Abstract
Transition nuclear proteins (TPs), the major proteins found in chromatin of condensing spermatids, are believed to be important for histone displacement and chromatin condensation during mammalian spermatogenesis. We generated mice lacking the major TP, TP1, by targeted deletion of the Tnp1 gene in mouse embryonic stem cells. Surprisingly, testis weights and sperm production were normal in the mutant mice, and only subtle abnormalities were observed in sperm morphology. Electron microscopy revealed large rod-like structures in the chromatin of mutant step 13 spermatids, in contrast to the fine chromatin fibrils observed in wild type. Steps 12–13 spermatid nuclei from the testis of Tnp1-null mice contained, in place of TP1, elevated levels of TP2 and some protamine 2 (P2) precursor. Most of the precursor was processed to mature P2, but high levels of incompletely processed forms remained in epididymal spermatozoa. Sperm motility was reduced severely, and ≈60% of Tnp1-null males were infertile. We concluded that TP1 is not essential for histone displacement or chromatin condensation. The absence of TP1 may partially be compensated for by TP2 and P2 precursor, but this dysregulation of nucleoprotein replacement results in an abnormal pattern of chromatin condensation and in reduced fertility.
The transformation of spermatids into spermatozoa (spermiogenesis) involves the most dramatic changes in chromatin structure and function that occur in any cell type. During the latter part of spermiogenesis, the nucleus elongates, transcription ceases, the histones are almost completely removed, and the chromatin appears as smooth fibers and then becomes highly condensed (1). In many animal and plant species, chromatin condensation is facilitated by the association of highly basic nuclear proteins, the protamines (2). The transition from histone-containing chromatin to the protamine-associated one seems to occur directly in fish and birds (2). However, in mammals (3), small, basic nuclear proteins appear when the histones are displaced and chromatin condensation is initiated; they are referred to as transition nuclear proteins (TPs), because they are subsequently replaced by protamines (3).
Although other TPs exist (4), TP1 and TP2 are the predominant ones found in rodent spermatids (5). TP1, a 6.2-kDa protein, consists of ≈20% each arginine and lysine and lacks cysteine (6, 7). TP2, a 13-kDa protein, consists of ≈10% each arginine and lysine and 5% cysteine (5). TP1 is expressed abundantly in most mammals (6) and is highly conserved, showing cDNA nucleotide and amino acid sequence homologies of 90% across species (8). The TPs are localized exclusively to nuclei of condensing spermatids (3, 9), and in the rat, constitute >90% of basic chromosomal proteins of these nuclei (10).
During human (11) and mouse (12) spermiogenesis, the TPs are replaced by two protamines, protamine 1 (P1) and protamine 2 (P2). Whereas P1 is synthesized as a mature protein, P2 is synthesized as the precursor. In mouse, mature P2 of 63 residues is derived from the precursor, pP2, of 106 residues (13) by sequential proteolysis that produces at least six identifiable intermediates (14). The genomic loci for the protamines are found in the same gene cluster as Tnp2 in all mammals examined (15), suggesting some functional relationship between the three proteins exists. Tnp1, however, is on a separate chromosome and is not clearly related to the other three proteins.
In vitro, TP1 decreases the melting temperature of DNA (16) and relaxes the DNA in nucleosomal core particles (17), which led to the proposal that TP1 reduces the interaction of DNA with the nucleosome core. In contrast, TP2 increases the melting temperature of DNA and compacts the DNA in nucleosomal cores, suggesting that it is a DNA-condensing protein (18). These apparently distinct functions of TP1 and TP2, as well as the absence of TP1 in humans with spermatid arrest (19), suggest that TP1 is essential for histone displacement and other aspects of spermatid development. To test these predictions, we generated mice lacking TP1 protein by targeted deletion of the Tnp1 gene in embryonic stem (ES) cells. In this paper, we describe the effects of this mutation on appearance, protein composition, and function of various stages of spermatids and sperm.
Materials and Methods
Construction of the Tnp1-Targeting Vector.
A Stratagene 129 mouse genomic library in the λ-FIX II vector was screened with Tnp1 cDNA (7). Positive clones were mapped with restriction enzymes (Fig. 1A). A 6.5-kb fragment was subcloned into a pBluescript SK(−) vector, the Tnp1 gene was removed and replaced with PGKneobpA cassette, and a modified pMC1-TK cassette also was cloned into the vector.
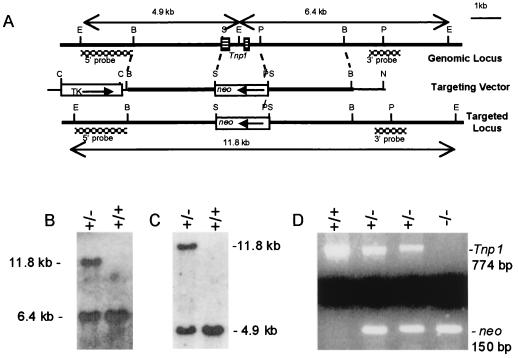
Figure 1.
Targeted deletion of the Tnp1 gene. (A) Graphic representation of the genomic Tnp1 locus, the targeting vector, and the targeted locus. Restriction sites: B, BamHI; C, ClaI; E, EcoRI; N, NotI; P, PstI; S, SalI. The SalI site is 2 bp downstream from the translational initiation codon, and the PstI site is downstream from the 3′ end of the cDNA. The positions of the probes used to distinguish the targeted locus from the endogenous locus and the EcoRI restriction fragments generated are indicated. (B and C) Southern blot genotyping of F1 mice (offspring of chimeras) was performed by probing EcoRI digests with the 3′ (B) or the 5′ probes (C). (D) PCR genotyping of Tnp1 and neo in F2 mice.
Generation of Tnp1-Mutant Mice.
The linearized Tnp1-targeting vector was electroporated into AB-1 ES cells (strain 129) that subsequently were cultured in the presence of G418 and 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil (FIAU) on mitotically inactivated STO fibroblasts. Resistant ES clones were screened by Southern blotting using probes external to the regions of vector homology to identify correctly targeted clones. The 3′ and 5′ probes were 970-bp HaeIII and 1.6-kb HpaII digestion products, respectively (Fig. 1A). Tnp1-mutant ES cells were microinjected into C57BL/6J (B6) blastocysts, which were transferred to pseudopregnant foster mothers.
Male chimeras from one ES cell clone were bred first with B6 females to produce F1 hybrid mice and then with 129 females. Offspring heterozygous for the Tnp1 knockout were identified by Southern blotting of tail DNA using external probes (Fig. 1 B and C) or by PCR using primers to amplify a portion of the neo gene (20). PCR results were in perfect agreement with Southern analysis. Mice produced by mating two Tnp1 heterozygotes were used in all experiments. In the case of the hybrid background, mice were of the F2 generation. Mice were genotyped with primers for amplification of both the neo gene and Tnp1 (upstream, 5′-GCAAGCAGGGGAACAGTCAAA-3′; downstream, 5′-GCTTTCGGAGGACCACATTCA-3′; nucleotides 942–962 and 1695–1715, respectively, in the Tnp1-genomic sequence) (Fig. 1D). The normal transmission frequencies of wild-type and null alleles indicated that the mutation did not affect viability or produce transmission ratio distortion. Most experiments were done with both hybrid and 129 backgrounds and, except where noted, no significant effects of genetic background were observed.
Preparation and Analysis of Nuclear Proteins.
Modifications to our standard procedures (10, 21, 22), as described below, were made primarily because of small amounts of available material.
Spermatogenic cells from five mice were separated by centrifugal elutriation using a Beckman JE-6B rotor (23). A fraction enriched in condensing spermatids was collected in 100 ml after raising the flow rate from 15.7 to 30.1 ml/min at a rotor speed of 3,000 rpm. Of the nuclei that should be sonication resistant (≥step 12) (24), 96–99% were steps 12 or 13 spermatids, the remainder being steps 14–16 spermatids. Cells were suspended in water containing protease inhibitors (10). After sonication, spermatid nuclei were layered over 2 ml of 5% sucrose in 0.2× concentration of MP buffer (5 mM MgCl2/5 mM sodium phosphate, pH 6.5) with 0.25% Triton X-100 and protease inhibitors and centrifuged at 740 × gmax for 5 min. Then the pellet was washed in 1 ml of MP buffer with 0.22 M NaCl.
For isolation of sonication-resistant spermatids (SRS), which represent steps 12–16, nuclei from testes from five mice were sonicated in 8 ml of water containing protease inhibitors (10). After centrifugation and resuspension in 2 ml of water, the SRS were mixed with 18 ml of 84% (wt/vol) sucrose, and 10 ml was layered over 7 ml of 84% (wt/vol) sucrose in each ultracentrifuge tube.
After incubation of nuclei in 200 μl of 0.5 mM PMSF, 1 μg/ml p-aminobenzamidine, and 10 mM DTT at 37°C for 7 to 10 min, basic proteins were extracted with 0.5 M HCl (9). Extracted proteins were precipitated with 25% trichloroacetic acid (TCA), except where noted differently.
Cauda epididymal sperm from one to three mice were sonicated (10), and nuclei were purified by centrifugation through 5% sucrose as described above. Nuclei were swelled with guanidine hydrochloride and DTT, and then proteins were dissociated with urea, mercaptoethanol, and NaCl (25). The DNA was precipitated by addition of HCl to 0.5 M and removed by centrifugation. The solutes were removed by centrifugal filtration (Centricon SR3; Amicon) or dialysis, and the protein, in 0.01 M HCl, was precipitated with TCA.
Proteins were separated initially by using acid/urea 15% polyacrylamide gels, but 18% gels were used later to resolve TP1 from the protamines. Several different protein loads were used for each sample, so that the Coomassie blue-stained bands were in the linear range. Gels were scanned with a Molecular Dynamics laser-scanning densitometer, and protein was quantified by using imagequant Version 5.0 software (Molecular Dynamics).
Electron Microscopy.
Testes were perfusion-fixed in vivo (26) and then dehydrated and embedded in epoxy. Selected areas were thin-sectioned and examined by electron microscopy.
Results
Targeted Deletion of Tnp1 Allele in the Mouse Germ Line.
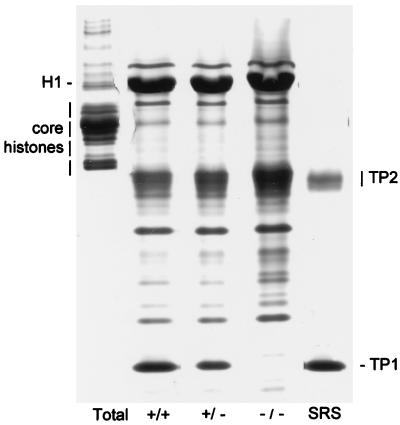
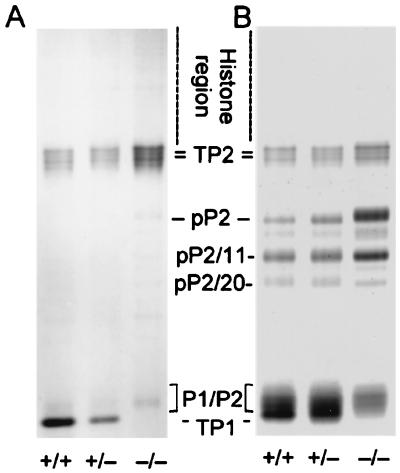
Essentially the entire Tnp1 gene was replaced by the neomycin-resistance expression cassette (Fig. 1A). Analysis of proteins in HCl extracts of testicular homogenates of Tnp1-null mice, which precipitated between 3.5% and 25% TCA and consisted largely of TP1, TP2, and histone 1 (H1), confirmed the absence of TP1 (Fig. 2). In heterozygotes, the level of TP1 (relative to H1) was reduced to 40% of that in control mice. The level of TP2 (relative to H1) was elevated 3-fold in the Tnp1-null mice but was not significantly increased in heterozygotes.
Figure 2.
Acid/urea gel of 3.5% TCA soluble proteins extracted from testes of wild-type, heterozygous, and Tnp1-null mice (B6 × 129 F2). Histones and TPs are identified in total testis basic nucleoproteins and in 3.5% TCA soluble proteins from sonication-resistant spermatid nuclei from wild-type mice. Additional bands are mostly cytoplasmic proteins. H1, histone 1.
Sperm Production and Chromatin Condensation in Tnp1-Mutant Males.
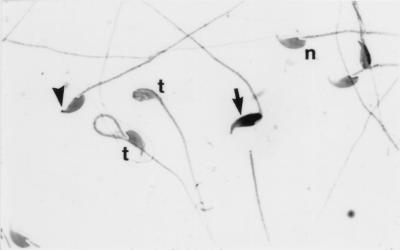
The growth, health, and appearance of Tnp1−/− mice were completely normal. Contrary to our expectations, their testis weights and sperm counts were not reduced (Table 1). Most cauda epididymal sperm in the Tnp1−/− mice appeared relatively normal (Fig. 3). However, frequencies of sperm in which the apex, the normally sharp point of the sperm nucleus, was bent or blunted and in which the nucleus stained more darkly with hematoxylin, which likely corresponded to those with less condensed chromatin as observed by electron microscopy (data not shown), were higher than those observed in the other genotypes. In addition, only 23% of sperm from the Tnp1−/− mice showed any movement, and most of those did not show forward progression (Table 1).
Table 1.
Organ weights and sperm characteristics of Tnp1-mutant males
| Tnp1 genotype | Seminal vesicle weight, mg | Testis weight without tunica, mg | Sperm heads per testis, × 106 | Sperm per two cauda epididymides, × 106 | Epididymal sperm heads that were detached, % | Epididymal sperm heads with blunted tips, % | Motility, % (forward progression) |
|---|---|---|---|---|---|---|---|
| +/+ | 330 ± 27 | 104 ± 2 | 18.6 ± 0.4* | 31 ± 2 | 3.9 ± 0.5 | 2.3 ± 0.5** | 59 ± 4** (good) |
| +/− | 326 ± 17 | 104 ± 3 | 19.8 ± 0.8 | 30 ± 2 | 3.5 ± 1.0 | 1.9 ± 0.4** | 60 ± 4** (good) |
| −/− | 323 ± 19 | 106 ± 2 | 21.1 ± 0.8 | 27 ± 1 | 3.6 ± 0.3 | 16.0 ± 2.6 | 23 ± 1 (poor) |
Mice from the 129 background were used at 24 weeks of age. n = 10 for each genotype. Values were significantly different from Tnp1−/− mice (Student's t test). *, P < 0.05; **, P < 0.005.
Figure 3.
Spermatozoa from a Tnp1−/− mouse (B6 × 129 F2). n, Normal sperm; arrowhead, sperm with blunted apex; arrow, dark-staining sperm; t, sperm with tails coiled around heads. (×900)
Tnp1-mutant males were mated with individual 7-week-old B6 females. The percentages that were fertile were significantly lower than those observed in wild types and heterozygotes (Table 2). Whereas the fertile Tnp1−/− males on the hybrid background had litter sizes equivalent to those of wild-type mice, those on the 129 background had significantly smaller litters.
Table 2.
Fecundity of the Tnp1-mutant males during an 8-week mating period
| Background (age at start of mating) | Tnp1 genotype | Fraction fertile, fertile/total | Litters per fertile pair, n | Mean litter size, n |
|---|---|---|---|---|
| B6 × 129 F2 (16 weeks) | +/+ | 10 /10** | 2.6 ± 0.2* | 6.6 ± 0.4 |
| +/− | 10 /10** | 2.1 ± 0.2 | 5.8 ± 0.5 | |
| −/− | 3 /10 | 1.7 ± 0.3 | 7.2 ± 1.2 | |
| 129 (9 weeks) | +/+ | 9 /10* | 2.4 ± 0.2* | 7.7 ± 0.3** |
| +/− | 10 /10** | 2.0 ± 0.1 | 7.5 ± 0.5** | |
| −/− | 4 /10 | 1.5 ± 0.3 | 1.6 ± 0.5 |
Values are significantly higher than those from Tnp1−/− (χ2 for fertility; Student's t test for other endpoints). *, P < 0.05; **, P < 0.005.
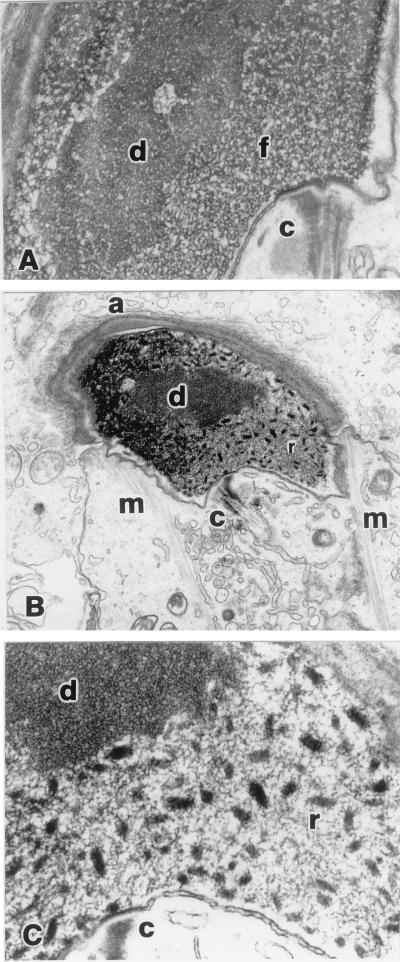
Light microscopic examination of testes of Tnp1−/− mice did not reveal any abnormalities except for retention of some sperm heads in stages IX–XI tubules, and chromatin condensation seemed completely normal. However, electron microscopy showed chromatin abnormalities beginning in step 12 in all Tnp1−/− mice tested. In addition to the fine fibrillar appearance of chromatin characteristic of wild-type mouse spermatids, rod-shaped chromatin condensation units (≈155 nm long and 65 nm across as measured in thin sections of randomly oriented units) appeared in the nuclei of all condensing spermatids from the Tnp1−/− mice (Fig. 4). After step 14, it became more difficult to observe the rod-shaped objects, because all of the chromatin became condensed and darkly stained (data not shown).
Figure 4.
Chromatin condensation in step 13 spermatids (B6 × 129 F2). (A) Wild type (×24,500). (B and C) Tnp1−/− mutant (×7,800 and ×39,000, respectively). Perinuclear structures are the centriolar region at the base of the flagellum (c), acrosome (a), and manchette (m). Within the nucleus, the central region of differential chromatin condensation (d), fibrillar chromatin (f), and rod-shaped regions of condensation (r) are indicated.
Nucleoprotein Transitions in Spermatids.
In steps 12 and 13, spermatids that were prepared from wild-type mice by elutriation followed by sonication, TP1 was the major protein, constituting 62% of the basic protein (Fig. 5A). TP2 was the next most abundant protein at 28%. P1 and P2 constituted only 10% of the protein, and it is likely that much of this was due to contamination by steps 14–16 spermatids, because no P2 precursors were detected. About 1% of the protein was histone.
Figure 5.
Acid/urea gel of nuclear proteins from enriched populations of steps 12 and 13 spermatids (A) and steps 12–16 spermatids (B) from wild-type, heterozygous, and Tnp1−/− mice (B6 × 129 F2). pP2 is the primary translation product of the Prm2 gene; pP2/11 and pP2/20 result from proteolytic cleavage before the 11th and 20th amino acids, respectively.
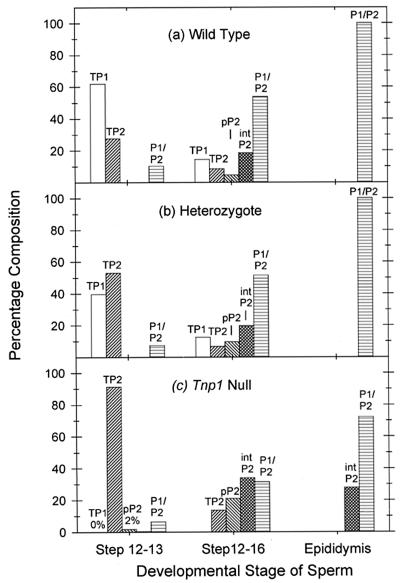
Analysis of SRS, which represent a combination of steps 12 and 13 and 14–16 spermatids, showed that these nuclei contained 74% protamines, 22% TPs, and <4% histones (Figs. 5B and 6). Because the sonication-resistant steps 12 and 13 spermatids represented 21% of the total SRS nuclei, the steps 14–16 spermatids must therefore have contained almost exclusively protamines and very little TPs. These results confirm that the TPs and the protamines normally appear sequentially in distinct stages.
Figure 6.
Percentage of basic nuclear proteins in developing sperm cells at different stages from wild-type (a), Tnp1+/− (b), and Tnp1−/− (c) mice (B6 × 129 F2).
Elevated TP2 Levels in Tnp1-Mutant Mice.
In steps 12 and 13 spermatids from Tnp1-null mice, the levels of histones were also very low (<4%; Fig. 5A), indicating that histone displacement occurred normally in the absence of TP1. The predominant protein was TP2, accounting for 91% of the basic nuclear protein. Full-length pP2 accounted for 2% of the protein in the Tnp1−/− mice in this experiment, whereas none was seen in the wild type or heterozygote (Fig. 6). In another experiment (data not shown), the level of pP2 in steps 12 and 13 spermatids was 11%. The pP2 is unlikely to be contamination from later spermatids because of the absence of the partially processed forms of P2 that are found in later stages. These results indicate some premature translation of the P2 mRNA in the Tnp1-null mice. The levels of P1 and mature P2, which may represent contamination from later stages, were not significantly different from those seen in wild type.
Abnormal P2 Processing in Tnp1-Mutant Mice.
Analysis of proteins from steps 12–16 spermatids confirmed that the level of histones, some of which could be contamination from other cells, was <6% in these cells in both heterozygote and Tnp1-null mice (Fig. 5B). The absence of TP1 and the elevation of TP2 in Tnp1-null mice, as compared with wild type and heterozygotes (Fig. 6), reflect events in steps 12 and 13 spermatids. The most noticeable nucleoprotein abnormality attributable to steps 14–16 spermatids of Tnp1-null mice was the elevation of P2 precursors (Fig. 6). In these mice, pP2 was 21% of the total nuclear protein, as compared with only 10% and 5% in heterozygous and wild-type males, respectively, and the ratio of pP2 to pP2/11 was higher (Fig. 5B), suggesting a deficiency in P2 processing.
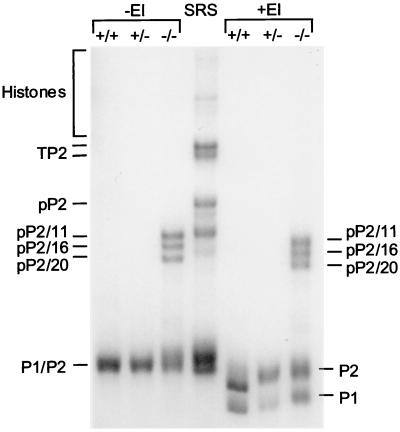
The precursor forms of P2 from the testes of Tnp1-null mice were further processed by the time the sperm reached the cauda epididymis, because the full-length precursor of P2 was absent and the major bands corresponded to pP2/11, pP2/16, and pP2/20 (Fig. 7). The precursors still constituted ≈30% of the protamines compared with undetectable levels in spermatozoa from wild-type and heterozygous mice (Fig. 6). The ratio of mature P2 to P1 was about 2 in wild-type and heterozygous mice, but seemed to be slightly reduced in the Tnp1-null mice. However, in these mice, the total amount of P2, including precursors, was about 3 times that of P1.
Figure 7.
Basic nuclear proteins from epididymal sperm from wild-type, heterozygous, and Tnp1−/− mice (129 background) and SRS from wild-type mice. Protein yields per cell were roughly similar for the three genotypes. Partially processed forms of P2 are labeled according to the convention in Fig. 5. That these bands are indeed protamines was confirmed by their insolubility in SDS (27). Part of the epididymal preparations were treated with ethyleneimine (+EI) (28) to separate P1 and the mature form of P2.
Discussion
TP1 is the major basic nuclear protein in steps 12 and 13 of mouse spermiogenesis. Therefore, the most surprising result from this study is that normal numbers of relatively normal sperm were produced in the Tnp1-null males, and, indeed, some of the mice were fertile. It seems that other nuclear proteins, primarily TP2, individually or in concert with pP2, were able to initiate chromatin condensation in steps 12 and 13 spermatids, even though the ultrastructural features were somewhat abnormal.
In addition, these proteins completely replaced histones in the developing sperm cells without the involvement of TP1. The present results suggest that the removal of histones may be an active process in step 12 spermatids and may generate a signal to synthesize basic chromatin proteins to neutralize the DNA. In Tnp1−/− mice, this removal might stimulate excess translation of TP2 and premature translation of pP2.
Thus, the signals that normally activate TP1 translation in step 12 spermatids can, in the absence of TP1, act on TP2 and P2 mRNA. This result, along with the fertility of the Tnp1−/− mice, suggests that TP1, although not part of the protamine gene cluster, is functionally related to TP2 and the protamines. Further support for the functional relationship among this family of four proteins is based on our preliminary observations of normal numbers of sperm produced and of fertility of most of the Tnp2−/− male mice (unpublished results).
The rod-shaped chromatin condensation units in the nuclei of the step 13 spermatids from the Tnp1-null males might be a consequence of the abnormal pP2 deposition. Toroidal DNA-protamine complexes of ≈80 nm in their major diameter have been observed in sperm (29) and in vitro reconstitution experiments (30). However, the rod-shaped objects observed here were larger and did not appear to have hollow centers. Although the abnormal chromatin condensation observed in Tnp1-null mice seemed not to affect the overall shape of the mature sperm nucleus, it may have contributed to generating the blunted tips of the sperm heads by reducing the rigidity of the fine apex of the sperm.
The deficiency observed in the processing of pP2 in the Tnp1-null mice was not as severe as in transgenic mice with premature translation of Prm1 mRNA, in which no mature P2 was formed (31). Our results are compatible either with pP2 being synthesized before the processing proteases appear or with partial saturation of the proteases by high levels of pP2, as well as with the previous suggestion (31) that processing of pP2 depends on its normal codeposition with P1. The Tnp1-mutant mice should be useful for studying the processing steps of the P2 precursors, which occur both in the testis and during epididymal transit (14).
Lack of TP1 reduces fertility, as indicated both by the decrease in the number of litters produced and, in the case of the inbred 129 males, by a decrease in litter size by 70% (Table 2). The reduced fertility was most likely a result of the poor motility, although abnormal sperm head morphology may also have contributed. The poor motility of sperm from Tnp1-null mice was unexpected, because TP1 is a chromosomal protein; we speculate that the disruption of the normal nuclear protein transition has some as yet unknown secondary effects on cytoplasmic structures or composition. The reduced fertility in Tnp1−/− mice on both the B6 × 129 F2 and 129 backgrounds is in agreement with the similar biochemical, quantitative, and morphological phenotypes observed on the different backgrounds.
Abnormality levels of P2 in sperm, including a decrease (32), an absence (33), and an aberrant ratio to P1 (34), have been implicated in human infertility. In addition, as in Tnp1-null mice, high levels of P2 precursors were detected in sperm of infertile men, whereas these levels were generally very low in fertile men (35). Our data suggest that defects in TP1 could be the cause of the elevated P2 precursors in spermatozoa. Although one study of the Tnp1 gene in 46 cases of male infertility failed to detect mutations (36), the phenotype of the Tnp1-null mice suggests that further studies are warranted.
Acknowledgments
We thank Drs. M. Zhao and B. Mohapatra for performing some experiments, R. Braun for discussions leading to these studies, K. Kleene for providing a Tnp1 cDNA plasmid, and W. Pagel for editorial assistance. We dedicate this paper to the memory of Miss Julia Yu. This work was supported by National Institutes of Health Grant HD-16843 and Core Grant CA-16672.
Abbreviations
- TP
transition nuclear protein
- P1
protamine 1
- P2
protamine 2
- ES
embryonic stem
- SRS
sonication-resistant spermatid
- TCA
trichloroacetic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kierszenbaum A L, Tres L L. J Cell Biol. 1975;65:258–270. doi: 10.1083/jcb.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliva R, Dixon G H. Prog Nucleic Acid Res Mol Biol. 1991;40:25–94. doi: 10.1016/s0079-6603(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 3.Meistrich M L. In: Histones and Other Basic Nuclear Proteins. Hnilica L S, Stein G S, Stein J L, editors. Orlando, FL: CRC; 1989. pp. 165–182. [Google Scholar]
- 4.Wouters-Tyrou D, Martinage A, Chevaillier P, Sautiere P. Biochimie. 1998;80:117–128. doi: 10.1016/s0300-9084(98)80018-7. [DOI] [PubMed] [Google Scholar]
- 5.Grimes S R, Meistrich M L, Platz R D, Hnilica L S. Exp Cell Res. 1977;110:31–39. doi: 10.1016/0014-4827(77)90266-x. [DOI] [PubMed] [Google Scholar]
- 6.Heidaran M A, Showman R M, Kistler W S. J Cell Biol. 1988;106:1427–1433. doi: 10.1083/jcb.106.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleene K C, Borzorgzadeh A, Flynn J F, Yelick P C, Hecht N B. Biochim Biophys Acta. 1988;950:215–220. doi: 10.1016/0167-4781(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 8.Kremling H, Luerssen H, Adham I M, Klemm U, Tsaousidon S, Engel W. Differentiation (Berlin) 1989;40:184–190. doi: 10.1111/j.1432-0436.1989.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 9.Kistler W S, Henriksén K, Mali P, Parvinen M. Exp Cell Res. 1996;225:374–381. doi: 10.1006/excr.1996.0188. [DOI] [PubMed] [Google Scholar]
- 10.Unni E, Meistrich M L. J Biol Chem. 1992;267:25359–25363. [PubMed] [Google Scholar]
- 11.Gusse M, Sautière P, Bélaiche D, Martinage A, Roux C, Dadoune J-P, Chevaillier P. Biochim Biophys Acta. 1986;884:124–134. doi: 10.1016/0304-4165(86)90235-7. [DOI] [PubMed] [Google Scholar]
- 12.Bellvé A R. Biochemistry. 1988;27:2890–2897. doi: 10.1021/bi00408a034. [DOI] [PubMed] [Google Scholar]
- 13.Yelick P C, Balhorn R, Johnson P A, Corzett M, Mazrimas J A, Kleene K C, Hecht N B. Mol Cell Biol. 1987;7:2173–2179. doi: 10.1128/mcb.7.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debarle M, Martinage A, Sautiere P, Chevaillier P. Mol Reprod Dev. 1995;40:84–90. doi: 10.1002/mrd.1080400111. [DOI] [PubMed] [Google Scholar]
- 15.Schlüter G, Celik A, Obata R, Schlicker M, Hofferbert S, Schlung A, Adham I M, Engel W. Mol Reprod Dev. 1996;43:1–6. doi: 10.1002/(SICI)1098-2795(199601)43:1<1::AID-MRD1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Singh J, Rao M R S. J Biol Chem. 1987;262:734–740. [PubMed] [Google Scholar]
- 17.Singh J, Rao M R S. Biochem Int. 1988;17:701–710. [PubMed] [Google Scholar]
- 18.Kundu T K, Rao M R S. Biochemistry. 1996;35:15626–15632. doi: 10.1021/bi961271i. [DOI] [PubMed] [Google Scholar]
- 19.Steger K, Klonisch T, Gavenis K, Behr R, Schaller V, Drabent B, Doenecke D, Nieschlag E, Bergmann M, Weinbauer G F. J Androl. 1999;20:747–754. [PubMed] [Google Scholar]
- 20.Gendron-Maguire M, Gridley T. Methods Enzymol. 1993;225:794–799. doi: 10.1016/0076-6879(93)25051-3. [DOI] [PubMed] [Google Scholar]
- 21.Platz R D, Meistrich M L, Grimes S R J. Methods Cell Biol. 1977;16:267–316. doi: 10.1016/s0091-679x(08)60107-7. [DOI] [PubMed] [Google Scholar]
- 22.Meistrich M L. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- 23.Grabske R J, Lake S, Gledhill B L, Meistrich M L. J Cell Physiol. 1975;86:177–190. doi: 10.1002/jcp.1040860119. [DOI] [PubMed] [Google Scholar]
- 24.Meistrich M L, Reid B O, Barcellona W J. Exp Cell Res. 1976;99:72–78. doi: 10.1016/0014-4827(76)90681-9. [DOI] [PubMed] [Google Scholar]
- 25.Balhorn R, Gledhill B L, Wyrobek A J. Biochemistry. 1977;16:4074–4080. doi: 10.1021/bi00637a021. [DOI] [PubMed] [Google Scholar]
- 26.Sprando R L. In: Histological and Histopathological Evaluation of the Testis. Russell L D, Ettlin R A, Sinha Hikim A P, Clegg E D, editors. Clearwater, FL: Cache River Press; 1990. pp. 277–280. [Google Scholar]
- 27.Elsevier S M, Noiran J, Carré-Eusèbe D. Eur J Biochem. 1991;196:167–175. doi: 10.1111/j.1432-1033.1991.tb15800.x. [DOI] [PubMed] [Google Scholar]
- 28.Balhorn R, Weston S, Thomas C, Wyrobek A J. Exp Cell Res. 1984;150:298–308. doi: 10.1016/0014-4827(84)90572-x. [DOI] [PubMed] [Google Scholar]
- 29.Allen M J, Lee C, Lee J D, IV, Pogany G C, Balooch M, Sickhaus W J, Balhorn R. Chromosoma. 1993;102:623–630. doi: 10.1007/BF00352310. [DOI] [PubMed] [Google Scholar]
- 30.Allen M J, Bradbury E M, Balhorn R. Nucleic Acids Res. 1997;25:2221–2226. doi: 10.1093/nar/25.11.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K, Haugen H S, Clegg C H, Braun R E. Proc Natl Acad Sci USA. 1995;92:12451–12455. doi: 10.1073/pnas.92.26.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belokopytova I A, Kostyleva E I, Tomilin A N, Vorob'ev V I. Mol Reprod Dev. 1993;34:53–57. doi: 10.1002/mrd.1080340109. [DOI] [PubMed] [Google Scholar]
- 33.de Yebra L, Ballescà J L, Vanrell J A, Bassas L, Oliva R. J Biol Chem. 1993;268:10553–10557. [PubMed] [Google Scholar]
- 34.Balhorn R, Reed S, Tanphaichitr N. Experientia. 1988;44:52–55. doi: 10.1007/BF01960243. [DOI] [PubMed] [Google Scholar]
- 35.de Yebra L, Ballescá J-L, Vanrell J A, Corzett M, Balhorn R, Oliva R. Fertil Steril. 1998;69:755–759. doi: 10.1016/s0015-0282(98)00012-0. [DOI] [PubMed] [Google Scholar]
- 36.Schlicker M, Schnulle V, Schneppel L, Vorob'ev V I, Engel W. Hum Reprod. 1994;9:2313–2317. doi: 10.1093/oxfordjournals.humrep.a138444. [DOI] [PubMed] [Google Scholar]