Abstract
Stimuli are translated to intracellular calcium signals via opening of inositol trisphosphate receptor and ryanodine receptor (RyR) channels of the sarcoplasmic reticulum or endoplasmic reticulum. In cardiac and skeletal muscle of amphibians the stimulus is depolarization of the transverse tubular membrane, transduced by voltage sensors at tubular–sarcoplasmic reticulum junctions, and the unit signal is the Ca2+ spark, caused by concerted opening of multiple RyR channels. Mammalian muscles instead lose postnatally the ability to produce sparks, and they also lose RyR3, an isoform abundant in spark-producing skeletal muscles. What does it take for cells to respond to membrane depolarization with Ca2+ sparks? To answer this question we made skeletal muscles of adult mice expressing exogenous RyR3, demonstrated as immunoreactivity at triad junctions. These muscles showed abundant sparks upon depolarization. Sparks produced thusly were found to amplify the response to depolarization in a manner characteristic of Ca2+-induced Ca2+ release processes. The amplification was particularly effective in responses to brief depolarizations, as in action potentials. We also induced expression of exogenous RyR1 or yellow fluorescent protein-tagged RyR1 in muscles of adult mice. In these, tag fluorescence was present at triad junctions. RyR1-transfected muscle lacked voltage-operated sparks. Therefore, the voltage-operated sparks phenotype is specific to the RyR3 isoform. Because RyR3 does not contact voltage sensors, their opening was probably activated by Ca2+, secondarily to Ca2+ release through junctional RyR1. Physiologically voltage-controlled Ca2+ sparks thus require a voltage sensor, a master junctional RyR1 channel that provides trigger Ca2+, and a slave parajunctional RyR3 cohort.
Keywords: contractility, excitation–contraction coupling, sarcoplasmic reticulum
Cytosolic [Ca2+] transients of excitable cells often require electrically initiated opening of ryanodine receptor (RyR) channels in the membrane of intracellular Ca2+ stores (1). Three isoforms of the RyR are controlled differently. In heart muscle RyR isoform 2 is activated by Ca2+ entering during the action potential [the calcium-induced calcium release (CICR) process] (2). In skeletal muscle and some neuronal terminals (3), RyR1 channels are opened by a mechanism that does not require Ca2+entry (4) and involves dihydropyridine receptors (DHPRs), sensors of voltage Vm of the plasma and T membranes (5). The present study addresses the activation and roles of the third RyR isoform.
Different activation mechanisms result in different Ca2+ transients. Whereas in cardiac and frog skeletal muscle the Vm-elicited signals are composed of discrete events called Ca2+ sparks, which involve the concerted action of multiple channels (6–8), in mammalian muscle channels act individually, so that the responses lack sparks (9, 10). Nonmammalian skeletal muscles express two RyR isoforms: α, homologous to RyR1, and β (11, 12), homologous to RyR3 (13, 14). β and RyR3 have greater homology with RyR2 than RyR1. Isoform 3 is largely absent from adult mammalian muscle (15). Therefore, isoforms 3 or β are candidates for a crucial role in spark production. Here we establish the minimal molecular endowment for Vm-operated sparks.
Like the adult amphibian, the late mammalian embryo and the neonate have RyR1 and RyR3 and produce Ca2+ sparks. These sparks, however, are Vm-independent (16). Therefore, the mere presence of RyR1 or α and RyR3 or β is not sufficient for production of Vm-controlled sparks. The present work identifies additional requisites.
In all, there are three basic phenotypes of local Ca2+ signaling: (i) Vm-controlled transients devoid of sparks in adult mammalian muscle, (ii) Vm-controlled signals built from sparks in frog twitch muscle, and (iii) a duality of Vm-independent sparks and responses to Vm that lack sparks in the mammalian embryo. To understand how two RyR channel isoforms determine these functional phenotypes we endowed muscles of an adult mammal with RyR3. To tell whether the consequent functional changes were specific to isoform 3 we then caused expression of extra RyR1.
Results
Expression of Foreign Channel Proteins at High Density in Adult Muscle.
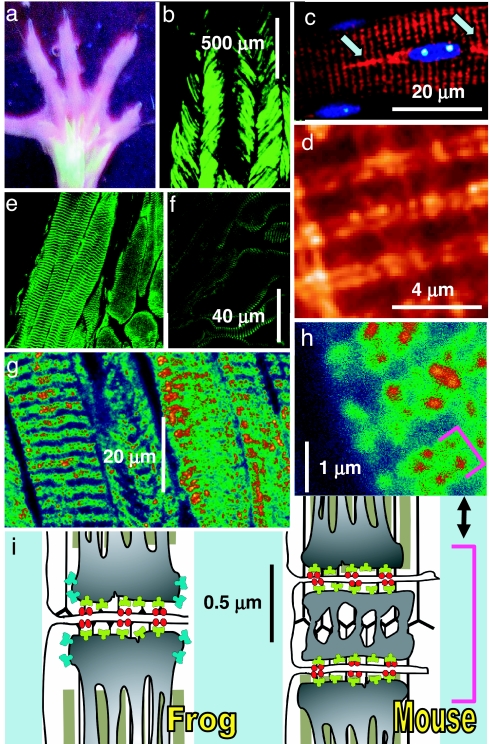
Transfection was achieved by injecting plasmid vectors into the hind paws of adult mice and then electroporating the paw muscles by pulses applied through acupuncture needles (17). Shown in Fig. 1 a and b are fluorescence images of a paw and a flexor digitorum brevis (FDB) expressing GFP cDNA delivered this way. The images confirm the high efficiency of transfection achieved by this method. Fig. 1 c and d show yellow fluorescence, F, of a muscle transfected with rabbit RyR1 cDNA tagged with yellow fluorescent protein (YFP). The protein appears correctly targeted to the double row of junctions characteristic of mammals (see Fig. 1i). One of the nuclei in Fig. 1c (blue) is flanked by fusiform areas of high F (arrows), indicating high RyR synthesis in paranuclear endoplasmic reticulum (18). A second nucleus lacks high F regions, coinciding with lower F in sarcomeres nearby. This segmental pattern of expression may be due to differences in transcribing activity at individual nuclei (19, 20). The other images are of muscles transfected with rabbit RyR3 (21). Immunolabeling with an antibody specific for RyR3 (22) shows immunoreactivity in transverse bands (Fig. 1e), which at higher magnification resolve into double rows of distinct foci (Fig. 1 g and h, bracket). As represented in Fig. 1i Right, in mammals one set of triadic junctions is present on each side of every Z disk, resulting in a doublet of closely placed triads (bracket). The staining therefore indicates that, like RyR1, RyR3 is targeted to triads, similar to RyR3 in muscles that natively express the two isoforms (Fig. 1i Left). In nonmammalian muscle that natively contains two RyR isoforms, RyR3 is believed to occupy “parajunctional” positions, close to but not in contact with the junctional channels (23). This placement is consistent with the known inability of RyR3 channels to interact structurally (24) or functionally (25) with DHPRs. Moreover, for reasons of symmetry RyR3 molecules are more likely to form homomeric arrays than mix with RyR1 at the junction (23). For all of these reasons, foreign RyR3 (blue in Fig. 1i) presumably occupy parajunctional locations in these transfected FDB fibers. Although the YFP fluorescence and immunofluorescence images (Fig. 1 d and h) are not strictly comparable, the wider, more diffuse staining in Fig. 1h appears consistent with a parajunctional location of RyR3.
Fig. 1.
Markers of expression in adult muscle. (a and b) Images of fluorescence from a mouse transfected with endoplasmic reticulum–GFP cDNA, showing expression in FDB and interosseus muscles (a) at efficiency close to 100% (b). (c) Overlay of confocal images of fluorescence of YFP (red) and blue fluorescence of a FDB fiber transfected with YFP-tagged RyR1 and stained with nuclear-selective Hoechst 33342. Arrows mark paranuclear areas of high YFP content. (d) YFP fluorescence at high magnification showing distribution in dual rows. (e) FDB transfected with RyR3 immunostained with anti-RyR3 Ab. (f) Immunostained control FDB. (g and h) FDB transfected and stained and at higher magnification to show doublets of stained rows. These rows correspond to triad junctions, as clarified by the diagram. (i) Location of triads and channels. In the mouse and other mammals there are two transverse tubules per sarcomere, located at the ends of the I band, so that two triads from neighboring sarcomeres (marked by the bracket) are close to each other; in amphibians, birds, and fish, a single triad is located at the center of the I band. DHPR (red) face RyR1 (green) in the T–SR junction. In nonmammals, RyR3 (blue) are located in the parajunctional SR membrane. The presence of doublets in the stained image (d and h, bracket) indicates that in transfected cells the foreign RyRs migrate to triads.
RyR3-Transfected Muscles Produce Ca2+ Sparks at Rest.
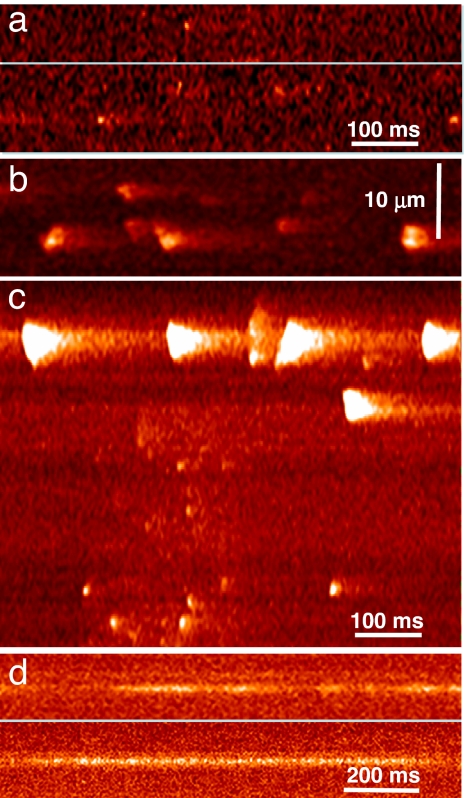
Intact adult mammalian fibers at rest do not show Ca2+ sparks when stimulated (9). Spontaneous events are observed only upon membrane stress, deformation, or damage (26). By contrast, 30% of cells from muscles of 16 mice transfected with RyR3 exhibited discrete spontaneous events (Fig. 2 a–c). Some intact fibers showed brief events (Fig. 2a). Their properties, listed in Table 1, were similar to sparks of intact frog fibers (8). Other intact fibers gave wider events (Fig. 2b), many of which were sequentially propagating, similar to those seen in frogs in the presence of SO42− (27, 28). Eleven RyR3 fibers that were patched and perfused exhibited a variety of events (as in Fig. 2c) including periodic “macrosparks,” wave-like events that usually originated near the fiber surface, at the “high F” nuclear flanking regions.
Fig. 2.
Spontaneous events in fibers from transfected muscle. (a) Line scans in an intact fluo-4-loaded RyR3-transfected fiber featuring small sparks. (b) Intact fiber from another mouse showing large and more frequent sparks, sometimes propagating in space. (c) Variety of events in a RyR3-transfected cell voltage-clamped at −80 mV. Note large events near surface. (d) Line scans of fluo-4 fluorescence in two cells transfected with RyR1 and voltage-clamped. Note narrow Ca2+“embers.” Line scanning was perpendicular to the fiber axis.
Table 1.
The discrete events of RyR-transfected cells
| RyR | Amplitude | FWHM, μm | Rise time, ms | FDHM, ms | No. of events (cells) |
|---|---|---|---|---|---|
| RyR3, intact | 1.51 ± 0.15 | 1.13 ± 0.11 | 7.97 ± 2.33 | 11.5 ± 2.62 | 23 (3) |
| RyR3, patch | 0.56 ± 0.03 | 2.30 ± 0.09 | 17.9 ± 0.87 | 29.3 ± 1.29 | 85 (5) |
| RyR1 | 0.22 ± 0.01 | 2.26 ± 0.15 | 30.5 ± 3.20 | 54.8 ± 3.73 | 33 (3) |
Events were detected automatically. Morphologic spark parameters are defined in SI Materials and Methods. All RyR1-transfected cells were patched and perfused with a solution containing 1 mM EGTA. RyR3 cells in the patched group included three cases with 1 mM EGTA and two cases with 5 mM EGTA in the pipette. FWHM, full width at half maximum; FDHM, full duration at half maximum.
Ten percent of cells from muscles of six mice transfected with either RyR1 or YFP-RyR1 exhibited also events, but narrow and long-lasting (Fig. 2d), with average properties (Table 1) comparable with the “lone embers” of skinned mammalian fibers (28). Approximately 20% of RyR1-transfected cells exhibited large events (similar to the macrosparks in Fig. 2c), strictly localized near nuclei. Neither WT cells (Fig. 3 a1 and c1) nor those transfected with other plasmids (endoplasmic reticulum–GFP, rabbit calsequestrin, and red protein-tagged calsequestrin silencer) displayed spontaneous events.
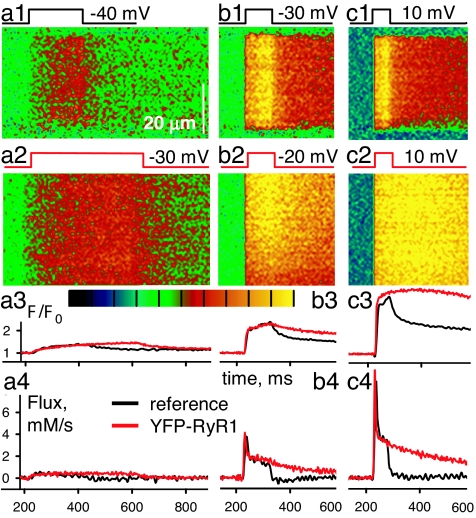
Fig. 3.
Response to voltage clamp of WT and RyR1-transfected fibers. (a1–c1) Line scan images of F(x,t)/F0(x) in a WT fiber held at −80 mV and subjected to pulses represented at top. (a2–c2) Corresponding images in a YFP-RyR1-transfected cell. Black corresponds to F/F0 = 0, and yellow corresponds to 2.5 (a and b) or 4 (c). (a3–c3) F/F0 for WT (black) or RyR1 (red). (a4–c4) Ca2+ release flux derived from the image-averaged fluorescence using removal parameters fitted to the WT records.
Ca2+ Sparks Respond to Vm Change in RyR3-Transfected Muscle.
Clearly, expression of RyR3 endows adult fibers with Ca2+ sparks. But are these insensitive to membrane depolarization, like those of the embryo, or are they Vm-operated, as in the frog? We answered this question by imaging the response to either action potentials in intact fibers or Vm pulses under whole-cell patch clamp. In most of the transfected fibers, the [Ca2+] increase accompanying action potentials was large and fast, conditions that prevent the identification of sparks. Serendipitously, some RyR3-transfected cells responded to electrical stimulation with Ca2+ transients that were weaker than usual (probably because of partial depolarization of the plasma membrane), as shown in Fig. 4a. The action potential-mediated responses were composed of sparks. As shown by the profile in Fig. 4f, the spontaneous sparks, which were observed in 25 RyR3 fibers, often were larger, appeared near the surface and were independent of the electrical stimuli.
Fig. 4.
Sparks activated by action potentials in RyR3-transfected muscle. (a) Line scan of a fiber from transfected FDB. The response to 0.5-ms field pulses (b) was composed of sparks. (c–f) Profiles of F/F0 at arrows. Large sparks occurring spontaneously near the surface (lower edge of image and profile f) were unaffected by Vm.
Exogenous RyRs crucially modified the response to voltage clamp. “Control” responses are in Fig. 3. In WT cells (Fig. 3 a1–c1) depolarizing pulses caused Ca2+ transients devoid of sparks, with a profile (black in Fig. 3 a3–c3) that increased during the pulse and dropped sharply afterward. This monotonic increase reflected a substantial steady level of Ca2+ release flux, sustained during the pulse. The release flux, derived from the Ca2+ transient, is plotted in Fig. 3 a4–c4. As reported earlier in experiments using photometry (29), the flux had an early peak, physiologically relevant, as it is representative of the activity elicited during the brief action potential of skeletal muscle. This peak was 2–2.5 times greater than the steady level reached immediately afterward (Fig. 3 b4 and c4, black traces).
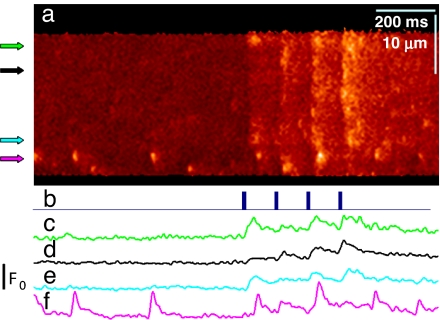
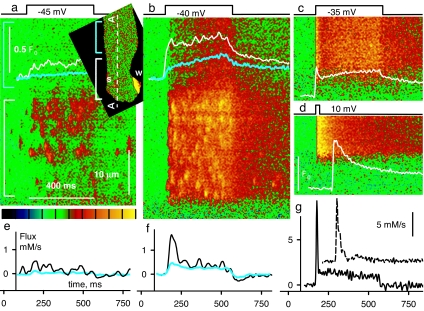
The response to Vm of RyR3-transfected cells is illustrated in Fig. 5. In Fig. 5a Inset is an x–y image of F/F0 in the resting cell, showing a large wave “w” near a protruding nucleus. Spontaneous sparks, “s,” which became frequent after the period of voltage stimulation used to generate the images in the figure, occurred only in the segment marked by the white bracket. Fig. 5 a and b shows responses to low Vm pulses, in scans along line AA in Fig. 5a Inset. Transients were largely constituted by sparks, of steeply Vm-dependent frequency. Responses at higher Vm, in a different cell, are in Fig. 5 c and d. Even though sparks, present at lower voltages, were no longer separable, there was a remarkable, qualitative difference with WT or RyR1 images at comparable Vm; the evolution of fluorescence (plotted in white) had an early maximum rather than a monotonic rise.
Fig. 5.
Response to voltage clamp of RyR3-transfected fibers. (a and b) F(x,t)/F0(x) in scans along line AA of fiber in Inset showing responses to Vm. In the segment marked by the white bracket the response consisted largely of sparks. In the rest of the image (cyan bracket in Inset and a) the response was eventless, like that of WT muscle (Fig. 3a1). White and cyan traces plot F/F0 averaged over x in the segments marked by color-matching brackets. (c and d) Responses to higher Vm in a second transfected cell. (e and f) Ca2+ release flux derived from the color-matched F/F0 traces in a and b. (g) Release flux derived from traces in c (solid) or d (dashed; note scale and horizontal shift for visibility in records at 10 mV). Unlike the WT (Fig. 3 b1 and c1), these responses show an early peak of fluorescence. Correspondingly, the peak in flux is more marked. Spatial scale is the same everywhere. Green in the color palette corresponds to F/F0 = 1, and yellow corresponds to 2.5 (a and b) or 3 (c and d).
To establish the isoform specificity of this change we examined the responses of fibers expressing foreign RyR1. Line scans of the responses to −30 mV (Fig. 3a2) or −20 mV (Fig. 3b2) are respectively similar to the WT responses to −40 or −30 mV (Fig. 3 a1 and b1), a voltage shift observed in five of six fibers. Sparks were not present in the responses of RyR1-transfected cells. In conclusion, Vm-operated sparks require the presence of RyR3.
Unexpectedly, the decay in fluorescence after the pulses was much slower in the RyR1 transfectants (red traces) compared with the WT (black traces). The corresponding release flux (red in Fig. 3 a4–c4) seemed to terminate more slowly (Fig. 3b4) or not at all (Fig. 3c4). This anomaly was found in three of three YFP-RyR1 and three of three RyR1-transfected cells examined.
The Collective Effect of Ca2+ Release Built from Sparks.
As can be seen in Fig. 5 a and b, only the 30-μm-long fiber segment within the white bracket produced sparks, spontaneous or in response to depolarization. In the adjacent region, bracketed in cyan in Fig. 5 a and Inset, the response was synchronous with the pulse (indicating good voltage control) but it was of smaller amplitude and did not show distinct sparks. In this segment the response was indistinguishable from that of WT muscle at a comparable Vm (Fig. 3 a1 and b1). Such segmental pattern of activity is probably due to transcription limited to individual nuclei (19, 20) (Fig. 1). The graphs in Fig. 5 e and f plot Ca2+ release flux derived from the Ca2+ transients in Fig. 5 a and b separately averaged over the two segments. At all voltages the flux in the segment with sparks (black trace) was much greater than in the area without sparks (cyan trace), which in turn was similar to that in reference or in RyR1-transfected fibers at a low Vm (Fig. 3). Vm-operated sparks therefore amplify release through RyR1, much as RyR2 amplify Ca2+ influx through L-type channels in cardiac muscle.
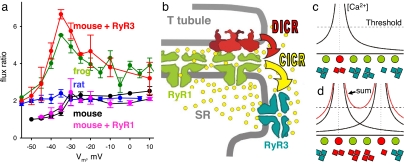
The amplification of Ca2+ release by sparks was greatest at the peak of the release flux waveform (as seen in Fig. 5 f and g), an effect that is best quantified through the peak/steady flux ratio, p/s. By this measure, the amplification of peak flux was most marked at intermediate Vm. For example at −35 mV (Fig. 5g) p/s reached a value of 7, while the corresponding value in the WT was ≈2 (Fig. 3b). Flux ratios in WT and transfected cells are compared in Fig. 6a. While the WT and RyR1 values (black or pink symbols) were mildly affected by voltage, p/s of transfected cells (red) was steeply Vm-dependent, with a prominent maximum at −35 mV.
Fig. 6.
Mechanisms of activation. (a) Ratio of peak over steady release flux vs. pulse voltage (Vm) in WT mouse (black, averages ± SEM, n = 4 fibers), RyR3-transfected fibers (red, n = 11 fibers and 8 mice), and YFP-RyR1 or RyR1 (pink, n = 6 fibers and 6 mice) compared with data from ref. 37 for rat EDL (blue) and frog semitendinosus (green). Note the similarity of frog and RyR3 mouse data. (b) A mechanism for Vm-operated sparks: T membrane depolarization is sensed by DHPRs (red), which activate underlying RyR1 by DICR. Ca2+ released through RyR1 then activates nearby RyR3, a CICR mechanism that may engulf a small cluster, to produce sparks. (c and d) The Vm dependence of amplification by CICR. Circles, junctional RyR1 operated by Vm; squares, RyR3 in parajunctional positions, assumed to open if local [Ca2+] rises above “threshold.” At low Vm (c) only one RyR1 is activated (red). Curves are profiles of cytosolic [Ca2+]; a cluster of RyR3 is activated by CICR (red). At higher Vm (d) two RyR1 channels are activated, but the number of open RyR3 channels (activated by the “sum” [Ca2+] profile) increases >2-fold. The diagram was modified from ref. 37.
Discussion
Ca2+ Sparks Controlled by Membrane Voltage Require Three Molecules.
The initial phase of the response to an action potential in skeletal muscle is largely a function of two molecules. As depicted in Fig. 6b, transverse tubule membrane depolarization is translated by DHPRs to a conformational signal for RyR to open. In avian and mammalian muscle this so-called DICR mechanism (depolarization-induced calcium release) has a strict requirement for RyR1 as effector (25, 30, 31). Analogously, spark-like syntillas are activated by depolarization in hypothalamic nerve terminals [which express RyR1 (3, 32)] but are Vm-independent in mouse chromaffin cells, which lack this isoform (33).
What happens after RyR1 activation is unclear. As in the heart, Vm-operated Ca2+ sparks of frog skeletal muscle are believed to involve CICR (34); CICR should therefore contribute a large fraction of total Ca2+ release in frog cells, where the response is largely made of sparks. On the other hand, after carefully measuring Ca2+ release stimulated by applied Ca2+ in skinned frog fibers, Ogawa et al. (35) concluded that CICR makes a negligible contribution physiologically. We show here that introducing RyR3 into mammalian muscle recreates the frog phenotype, characterized by Vm-dependent sparks. From this observation it follows that RyR3 are required for the production of sparks physiologically operated by Vm. Because the present transfection places the RyR3 channels at the triad, their activation during Vm-operated sparks is probably triggered by Ca2+ released through RyR1. That the introduction of extra RyR1 molecules fails to reproduce the effect establishes Vm-operated sparks as a specific functional product of isoform 3.
We also showed that the physiologically relevant early peak in flux is potentiated by RyR3, which implies that CICR made a significant contribution to sarcoplasmic reticulum (SR) Ca2+ release in our RyR3-transfected cells. A similar component is probably present in native two-isoform cells.
The results are consistent with the three-molecule mechanism depicted in Fig. 6b whereby the initial DICR step is amplified, via CICR, by release through RyR3. This amplification enhances the concentration gradient that propels Ca2+ from release to target control sites on troponin C in areas where contractile filaments overlap. As illustrated in Fig. 1i, in nonmammalian species those sites are located farther away and are therefore harder to reach. A similar amplification by RyR3 of a primary Ca2+ signal (produced by InsP3 receptors) may explain the contractile response of the pulmonary artery to hypoxia (36).
Amplification by CICR Has a “Signature.”
Both native two-isoform and RyR3-transfected muscles share a feature predicted for CICR. As illustrated in Fig. 5, the excess release in fibers transfected with RyR3 is greatest within the early peak of release flux, and the ratio of peak over steady flux is a sensitive measure of the effect. It is well known that this ratio differs sharply between frog and mammal (37). In Fig. 6a are plots of average ratios vs. Vm for frog (green symbols) and rat muscle (blue). p/s for the rat is lower at all voltages and quite constant. The frog values exhibit instead a steep nonlinear increase at low Vm and reach a maximum at intermediate voltages. There are obvious similarities between rat and WT or RyR1-transfected mouse ratios (black or pink) on one side, and frog and RyR3-transfected mouse ratios (red) on the other. The two systems containing RyR3 exhibit a signature mode at intermediate Vm.
A simple explanation of the Vm dependence [which updates a model of Shirokova et al. (37)] is presented in Fig. 6 c and d. Vm-operated RyR1 channels of the T-SR junction are depicted as a row of circles. Fig. 6c represents activation at a low Vm, which opens only one RyR1 channel in the group (red). The curves plot the profile of cytosolic [Ca2+] near the open channel. Among RyR3 channels located near the junction (squares) only those in the presence of a [Ca2+] above their activation threshold are open (red). At a higher Vm (Fig. 6d), addition of domains of elevated cytosolic [Ca2+] from two open RyR1 channels will lead to activation of RyR3 in numbers greater than the sum of those activated by the RyR1 opening individually. Therefore, at low Vm the gain provided by CICR over DICR will increase with voltage. At greater Vm the gain will decrease, because increasing DICR becomes proportionally less effective as a stimulus for CICR when most RyR3 are open. The recreation in a mammalian context, through addition of a Ca2+-sensitive channel, of a response with quantitative features predicted by CICR, constitutes strong evidence of the involvement of CICR in these two-isoform systems.
Inactivation of Ca2+ Release and Termination of Sparks.
As important for function as a rapid rise of the Ca2+ transient is its termination. This requires rapid restoration of the closed state of the RyRs, which in skeletal muscle is promoted by a Ca2+-dependent inactivation process (38, 39). The preservation of the closed state at rest is assured by basal inhibitory effects of Mg2+ (40, 41) and various proteins (12, 42, 43). Large events occurring spontaneously near nuclei in RyR1- or RyR3-transfected cells imply that newly synthesized channels spend more time open. By contrast, channels that have taken positions near junctions (as evidenced by their subordination to Vm) are stable, not opening until commanded by depolarization. Because basal inhibition works well for these channels, it must be associated with the interactions that enable control by Vm. Because this inhibition operates in transfected mice as well as in frogs, its mechanism must be fundamental, not dependent on species context. Stochastic attrition (44), the random turn-off of channels in a group, may be such mechanism. The effectiveness of attrition as an agent of event termination is steeply dependent on the size of the channel cluster (45). Because spontaneous events arise at arrays of newly synthesized RyRs, which must be extensive to support waves, attrition will affect them minimally; it should become increasingly effective as an inhibitory mechanism as these channels are dispersed in smaller clusters along triads in the SR.
Foreign RyR1 Channels Hinder the Response to Vm.
The expression of foreign RyR1, YFP-tagged or not, failed to induce sparks but had two reproducible effects: a shift in the sensitivity to Vm and an apparent loss of the ability to rapidly terminate Ca2+ release upon repolarization. The demonstration of the second effect required an estimation of release flux, which relied on the assumption that “Ca2+ removal” properties and SR Ca2+ content remained unchanged. If this was not the case, then the anomalous “off” of the Ca2+ transients could have other explanations, for example a full depletion of Ca2+ in the SR during the pulses. In any case, impairment of control by Vm seems plausible upon incorporating slightly different and/or excessive channels to the junctional RyR1 lattice.
The efficient expression of a functional foreign channel protein, similar in density to that demonstrated for other proteins by DiFranco et al. (17), is unprecedented for adult muscle. As pointed out by these authors, the efficiency of this technique makes it suitable for the production of secreted proteins in gene therapy and structural and biochemical studies. The present success in reconstruction of a physiologically relevant signaling cascade portends a more systematic approach to structure-function studies, namely silencing of native proteins (46) combined with expression of their mutated substitutes plus genetically encoded, organelle-targeted sensors (47), in the cellular context of adult muscle.
Materials and Methods
All procedures and solutions are described in detail in supporting information (SI) Materials and Methods. Briefly, hind paws of adult mice were transfected as described in ref. 17. Animals were killed, and FDB were surgically removed ≈8 days later. Fibers were separated enzymatically and treated for immunohistochemistry as described in ref. 46. Voltage clamp was carried out with ≈0.8-MΩ pipettes containing a Cs glutamate-based solution. RyR1- or RyR3-transfected cells were identified by their spontaneous events after loading with fluo-4 AM. YFP-RyR1-transfected cells were identified by yellow fluorescence, then patched and loaded with X-rhod-2 through the pipette. Confocal imaging and derivation of [Ca2+] (t) and Ca2+ release flux were as described in refs. 10 and 37, respectively.
Supplementary Material
Acknowledgments
We thank M. DiFranco and J. L. Vergara (University of California, Los Angeles) for advice on the electrotransfer technique; S. R. Wayne Chen (University of Calgary, Calgary, AB, Canada) for the RyR3 plasmid; P. D. Allen (Harvard University, Cambridge, MA) for the YFP-RyR1; T. Murayama (Juntendo University, Tokyo, Japan) and V. Sorrentino (University of Milan, Milan, Italy) for anti-RyR antibodies; C. Franzini-Armstrong (University of Pennsylvania, Philadelphia, PA) for encouragement; J. Eu (Duke University, Durham, NC) for advice on immunostaining; X. Xu, Y. Chu, D. Santiago, and J. Tang (Rush University) for hands-on help, and T. DeCoursey (Rush University) for reading the manuscript. This work was supported by grants from Rush University (to J.Z.), Programa de Desarrollo de Las Ciencias Básicas of Uruguay (to G.B.), and National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health (to G.M. and E.R).
Abbreviations
- SR
sarcoplasmic reticulum
- DHPR
dihydropyridine receptor
- RyR
ryanodine receptor
- CICR
calcium-induced calcium release
- DICR
depolarization-induced calcium release
- FDB
flexor digitorum brevis
- YFP
yellow fluorescent protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700748104/DC1.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Fabiato A. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.De Crescenzo V, ZhuGe R, Valazquez-Marrero C, Lifshitz LM, Custer E, Carmichael J, Lai FA, Tuft RA, Fogarty KE, Lemos JR, et al. J Neurosci. 2004;24:1226–1235. doi: 10.1523/JNEUROSCI.4286-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 5.Ríos E, Brum G. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Lederer WJ, Cannell MB. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 7.Tsugorka A, Ríos E, Blatter LA. Science. 1995;269:1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- 8.Baylor SM. Cell Calcium. 2005;37:513–530. doi: 10.1016/j.ceca.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Shirokova N, Garcia J, Ríos E. J Physiol. 1998;512:377–384. doi: 10.1111/j.1469-7793.1998.377be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csernoch L, Zhou J, Stern MD, Brum G, Ríos E. J Physiol. 2004;557:43–58. doi: 10.1113/jphysiol.2003.059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivares EB, Tanksley SJ, Airey JA, Beck CF, Ouyang Y, Deerinck TJ, Ellisman MH, Sutko JL. Biophys J. 1991;59:1153–1163. doi: 10.1016/S0006-3495(91)82331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murayama T, Ogawa Y. J Biochem (Tokyo) 1992;112:514–522. doi: 10.1093/oxfordjournals.jbchem.a123931. [DOI] [PubMed] [Google Scholar]
- 13.Giannini G, Clementi E, Ceci R, Marziali G, Sorrentino V. Science. 1992;257:91–94. doi: 10.1126/science.1320290. [DOI] [PubMed] [Google Scholar]
- 14.Hakamata Y, Nakai J, Takeshima H, Imoto K. FEBS Lett. 1992;312:229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- 15.Sorrentino V. Adv Pharmacol. 1995;33:67–90. doi: 10.1016/s1054-3589(08)60666-3. [DOI] [PubMed] [Google Scholar]
- 16.Shirokova N, Shirokov R, Rossi D, Gonzalez A, Kirsch WG, Garcia J, Sorrentino V, Ríos E. J Physiol. 1999;521:483–495. doi: 10.1111/j.1469-7793.1999.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiFranco M, Neco P, Capote J, Meera P, Vergara JL. Protein Expression Purif. 2006;47:281–288. doi: 10.1016/j.pep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Kaisto T, Metsikko K. Exp Cell Res. 2003;289:47–57. doi: 10.1016/s0014-4827(03)00231-3. [DOI] [PubMed] [Google Scholar]
- 19.Gussoni E, Blau HM, Kunkel LM. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 20.Vilquin JT, Kennel PF, Paturneau-Jouas M, Chapdelaine P, Boissel N, Delaere P, Tremblay JP, Scherman D, Fiszman MY, Schwartz K. Gene Ther. 2001;8:1097–1107. doi: 10.1038/sj.gt.3301484. [DOI] [PubMed] [Google Scholar]
- 21.Chen SR, Li X, Ebisawa K, Zhang L. J Biol Chem. 1997;272:24234–24246. doi: 10.1074/jbc.272.39.24234. [DOI] [PubMed] [Google Scholar]
- 22.Kubota M, Narita K, Murayama T, Suzuki S, Soga S, Usukura J, Ogawa Y, Kuba K. Cell Calcium. 2005;38:557–567. doi: 10.1016/j.ceca.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Felder E, Franzini-Armstrong C. Proc Natl Acad Sci USA. 2002;99:1695–1700. doi: 10.1073/pnas.032657599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Protasi F, Takekura H, Wang Y, Chen SR, Meissner G, Allen PD, Franzini-Armstrong C. Biophys J. 2000;79:2494–2508. doi: 10.1016/S0006-3495(00)76491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fessenden JD, Wang Y, Moore RA, Chen SR, Allen PD, Pessah IN. Biophys J. 2000;79:2509–2525. doi: 10.1016/S0006-3495(00)76492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, et al. Nat Cell Biol. 2005;7:525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Brum G, Gonzalez A, Launikonis BS, Stern MD, Ríos E. J Gen Physiol. 2005;126:301–309. doi: 10.1085/jgp.200509353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Brum G, Gonzalez A, Launikonis BS, Stern MD, Ríos E. J Gen Physiol. 2003;122:95–114. doi: 10.1085/jgp.200308796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ursu D, Schuhmeier RP, Melzer W. J Physiol. 2005;562:347–365. doi: 10.1113/jphysiol.2004.073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Airey JA, Deerinck TJ, Ellisman MH, Houenou LJ, Ivanenko A, Kenyon JL, McKemy DD, Sutko JL. Dev Dyn. 1993;197:189–202. doi: 10.1002/aja.1001970304. [DOI] [PubMed] [Google Scholar]
- 31.Ward CW, Protasi F, Castillo D, Wang Y, Chen SR, Pessah IN, Allen PD, Schneider MF. Biophys J. 2001;81:3216–3230. doi: 10.1016/S0006-3495(01)75957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Crescenzo V, Fogarty KE, Zhuge R, Tuft RA, Lifshitz LM, Carmichael J, Bellve KD, Baker SP, Zissimopoulos S, Lai FA, et al. J Neurosci. 2006;26:7565–7574. doi: 10.1523/JNEUROSCI.1512-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ZhuGe R, DeCrescenzo V, Sorrentino V, Lai FA, Tuft RA, Lifshitz LM, Lemos JR, Smith C, Fogarty KE, Walsh JV., Jr Biophys J. 2006;90:2027–2037. doi: 10.1529/biophysj.105.071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein MG, Cheng H, Santana LF, Jiang YH, Lederer WJ, Schneider MF. Nature. 1996;379:455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa Y, Murayama T, Kurebayashi N. Front Biosci. 2002;7:d1184–d1194. doi: 10.2741/A832. [DOI] [PubMed] [Google Scholar]
- 36.Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirokova N, Garcia J, Pizarro G, Ríos E. J Gen Physiol. 1996;107:1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider MF, Simon BJ. J Physiol. 1988;405:727–745. doi: 10.1113/jphysiol.1988.sp017358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jong DS, Pape PC, Chandler WK, Baylor SM. J Gen Physiol. 1993;102:333–370. doi: 10.1085/jgp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meissner G, Darling E, Eveleth J. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- 41.Lamb GD. Front Biosci. 2002;7:d834–d842. doi: 10.2741/A815. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J, Yi J, Launikonis BS, Royer L, Ríos E. Am J Physiol. 2006;290:C539–C553. doi: 10.1152/ajpcell.00592.2004. [DOI] [PubMed] [Google Scholar]
- 43.Murayama T, Ogawa Y. J Biol Chem. 2001;276:2953–2960. doi: 10.1074/jbc.M005809200. [DOI] [PubMed] [Google Scholar]
- 44.Stern MD. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern MD, Cheng H. Cell Calcium. 2004;35:591–601. doi: 10.1016/j.ceca.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Xu L, Duan H, Pasek DA, Eu JP, Meissner G. J Biol Chem. 2006;281:15572–15581. doi: 10.1074/jbc.M600090200. [DOI] [PubMed] [Google Scholar]
- 47.Rudolf R, Magalhaes PJ, Pozzan T. J Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.