak Na+ current is reduced by SKF 81297 application. Currents were elicited by a 40-ms pulse to −20 mV from a holding potential of −70 mV once every 2 s. (B) Example Na+ currents from the same cell as in A before and after SKF 81297 perfusion. (C) Control experiment demonstrating stability of peak current amplitude in the absence of SKF 81297 perfusion. (D) Example Na+ currents from the same cell as in C.
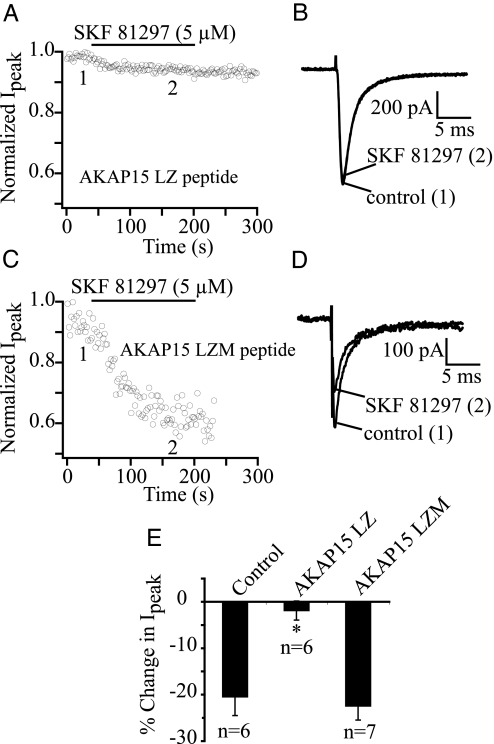
To test the functional role of the AKAP15–Na+ channel interaction in dopaminergic neuromodulation of pyramidal cell Na+ currents, we recorded whole-cell Na+ currents from acutely dissociated hippocampal pyramidal cells with a small competing peptide (AKAP15 LZ; 100 μM) in the patch pipette (27). This peptide contains the leucine zipper-like region of AKAP15, which interacts with LI–II of the Na+ channel in vitro (Fig. 2). When AKAP15 LZ was included in the pipette, and 5 min were allowed for intracellular dialysis, SKF 81297 no longer effectively modulated the hippocampal pyramidal cell Na+ current (Fig. 5 A, B, and E; n = 6). As a negative control, experiments were performed with a mutant control peptide (AKAP15 LZM; 100 μM). The AKAP15 LZM peptide contains leucine-to-alanine mutations, which inhibit the binding of AKAP15 to LI–II of Nav1.2. When the AKAP15 LZM peptide was included in the pipette, and 5 min were allowed for intracellular dialysis, SKF 81297 application reduced Na+ current amplitude 22.5 ± 3.02% (Fig. 5 C–E; n = 7). Thus, the block of SKF 81297 modulation by the AKAP15 peptide is attributable to its inhibition of AKAP15 anchoring to the channel and not to nonspecific effects of the peptide itself. Altogether, our data show that targeting of PKA to Na+ channels via a modified leucine zipper interaction between AKAP15 and LI–II of Nav1.2 plays an essential role in dopaminergic neuromodulation of brain Na+ channels.
Fig. 5.
Block of dopaminergic modulation of hippocampal Na+ current by a peptide inhibitor of the AKAP15/Nav1.2 leucine zipper interaction. (A) Effect of intracellular dialysis of AKAP15 LZ peptide (100 μM) on SKF 81297 reduction of Na+ current amplitude. Currents were elicited by a 40-ms pulse to −20 mV from a holding potential of −70 mV once every 2 s. (B) Example Na+ currents from the same cell as in A before and after SKF 81297 perfusion. (C) Efect of intracellular dialysis of AKAP15 LZM peptide (100 μM) on SKF 81297 reduction of Na+ current amplitude. (D) Example Na+ currents from the same cell as in C before and after SKF 81297 perfusion. (E) Bar graph summarizing data from control (no peptide) and peptide experiments.
Discussion
Dopaminergic Reduction of Na+ Current in Hippocampal Pyramidal Neurons Requires PKA Anchored by AKAP15 via a Leucine Zipper Interaction.
Neuromodulation of Na+ channels through protein phosphorylation is an important form of neuronal plasticity that can alter the input–output relationship of a cell by reducing its overall excitability (2). Here we show that, in hippocampal pyramidal cells, dopamine modulation of whole-cell Na+ current via PKA phosphorylation requires PKA to be specifically anchored to the channel by AKAP15 via a leucine zipper-like motif located adjacent to the sites of phosphorylation in LI–II of Nav1.2 channels.
Coimmunoprecipitation experiments from transfected cells demonstrate that AKAP15 interacts with the N terminus of the intracellular loop connecting domains I and II of the channel α subunit between amino acids 428 and 519, but not with other regions of LI–II. Secondary structure prediction analysis indicates that this region of the channel is helical and has a high probability of forming a coiled coil. Mutation of a heptad repeat of leucines to alanine at positions 42 and 49 of AKAP15 inhibited its coimmunoprecipitation by the N terminus of LI–II. Similar alanine mutations of a heptad repeat of hydrophobic amino acids in the N terminus of LI–II of Nav1.2 (F446A/L453A) also caused an inhibition of the coimmunoprecipitation of AKAP15 by this region of the channel. Moreover, whole-cell voltage clamp experiments in which a dominant-negative peptide that contains the leucine zipper-like motif of AKAP15 was dialyzed into hippocampal pyramidal cells show that the reduction in peak Na+ current caused by the specific D1-like dopamine receptor agonist SKF 81297 requires the specific interaction of AKAP15 with brain Na+ channels. In contrast, a peptide containing alanine mutations of the leucines at positions 42 and 49 of AKAP15 did not inhibit the dopaminergic neuromodulation of whole-cell Na+ currents. Taken together, these data indicate that AKAP15 and brain Na+ channels interact through a leucine zipper-like motif and that this specific interaction is necessary for the dopamine-induced PKA regulation of Na+ channels in hippocampal pyramidal cells. Thus, dopaminergic neuromodulation of hippocampal Na+ channels requires a functional signaling complex composed of the Na+ channel α subunit, AKAP15, and PKA.
Leucine Zipper Interactions and Ion Channels.
Our results show that Nav1.2 channels contain a leucine zipper-like motif that plays a role in mediating binding to the kinase anchoring protein AKAP15. Modified leucine zipper interactions have previously been shown to play a role in the regulation of a variety of other ion channels (29, 30). AKAP15 is known to bind to the C terminus of skeletal muscle Cav1.1 channels via a modified leucine zipper (27). This interaction is required for effective voltage-dependent potentiation of the channels through PKA phosphorylation of sites on the C terminus (27). Similarly, Cav1.2 channels in cardiac myocytes also target AKAP15 to the C terminus of the channel via a modified leucine zipper (28). Disrupting this interaction prevents β-adrenergic receptor- and PKA-dependent regulation of L-type Ca2+ currents in ventricular myocytes (28). Yotiao (37, 38), another AKAP, targets PKA and protein phosphatase 1 (PP1) to hKCNQ1 cardiac K+ channels via a leucine zipper (39). Interestingly, an inherited long QT syndrome mutation (hKCNQ1-G589D) disrupts the leucine zipper in hKNCNQ1 and prevents β-adrenergic regulation of the slow outward K+ current (39). Thus, a mutation in an ion channel leucine zipper motif that disrupts a macromolecular signaling complex may play a role in human disease.
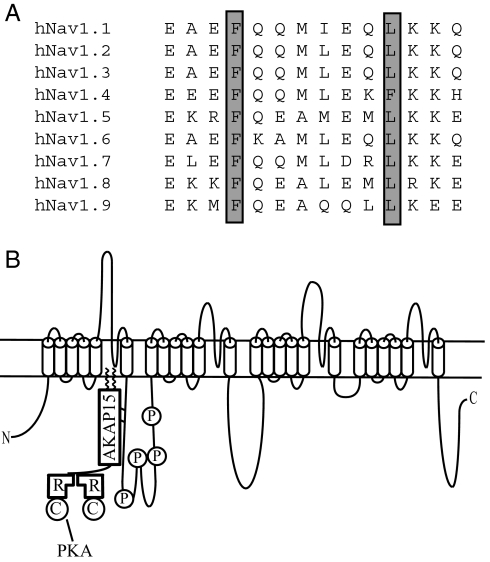
The leucine zipper-like motif in Nav1.2 is shorter than a “classical” leucine zipper. In addition, one of the hydrophobic amino acids involved in the interaction between the channel and AKAP15 is phenylalanine, not leucine or isoleucine. Phenylalanine residues are also present in the modified leucine zipper motif of Cav1.2 and Cav1.4 channels, suggesting that this may be common in the modified leucine zipper interactions of ion channels. The affinity of modified leucine zipper interactions involving phenylalanine would be expected to be lower than leucine, which may allow reversible association of AKAP15 with ion channels in a cellular context. The alanine mutations we made inhibited the coimmunoprecipitation of AKAP15, demonstrating that those residues are involved in the interaction between Nav1.2 and AKAP15. The leucine zipper-like motif that we identified is highly conserved in the Nav1 family of voltage-gated Na+ channels (Fig. 6A), suggesting that it may be involved in PKA regulation in this large group of proteins. In addition to Nav1.2, the Nav1.1, Nav1.7, and Nav1.8 channels expressed primarily in neurons and the Nav1.5 channel expressed primarily in heart are all regulated by PKA (40–43). In contrast, the Nav1.6 channel expressed primarily in neurons and the Nav1.4 channel expressed primarily in skeletal muscle are not regulated by PKA (40, 41). Because all of these channels retain the modified leucine zipper motif, differences in the serine residues that are the targets for PKA phosphorylation are likely to be responsible for the differential regulation.
Fig. 6.
Conserved leucine zipper-like motifs in the Nav1 family of voltage-gated Na+ channels. (A) Amino acid sequence alignment of the leucine zipper-like region identified in Nav1.2 with other members of the Nav1 family. (B) Model of the leucine zipper-like interaction between AKAP15 and LI–II of Nav1.2. AKAP15 directly interacts with the N terminus of LI–II in close proximity to the PKA phosphorylation sites.
Functional Implications of AKAP15 in Dopaminergic Modulation of Na+ Channels.
AKAPs act to compartmentalize and add specificity to PKA signaling (22, 24, 44, 45), which is known to control numerous physiological processes in neurons, including the modulation of multiple classes of ion channels (46). Specific anchoring of PKA ensures that the appropriate target is phosphorylated in response to a particular signal. In the case of Na+ channels in hippocampal pyramidal cells, normal dopamine signaling is achieved only when PKA is anchored directly to the LI–II of the channel. Although nonanchored PKA is capable of phosphorylating and modulating Na+ channels, as is the case in nonneuronal expression systems that do not contain AKAP15 such as Xenopus oocytes, it seems likely that rapid modulation on the millisecond timescale in neurons requires that the kinase is anchored in close proximity to the phosphorylation sites. LI–II of Nav1.2 contains numerous PKA phosphorylation sites, with S573 phosphorylation accounting for the majority of channel modulation (8, 9, 14). Thus, our current work suggests a mechanism by which G protein-coupled signaling pathways can rapidly and efficiently modulate neuronal excitability through local protein phosphorylation of Na+ channels by specifically anchored PKA.
Materials and Methods
Molecular Biology.
Sequences corresponding to the intracellular loop between domains I and II (LI–II) of rat Nav1.2a (p428–753, p428–519, p509–600, p590–681) were amplified by PCR by using specific primers and cloned into pcDNA3.1 myc-His A (Invitrogen, Carlsbad, CA). An N-terminal lipid anchor (the myristoylation/palmitoylation sequence of AKAP15; MGALCC) was included to direct plasma membrane localization, as described in ref. 27. The p671–753 construct was amplified by PCR, including a C-terminal lipid anchor [the CAAX motif from RhoF; CLLL (47)] and an N-terminal myc tag, and was cloned into pcDNA3.1 (Invitrogen). AKAP15 and AKAP15 LZM (L42A/L49A) were constructed as described and cloned into pcDNA3.1 (27). p428–519 (F446A/L453A) double mutants and p428–519 (F446A/L450A/L453A) triple mutants were generated by PCR by using the QuickChange method (Stratagene) and cloned into pcDNA3.1 myc-His A. All constructs were confirmed by sequencing.
Coimmunoprecipitation Experiments.
tsA-201 cells were cultured in DMEM/Ham's F-12 supplemented with 10% FBS, 100 units/ml penicillin and streptomycin, and plated on 15-cm dishes. Cells were transfected with 50 μg of an equimolar ratio of plasmid cDNAs by the calcium phosphate method. After 48 h, cells were rinsed in PBS and solubilized in lysis buffer [25 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EGTA, 1 mg/ml BSA, 0.1% Triton X-100, and protease inhibitors (Complete Mini; Roche Diagnostics Indianapolis, IN)]. Four micrograms of anti-myc Ab (Zymed, San Francisco, CA) or IgG were added to the samples, which were rotated for 2 h at 4°C. Prewashed Protein A Sepharose beads (2.5 mg) were added, and the samples were rotated for an additional 1 h. The beads were then spun down and washed extensively in lysis buffer and once in 10 mM Tris (pH 7.4). The immunoprecipitates were then processed by SDS/PAGE and transferred to nitrocellulose membranes for immunoblotting with an anti-AKAP15 Ab, which was prepared as described (25).
Acute Dissociation of Hippocampal Neurons.
Hippocampal neurons from 21- to 28-d postnatal mice were acutely isolated, as described (8). All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Washington. Mice were decapitated under halothane anesthesia. Brains were removed and iced in dissociation solution (82 mM Na2SO4, 30 mM K2SO4, 5 mM MgCl26H2O, 10 mM Hepes, 10 mM d-glucose, pH 7.4) bubbled with 95% O2 and 5% CO2. Slices (400 μm) were cut, transferred to a holding solution (126 mM NaCl, 2.5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, 1 mM pyruvic acid, 0.2 mM l-ascorbic acid, 0.2 mM ω-N-nitro-l-arginine, pH 7.2), bubbled with 95% O2 and 5% CO2, and allowed to rest for at least 1 h. Portions of the hippocampus were dissected and placed in a treatment chamber containing protease type XIV (1 mg/ml; Sigma, St. Louis, MO) in Hepes-buffered Hanks' balanced salt solution (Sigma), pH 7.4, at 37°C for 15–20 min. After treatment, tissue was washed three times in dissociation solution with BSA (1 mg/ml; Sigma) and trypsin inhibitor (1 mg/ml; Fluka Biochemica, Buchs, Switzerland) followed by several rinses in Tyrode's solution. Tissue was then manually triturated by passing it through several fire-polished Pasteur pipettes of decreasing diameter. Dissociated cells were plated in 35-mm tissue culture dishes and allowed to settle for 10 min before the bathing solution was changed to normal extracellular recording solution.
Whole-Cell Voltage Clamp Recording.
Na+ currents were recorded from pyramidally shaped hippocampal neurons with, at most, one or two short processes. Electrodes were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) and had resistances of 3–5 MΩ in the bath. A low Na+ extracellular recording solution was used to reduce current magnitude and improve voltage control. It contained 20 mM NaCl, 116 mM glucose, 10 mM Hepes, 1 mM BaCl2, 2 mM MgCl2, 55 mM CsCl, 1 mM CdCl2, 1 mM CaCl2, 20 mM tetraethylammonium–Cl, pH 7.2, with NaOH. The intracellular recording solution contained 189 mM N-methyl d-glucamine, 40 mM Hepes, 4 mM MgCl2, 1 mM NaCl, 0.1 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate, (BAPTA), 25 mM phosphocreatine, 2 mM Na2ATP, 0.2 mM Na3GTP, and 0.1 mM leupeptin, pH 7.2, with H2SO4. Concentrated stocks of SKF 81297 (Sigma) were prepared in water and frozen in aliquots. AKAP15 LZ peptide (37-ENAVLKAVQQYLEETQN-55) and AKAP15 LZM peptide (37-ENAVAKAVQQYAEETQN-55) were synthesized and prepared by Genemed Synthesis Inc. (San Francisco, CA). Bold letters in the LZ peptide sequence denote the heptad repeat of leucine residues that were mutated to alanines in the LZM peptide. Recordings were made by using an Axopatch ID voltage clamp (Molecular Devices, Union City, CA). Pharmacological compounds were applied by using a multibarrel gravity-fed “sewer pipe” system. The application capillaries were positioned several hundred micrometers away from the cell under study, and the solutions changed by manually altering the position of the capillary. The effects of SKF 81297 in the absence or presence of peptides was examined at least 5 min after establishing the whole-cell configuration. All data are expressed as mean ± SEM of n cells. Statistical significance was tested using Student's t test. Values of P< 0.01 were considered significant.
Acknowledgments
We thank Dr. G. S. McKnight (University of Washington), Dr. A. R. Cantrell (University of Tennessee, Memphis, TN), and Dr. A. L. Goldin, (University of California, Irvine, CA) for critical comments on a draft of the manuscript. This research was supported by National Institutes of Health Grant NS 15751 (to W.A.C.).
Abbreviations
- AKAP
A kinase-anchoring protein
- LZM
leucine zipper mutant
- PP
protein phosphatase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 2.Cantrell AR, Catterall WA. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- 3.Costa MR, Casnellie JE, Catterall WA. J Biol Chem. 1982;257:7918–7921. [PubMed] [Google Scholar]
- 4.Costa MR, Catterall WA. J Biol Chem. 1984;259:8210–8218. [PubMed] [Google Scholar]
- 5.Rossie S, Catterall WA. J Biol Chem. 1987;262:12735–12744. [PubMed] [Google Scholar]
- 6.Li M, West JW, Lai Y, Scheuer T, Catterall WA. Neuron. 1992;8:1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- 7.Smith RD, Goldin AL. J Neurosci. 1996;16:1965–1974. doi: 10.1523/JNEUROSCI.16-06-01965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantrell AR, Smith RD, Goldin AL, Scheuer T, Catterall WA. J Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RD, Goldin AL. J Neurosci. 1998;18:811–820. doi: 10.1523/JNEUROSCI.18-03-00811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ., Jr Neuropsychopharmacology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- 13.Stoof JC, Kebabian JW. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 14.Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Mol Cell Neurosci. 2002;21:63–80. doi: 10.1006/mcne.2002.1162. [DOI] [PubMed] [Google Scholar]
- 15.Tibbs VC, Gray PC, Catterall WA, Murphy BJ. J Biol Chem. 1998;273:25783–25788. doi: 10.1074/jbc.273.40.25783. [DOI] [PubMed] [Google Scholar]
- 16.Cantrell AR, Tibbs VC, Westenbroek RE, Scheuer T, Catterall WA. J Neurosci. 1999;19:RC21. doi: 10.1523/JNEUROSCI.19-17-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossie S, Gordon D, Catterall WA. J Biol Chem. 1987;262:17530–17535. [PubMed] [Google Scholar]
- 18.Rossie S, Catterall WA. J Biol Chem. 1989;264:14220–14224. [PubMed] [Google Scholar]
- 19.Murphy BJ, Rossie S, De Jongh KS, Catterall WA. J Biol Chem. 1993;268:27355–27362. [PubMed] [Google Scholar]
- 20.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 21.Hausken ZE, Coghlan VM, Hastings CA, Reimann EM, Scott JD. J Biol Chem. 1994;269:24245–24251. [PubMed] [Google Scholar]
- 22.Hausken ZE, Scott JD. Biochem Soc Trans. 1996;24:986–991. doi: 10.1042/bst0240986. [DOI] [PubMed] [Google Scholar]
- 23.Newlon MG, Roy M, Hausken ZE, Scott JD, Jennings PA. J Biol Chem. 1997;272:23637–23644. doi: 10.1074/jbc.272.38.23637. [DOI] [PubMed] [Google Scholar]
- 24.Gray PC, Scott JD, Catterall WA. Curr Opin Neurobiol. 1998;8:330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 25.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, III, Scheuer T, Catterall WA, Murphy BJ. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 26.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 28.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Proc Natl Acad Sci USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulme JT, Scheuer T, Catterall WA. J Mol Cell Cardiol. 2004;37:625–631. doi: 10.1016/j.yjmcc.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Kass RS, Kurokawa J, Marx SO, Marks AR. Trends Cardiovasc Med. 2003;13:52–56. doi: 10.1016/s1050-1738(02)00211-6. [DOI] [PubMed] [Google Scholar]
- 31.Lupas A, Van Dyke M, Stock J. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 32.Landschulz WH, Johnson PF, McKnight SL. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 33.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 34.Simmerman HK, Kobayashi YM, Autry JM, Jones LR. J Biol Chem. 1996;271:5941–5946. doi: 10.1074/jbc.271.10.5941. [DOI] [PubMed] [Google Scholar]
- 35.Cantrell AR, Scheuer T, Catterall WA. J Neurosci. 1999;19:5301–5310. doi: 10.1523/JNEUROSCI.19-13-05301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantrell AR, Ma JY, Scheuer T, Catterall WA. Neuron. 1996;16:1019–1026. doi: 10.1016/s0896-6273(00)80125-7. [DOI] [PubMed] [Google Scholar]
- 37.Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. J Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 39.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 40.Frohnwieser B, Chen LQ, Schreibmayer W, Kallen RG. J Physiol. 1997;498:309–318. doi: 10.1113/jphysiol.1997.sp021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijayaragavan K, Boutjdir M, Chahine M. J Neurophysiol. 2004;91:1556–1569. doi: 10.1152/jn.00676.2003. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. J Physiol. 1999;516:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin CS. Biochim Biophys Acta. 1994;1224:467–479. [PubMed] [Google Scholar]
- 45.Dell'Acqua ML, Scott JD. J Biol Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- 46.Brandon EP, Idzerda RL, McKnight GS. Curr Opin Neurobiol. 1997;7:397–403. doi: 10.1016/s0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- 47.Zacharias DA, Violin JD, Newton AC, Tsien RY. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]