Abstract
Background
It is hypothesized that low selenium concentrations are associated with an increased risk of cardiovascular disease and that selenium supplements prevent coronary heart disease.
Objective
The objective was to perform a meta-analysis on the association of selenium biomarkers with coronary heart disease endpoints in observational studies and on the efficacy of selenium supplements in preventing coronary heart disease endpoints in randomized trials.
Design
The MEDLINE and the Cochrane Library databases were searched for studies conducted from 1966 through 2005. Relative risks were pooled by using an inverse-variance weighted random-effects model.
Results
Twenty-five observational studies (14 cohort and 11 case-control studies) that measured blood or toenail selenium concentrations and 6 randomized trials that evaluated supplements containing selenium met our inclusion criteria. The pooled relative risk in a comparison of the highest with the lowest selenium concentration categories was 0.85 (95% CI: 0.74, 0.99) in cohort studies and 0.43 (0.29, 0.66) in case-control studies. In observational studies, a 50% increase in selenium concentrations was associated with a 24% (7%, 38%) reduction in coronary heart disease risk. In randomized trials, the pooled relative risk in a comparison of supplements containing selenium with placebo was 0.89 (0.68, 1.17).
Conclusions
Selenium concentrations were inversely associated with coronary heart disease risk in observational studies. Because observational studies have provided misleading evidence for other antioxidants, the validity of this association is uncertain. Few randomized trials have addressed the cardiovascular efficacy of selenium supplementation, and their findings are still inconclusive. Evidence from large ongoing trials is needed to establish low selenium concentrations as a cardiovascular disease risk factor. Currently, selenium supplements should not be recommended for cardiovascular disease prevention.
Keywords: Selenium, coronary heart disease, atherosclerosis, meta-analysis, systematic review
INTRODUCTION
Selenium is an essential trace mineral involved in protection against oxidative damage via selenium-dependent glutathione peroxidases and other selenoproteins (1). Current recommendations on dietary intake of selenium are based on optimizing the activity of plasma glutathione peroxidases (2). The recommended dietary allowance for selenium that is estimated to be sufficient to meet the nutritional needs of nearly all healthy adults is 55 μg/d (2, 3). Plant foods, meat, and seafood are the major dietary sources of selenium, predominantly as selenomethionine and selenocysteine, but the selenium content of foods varies geographically depending on soil and water concentrations and use of selenium-containing fertilizers (4–8). For this reason, dietary assessment methods are inappropriate for estimating selenium exposure (6) and observational studies of selenium status are based on biomarkers such as toenail, blood, erythrocyte, or serum and plasma selenium concentrations (7–9).
Because of its antioxidant properties, it has long been hypothesized that selenium may prevent cardiovascular and other chronic diseases. Selenium supplementation increases enzymatic antioxidant activity (10–12) and decreases lipid peroxidation (12–14). The effect of selenium on atherosclerotic cardiovascular disease, however, is uncertain. Observational studies (15–28) investigating the association of low selenium concentrations with cardiovascular outcomes and randomized trials (14, 29–33) investigating whether selenium supplements prevent coronary heart disease have been inconclusive, but the evidence has not been appraised systematically.
The objective of the present meta-analysis was to synthesize results from observational studies of the association of selenium biomarkers with coronary heart disease endpoints and from results of clinical trials of the efficacy of selenium supplements in preventing coronary heart disease endpoints.
METHODS
We searched MEDLINE for observational studies and randomized trials investigating the relation of selenium with coronary heart disease. We used free text and the Medical Subject Headings (MeSH) terms “selenium,” “selenite,” “selenate,” “cardiovascular disease,” “Khesan disease,” “myocardial infarction,” “stroke,” “peripheral arterial disease,” and “mortality.” The search period was January 1966 through March 2006; no language restrictions were added. We also searched the Cochrane Central Register of Controlled Trials and reviewed the reference lists of relevant original papers and review articles.
We aimed to identify all observational studies that assessed the association of selenium concentrations in blood or toenails with clinical coronary heart disease outcomes and all randomized trials that assessed the efficacy of selenium supplements, either alone or in combination with other vitamins or minerals, for preventing coronary heart disease ( Figure 1). Our exclusion criteria were the following: 1) no original research (reviews, editorials, nonresearch letters); 2) studies not conducted in humans; 3) case reports or case series; 4) ecologic studies; 5) lack of data on selenium exposure; 6) studies of angiographically defined endpoints or of angina pectoris as the endpoint; 7) studies of other cardiovascular outcomes such as heart failure, stroke, peripheral arterial disease, or nonatherosclerotic heart disease; and 8) observational studies conducted in populations of patients with coronary heart disease at baseline. We additionally excluded a small autopsy-based study (21 case and 22 control subjects) that did not measure any of the standard selenium biomarkers (34). For populations originating several reports, the publication with the longest follow-up was selected (26, 33, 35).
FIGURE 1.
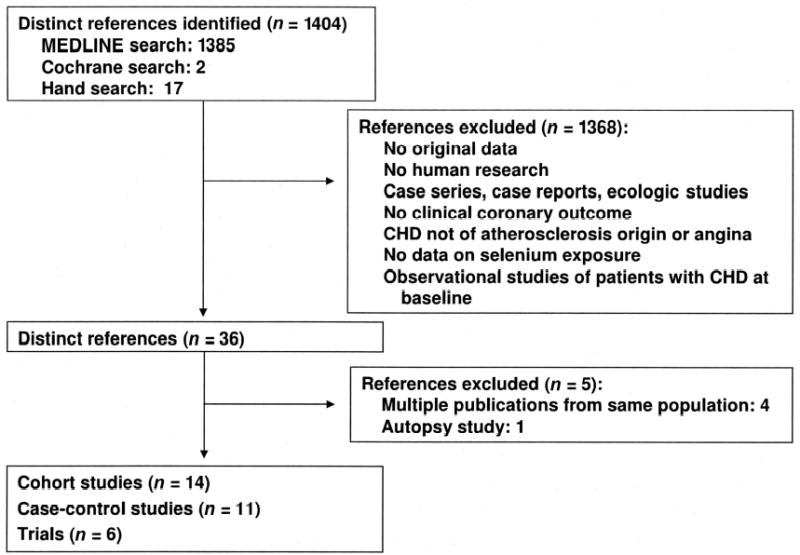
Flow diagram of study selection process. CHD, coronary heart disease.
Two investigators (GF-M and AN-A) independently reviewed search results and selected articles to determine eligibility and to abstract study data. They resolved discrepancies by consensus. The investigators of the original studies were contacted if relevant information on eligibility or key study data were not available in the published report. For observational studies, the criteria used by Longnecker et al (36) were adapted to assess study quality (Appendix A). For randomized trials, we used the quality criteria of Jadad et al (37).
The a priori selected endpoint was coronary heart disease, which was defined as any combination of fatal or nonfatal coronary heart disease and myocardial infarction. Studies reporting only total cardiovascular endpoints were also included, because coronary heart disease is the major contributor to cardiovascular disease in many populations.
Statistical analysis
Observational studies and randomized trials were analyzed separately. For observational studies, measures of association (odds ratios, relative risks, or hazard ratios) and their 95% CIs were abstracted or derived by using data reported in the publications. When several measures of association were reported, we selected the measure obtained from the model with the highest number of categories for selenium exposure first and the measure adjusted for most covariates second. For studies that categorized selenium exposure, we compared the risk of coronary heart disease in the highest with the lowest selenium category. For one study that analyzed selenium only as a continuous variable (25), we derived the relative risk associated with an increase of one SD in selenium concentrations in noncase subjects. For studies reporting only mean selenium concentrations in case and noncase subjects (16, 28, 38–47), we used linear discriminant function methods (48) to calculate the relative risk in a comparison of the 75th to the 25th percentiles of the selenium distribution in non-case subjects, assuming a normal distribution for selenium.
To pool relative risk estimates from individual studies, we used an inverse-variance weighted random-effects model. Heterogeneity was quantified with the I2 statistic (49), which describes the proportion of total variation in study estimates due to heterogeneity. We used meta-regression to evaluate whether results were different by selenium concentrations in the reference category (> or <70 μg selenium/L), study design (cohort compared with case-control), selenium biomarker (serum compared with other), outcome (mortality only compared with mortality or morbidity outcomes), or country (European compared with other). Because study design was the only significant determinant of heterogeneity, we separated the analyses for prospective cohort and case-control studies.
For observational studies that reported ≥3 categories of exposure, we additionally conducted a random-effects dose-response meta-analysis using the methods of Greenland and Longnecker (50). Because selenium concentrations in the reference categories differed across studies, study-specific results were pooled in terms of relative changes in selenium concentrations with respect to the reference category. We evaluated departures from the linear trend by testing for a quadratic term in the dose-response meta-analysis (50).
Clinical trials were analyzed according to the intention-to-treat principle. We computed relative risks and 95% CIs of coronary heart disease in a comparison of participants assigned to supplements containing selenium with those assigned to control supplements. We used an inverse-variance weighted random-effects model to pool relative risk estimates.
For both observational studies and clinical trials, we assessed the relative influence of each study on pooled estimates by omitting one study at a time. Finally, we assessed publication bias using funnel plots (51). Statistical analyses were conducted with Stata version 8 (STATA Corp, College Station, TX) and with S-PLUS version 7 (Insightful Corporation, Seattle, WA).
RESULTS
Meta-analysis of observational studies
Fourteen prospective cohort studies (15–28) ( Table 1) and 11 case-control studies (38, 40–47, 52, 53) ( Table 2) met our inclusion criteria (Figure 1). The studies were published between 1982 and 2005. Most studies, except 4 (23, 26, 27, 52), were performed in Europe. The number of case subjects varied between 22 (28) and 683 (53). One cohort study (28) and 7 case-control studies (38, 40, 43– 47) did not control for potential confounders. Cohort studies tended to fulfill pre specified quality criteria, whereas case-control studies varied widely (Appendix 1).
TABLE 1.
Prospective cohort studies of selenium and coronary heart disease (CHD)1
| Selenium concentration |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year | Country | Population | Men | Mean age | Endpoint ascertainment | Follow-up | Outcome | No. of case subjects /noncase subjects | Selenium assessment (technique) | Case subjects | Noncase subjects |
| % | y | y | μg/L | ||||||||
| Salonen, 1982 (15) | Finland | General population Eastern Finland | 73 | 50 | Hospital records, death certificate | 7 | CHD mortality | 95/95 | Serum (AAS) | 51.8 ± 13.82 | 55.3 ± 14.7 |
| Miettinen, 1983 (16) | Finland | Men with high CVD risk | 100 | 48 | Chest pain, cardiac enzyme, ECG | 5–7 | AMI incidence | 33/64 | Serum (AAS) | 71.6 ± 13.7 | 72.9 ± 14.4 |
| Virtamo, 1985 (17) | Finland | Rural men | 100 | 55–74 | Clinical exam, death certificate, ECG | 5 | CHD mortality | 30/591 | Serum (AAS) | NR | NR |
| Salonen, 1985 (18) | Finland | Eastern Finland Heart Survey | 75 | 54 | Death certificate | 5 | CHD mortality | 92/92 | Serum (AAS) | 62.0 | 68.0 |
| Ringstad, 1986 (19) | Norway | First Tromsø Heart Study | 100 | 20–49 | Death certificate or chest pain, enzyme, ECG | 8 | AMI incidence | 99/99 | Serum (AAS) | 130.7 ± 21.2 | 125.9 ± 22.0 |
| Kok, 1987 (20) | Netherlands | General population | 56 | 67 | Death certificate | 9 | CVD mortality | 84/168 | Serum (NAA) | 125.1 3 28.4 | 126.5 3 28.5 |
| Ringstad, 1987 (21) | Norway | Second Tromsø Heart Study | 100 | 46 | Hospital records, death certificates | 6 | AMI incidence | 59/59 | Serum (AAS) | 123.6 ± 16.5 | 127.7 ± 21.4 |
| Suadicani 1992 (22) | Denmark | Copenhagen Male Study | 100 | 63 | Hospital records, death Certificates | 3 | CHD incidence | 107/2715 | Serum (AAS) | 92.1 ± 22.0 | 93.7 ± 21.2 |
| Salvini, 1995 (23) | USA | Physicians’ Health Study | 100 | 40–84 | Questionnaires, hospital records, death certificates | 5 | AMI incidence | 186/186 | Serum (NAA) | 114.4 ± 15.13 | 113.2 ± 15.73 |
| Marniemi, 1998 (24) | Finland | General elderly population | 53 | ≥65 | Death certificates | 13 | CVD mortality | 142/202 | Serum (AAS) | 78.1 ± 23.0 | 79.5 ± 25.2 |
| Kilander, 2001 (25) | Sweden | Men born in Uppsala in 1920–1924 | 100 | 50 | Death certificate | 25 | CVD mortality | 301/1727 | Serum (AAS) | NR | NR |
| Yoshizawa, 2003 (26) | USA | Health Professionals Follow-Up Study | 100 | 62 | Questionnaires, medical records, death certificates | 5 | CHD incidence | 470/465 | Toenails (NAA) | 0.95 ± 0.433 | 0.93 ± 0.293 |
| Wei, 2004 (27) | China | General population trial of Linxian | 55 | 57 | Monthly follow-up | 15 | CHD mortality | 116/987 | Serum (AAS) | NR | NR |
| Akbaraly, 2005 (28) | France | Etude du Vieillissement Arteriel (EVA) | 41 | 65 | Death certificate, Hospital records | 9 | CVD mortality | 22/1367 | Serum (AAS) | 83.7 ± 15.7 | 86.0 ± 15.7 |
AAS, atomic absorption spectroscopy; ECG, electrocardiogram; AMI, acute myocardial in fraction; NR, not reported; CVD, cardiovascular disease; NAA, neutron activation analysis.
± SD (all such values).
In μg/g.
TABLE 2.
Case-control studies of selenium and coronary heart disease (CHD)1
| Selenium concentration2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study, year | Country | Percentage of men among control subjects | Mean age of control subjects | Type of control subjects | Source of case subjects | Outcomes | No. of case subjects/control subjects | Selenium assessment (technique) | Case subjects | Control subjects |
| % | y | |||||||||
| Oster, 1986 (38) | Germany | 100 | 52 | University employees | University health care center | AMI incidence | 49/41 | Serum (AAS) | 56.0 ± 15.03 | 78.0 ± 11.0 |
| Auzepy, 1987 (40) | France | 60 | 34 | Nursing and medical staff | Hospital | AMI incidence | 31/48 | Serum (AAS) | 73.6 ± 13.0 | 84.7 ± 12.4 |
| Salonen, 1988 (41) | Finland | 100 | 54 | Kuopio Ischemic Heart Disease Study | Kuopio Ischemic Heart Disease Study | CHD prevalence | 175/449 | Serum (AAS) | 81.5 ± 19.2 | 85.0 ± 21.6 |
| Kok, 1989 (42) | Netherlands | 70 | 59 | General population | Hospital | AMI incidence | 84/84 | Plasma | 100.8 ± 27.5 | 106.8 ± 23.8 |
| Erythrocytes | 0.54 ± 0.094 | 0.59 ± 0.184 | ||||||||
| Toenail (NAA) | 0.70 ± 0.185 | 0.78 ± 0.185 | ||||||||
| Beaglehole, 1990 (52) | New Zealand | 60 | 52 | General population | Monica Project Registry | AMI incidence | 252/838 | Whole blood (fluorimetry) | 82.7 ± 20.2 | 88.2 ± 20.7 |
| Thiele, 1995 (43) | Germany | NR | NR | Healthy blood donors | Hospital | AMI incidence | 83/62 | Serum (AAS) | 71.0 ± 13.4 | 78.9 ± 13.4 |
| Whole blood | 86.8 ± 15.8 | 105.8 ± 13.4 | ||||||||
| Kardinaal, 1997 (53) | 8 European countries and Israel | 100 | 53 | General population and clinic based | Coronary unit | AMI incidence | 683/729 | Toenail (NAA) | 0.55 ± 0.49–0.695,6 | 0.59 ± 0.49–0.725,6 |
| Coudray, 1997 (44) | France | 40 | 65 | General population | Surveys | AMI prevalence | 36/498 | Plasma (AAS) | 88.4 ± 16.6 | 85.2 ± 15.0 |
| Navarro- Alarcon, 1999 (45) | Spain | NR | NR | NR | Hospital | CHD prevalence | 50/130 | Serum (AAS) | 55.5 ± 16.7 | 74.9 ± 27.3 |
| Bor, 1999 (46) | Turkey | 83 | 51 | NR | Emergency room | AMI incidente | 27/24 | Plasma | 63.7 ± 12 | 82.2 ± 14.6 |
| Erythrocytes (fluorimetry) | 0.48 ± 0.044 | 0.51 ± 0.034 | ||||||||
| Zachara, 2001 (47) | Poland | 62 | 57 | NR | Coronary unit | AMI incidente | 49/58 | Plasma | 53.8 ± 18.3 | 52.5 ± 13.6 |
| Whole blood (fluorimetry) | 71.4 ± 18.2 | 73.1 ± 18.0 | ||||||||
AMI, acute myocardial infarction; AAS, atomic absorption spectroscopy; NAA, neutron activation analysis.
Measured in μg/L, unless otherwise specified.
± SD (all such values).
Measured in μg/g hemoglobin.
Measured in μg/g.
Median (25th and 75th percentiles).
Except for 3 cohort (21, 23, 24) and 2 case-control (44, 47) studies, most studies found an inverse association of selenium with the risk of coronary heart disease ( Figure 2). The pooled relative risk in a comparison of the highest to the lowest category of selenium concentration was 0.85 in cohort studies (95% CI: 0.74, 0.99; P for heterogeneity = 0.33; I2 = 5%) and 0.43 in case-control studies (95% CI: 0.29, 0.66; P for heterogeneity < 0.001; I2 = 88%). Other sources of heterogeneity investigated, including the influence of selenium concentrations of the reference category, were minor and not statistically significant. Specifically, we used a meta-regression model to evaluate whether the relative risk of coronary heart disease in a comparison of the highest and lowest categories of selenium exposure were similar in studies with plasma or serum selenium concentrations in the reference category > or <70 μg/L. The relative risks in both types of studies were similar and the difference was not statistically significant (difference in log relative risk: 0.07; 95% CI:−0.51, 0.64; P = 0.82).
FIGURE 2.
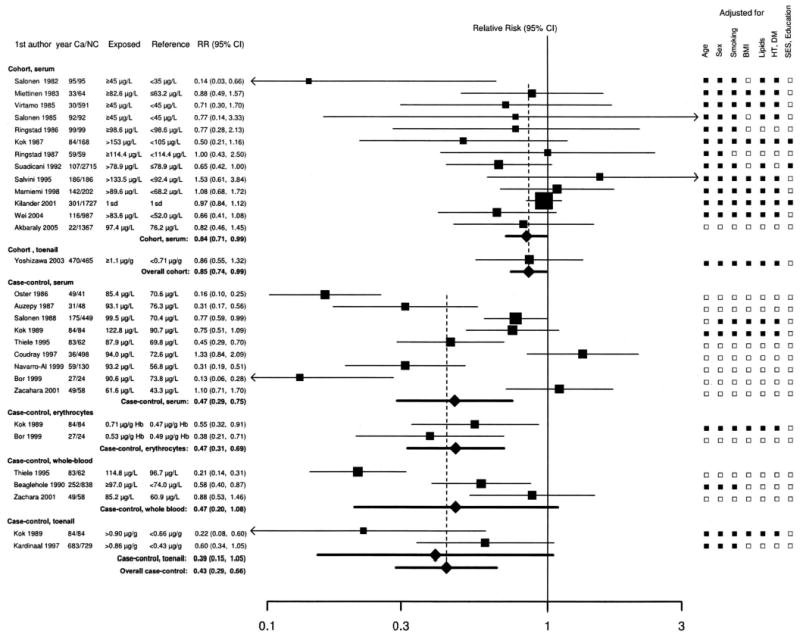
Meta-analysis of the association of selenium with coronary heart disease in observational studies. Studies are divided by study design (cohort or case-control) and by selenium biomarker (serum, toenail, erythrocyte, or whole blood). Relative risks (RRs) correspond to comparisons of extreme categories of exposure within each study. The area of each square is proportional to the inverse of the variance of the log RR. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from inverse-variance weighted random-effects models. For case-control studies with multiple biomarkers, we used the biomarker with the longest half-life (toenail > whole blood and erythrocyte > serum) to measure the overall RR. Ca, case subjects; NC, noncase subjects; DM, diabetes mellitus; HT, hypertension; SES, socioeconomic status; Hb, hemoglobin. ▪ Indicates categories that were adjusted for; □indicates categories that were not adjusted for.
In sensitivity analyses, exclusion of individual studies did not modify the estimates substantially, with pooled relative risks ranging from 0.78 to 0.90 in cohort studies and from 0.41 to 0.59 in case-control studies. Funnel plots did not suggest the presence of publication or related biases (not shown).
For studies with ≥3 selenium categories, the dose-response meta-analysis showed a decreasing trend of coronary heart disease risk with increasing selenium concentrations ( Figure 3). The pooled relative risk associated with a 50% increase in selenium concentrations was 0.76 (95% CI: 0.62, 0.93; P for heterogeneity = 0.06). Adding a quadratic term to the model did not significantly improve model fit (P = 0.64).
FIGURE 3.
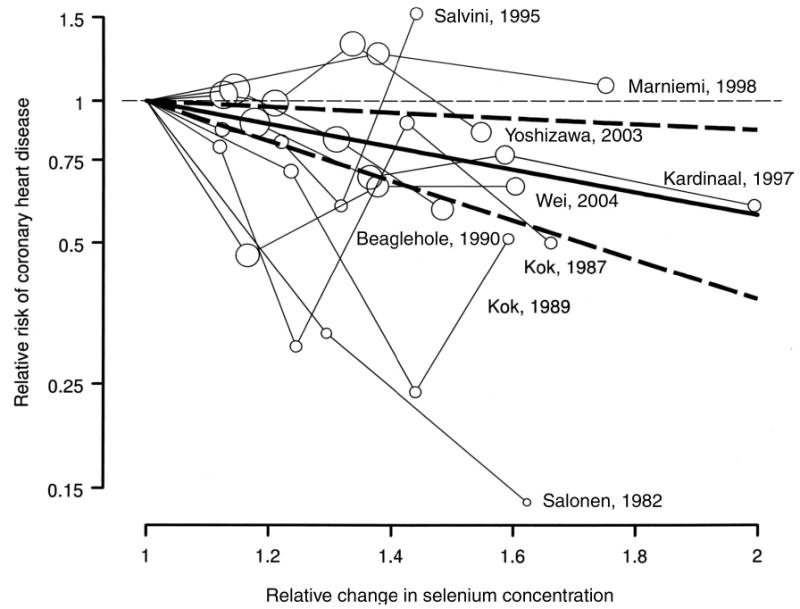
Dose-response meta-analysis of selenium and coronary heart disease in observational studies (shown by first author and year of publication). The pooled linear risk trend (thick solid line) and its 95% CI (dashed lines) were obtained by a random-effects dose-response meta-analysis. Circles are inversely proportional to the variance of log relative risks.
Meta-analysis of randomized trials
Six trials (14, 29–33), published between 1989 and 2004, met our inclusion criteria ( Table 3). These trials randomly assigned a total of 17 766 participants. Four trials used selenium combined with other vitamins or minerals (14, 30, 32, 54), and 2 trials used selenium alone (29, 33). Selenium doses were 75 μg/d (54), 100 μg/d (14, 29, 30, 32), or 200 μg/d (33). Only one trial used selenite (30), whereas 3 trials used selenium yeast (29, 32, 33). In 2 trials, the form of selenium was not specified. All trials were placebo-controlled, and all except one (30) were double-blinded. The length of follow-up ranged from 0.5 to 7.6 y.
TABLE 3.
Randomized trials of selenium supplementation and risk of coronary heart disease (CHD)1
| First author, year | Country | Population | Men | Mean age | Selenium form (dose μg/d) | Selenium combined with other vitamins or minerals | Factorial design (factorial intervention) | Placebo-controlled | Double-blind | Follow-up | Outcomes | Quality score2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | y | y | ||||||||||
| Korpela, 1989 (29) | Finland | Patients with AMI | 77 | 57 | Selenium yeast (100) | No | No | Yes | Yes | 0.5 | CHD incidence | 2 |
| Kuklinski, 1994 (30) | Germany | Patients with AMI | NR | NR | Sodium selenite (100) | Yes (100 mg coenzyme Q10, 15 mg Zn, 1 mg vitamin A, 2 mg vitamin B-6, 90 mg vitamin C, 15 mg vitamin E) | No | Yes | No | 1.0 | AMI mortality | 1 |
| Brown, 2001 (14) | Canada and USA | Patients with CHD | 87 | 53 | Selenium yeast (100) | Yes (800 IU vitamin E, 1000 mg vitamin C, 25 mg β-carotene) | Yes (10 mg simvastatin, 250–1000 mg niacin) | Yes | Yes | 3.2 | CVD incidence | 5 |
| You, 2001 (31) and Gaul, 1998 (54) | China | Residents in Linqu | 51 | 47 | NR (75) | Yes (200 IU vitamin E, 500 mg vitamin C, 15 mg β-carotene) | Yes (800 mg garlic extract, 4 mg garlic oil) | Yes | Yes | 3.3 | CVD mortality | 5 |
| Hercberg, 2004 (32) | France | Healthy adults | 39 | 48 | Selenium yeast (100) | Yes (30 mg vitamin E, 120 mg vitamin C, 6 mg β-carotene, 20 mg Zn) | No | Yes | Yes | 7.5 | CHD incidence | 5 |
| Stranges, 2006 (33) | USA | Patients with skin carcinoma and CVD- Free | 71 | 62 | Selenium yeast (200) | No | No | Yes | Yes | 7.6 | CHD incidence | 5 |
AMI, acute myocardial infarction; NR, not reported; CVD, cardiovascular disease.
Quality score based on criteria by Jadad et al (37). Score ranges from 0 (lowest quality) to 5 (highest quality).
The pooled relative risk in a comparison of selenium supplementation to placebo across all trials was 0.89 (95% CI: 0.68, 1.17; P for heterogeneity = 0.22; I2 = 40%) ( Figure 4). Exclusion of any individual trial did not substantially change the overall pooled relative risk estimates, which ranged from 0.63 to 0.92.
FIGURE 4.
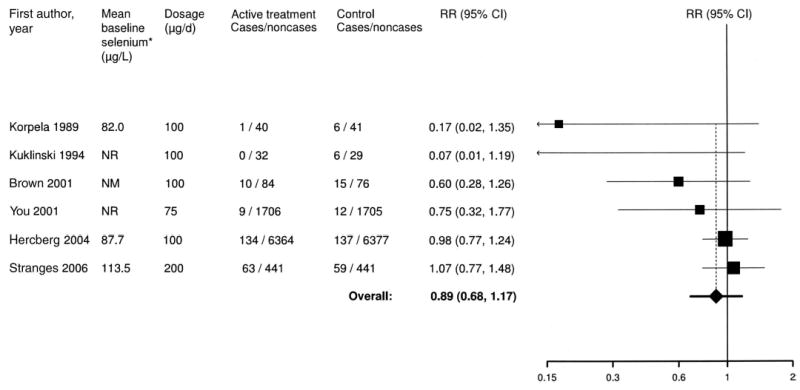
Meta-analysis of selenium and coronary heart disease in randomized trials. *Baseline selenium concentrations were measured in serum (Korpela et al 1989 and Hercberg et al 2004 studies) and plasma (Stranges et al 2006 study). NM, not measured; NR, not reported; RR, relative risk.
DISCUSSION
In the present meta-analysis, we identified a moderate but statistically significant inverse association between selenium concentrations in several tissues and coronary heart disease outcomes in observational studies. A 50% increase in selenium concentrations was associated with a 24% reduced risk of coronary events. The validity of this association, however, is uncertain, because observational studies have been unreliable in determining the cardiovascular effects of other antioxidants and vitamins, such as β-carotene, vitamin E, and folate (55). Few randomized controlled trials have addressed the effect of selenium supplementation on clinical endpoints. In these trials, participants taking supplements containing selenium had a nonsignificant 11% reduction in coronary events, but the trials were small and selenium was given in combination with other vitamins or minerals in all but 2 trials. Overall, the evidence is still inadequate to establish a protective role of selenium in coronary heart disease.
Biological plausibility
Selenium, a constituent of selenoproteins as selenocysteine, has important antioxidant properties (1, 56, 57). Selenoproteins with antioxidant functions include glutathione peroxidases, which reduce hydrogen peroxide and lipid and phospholipid hydroperoxides; thioredoxin reductases, which help regenerate antioxidant systems and maintain the intracellular redox status (1); and selenoprotein P, which may protect endothelial cells against peroxynitrite and lipid peroxidation (58, 59). In selenium-deficient humans, selenium supplementation increases enzymatic antioxidant activity (10–12, 60) and decreases lipid peroxidation (12–14). In addition, selenium may reduce the production of inflammatory prostaglandins and leukotrienes by neutralizing peroxide intermediates (1).
Low selenium concentrations may also increase cardiovascular disease risk through other mechanisms. By shifting prostaglandin synthesis from prostacyclin to thromboxane, low selenium may increase platelet aggregability and vasoconstriction (1, 56, 61). Randomized trials of selenium supplementation on platelet function, blood pressure levels, and lipid profile, however, have been contradictory (12, 14, 62, 63). Finally, selenium may protect the cardiovascular system from toxic metals that have been implicated in atherogenesis, such as mercury, cadmium, and arsenic, by preventing metal-induced oxidative damage or by forming inactive complexes with metals (56, 64, 65).
Selenium supplementation decreased the incidence of Keshan disease, a congestive cardiomiopathy that mostly affects children and young women in some selenium-poor areas of China (1, 66). However, whether selenium deficiency results in increased atherosclerosis is unclear (1, 56, 67).
Low selenium concentration as a cardiovascular disease risk factor
Biomarkers of selenium, such as toenail, blood, erythrocyte, and serum or plasma selenium concentrations (7–9), have all been shown to reflect selenium exposure (7, 8). However, the interpretation of biomarkers is complex because selenium concentrations depend not only on exposure, but also on the form of selenium intake, on selenium metabolism, and on pathophysiological responses to conditions associated with increased oxidative stress or inflammation. Consequently, although selenium concentrations are correlated with intake, the comparability of different biomarker concentrations observed in different studies is uncertain. In addition, selenium in blood and other tissues is present as selenocysteine in selenoproteins, which are maximized at plasma selenium concentrations between 70 and 90 μg/L, and as selenomethionine in proteins that contain methionine, with no apparent maximum concentration (68, 69). As a result, high selenium concentrations may reflect selenomethionine incorporated nonspecifically in proteins instead of methionine and may thus be considered primarily a marker of high dietary intake of plant-derived foods grown in selenium-rich soils. None of the observational studies included in the present review provided information on the selenium content of plant-derived foods or other food items. In addition, selenomethionine and selenium yeast supplements also increase seleniomethionine concentrations without increasing selenoprotein activity in populations with adequate selenium intakes (70). In most observational studies included in this meta-analysis, serum and whole-blood selenium concentrations in the highest category of exposure were >80 μg selenium/L. In some studies, the cutoff for the reference category was also >80 μg selenium/L (19–21, 23, 42, 43).
The prospective cohort studies summarized in the present meta-analysis show, in the aggregate, a moderate inverse association between selenium concentrations and coronary heart disease endpoints. This inverse association appeared to be linear throughout the range of selenium concentrations and was observed in populations from different countries with different baseline selenium concentrations. In our dose-response meta-analysis, we estimated that a 50% increase in selenium concentrations was associated with a 24% decreased risk of coronary heart disease. In trials, a dose of 100 μg selenium/d increased blood selenium from 82 to 122 μg/L (a 49% increase) (29), whereas a dose of 200 μg/d increased blood selenium from 67 to 190 μg/L (a 184% increase) (71). Most trials in our meta-analysis used doses of ≥100 μg selenium/d, yet the overall reduction in coronary heart disease was only 11%. Thus, observational studies may also overestimate the association between selenium and coronary heart disease.
The different characteristics of subjects receiving high and low selenium diets or selenium supplements, factors affecting selenium concentrations, residual confounding by socioeconomic status, education, or other cardiovascular risk factors, and selective publication of studies that show an inverse association could contribute to create the inverse association observed between selenium concentrations and coronary heart disease. A better understanding of the determinants of selenium intake and selenium concentrations is needed before low selenium concentrations can be established as a cardiovascular risk factor on the basis of observational evidence.
Is the use of selenium supplements justified for cardiovascular disease prevention?
The difficulties in interpreting the findings of observational studies of antioxidants and coronary endpoints highlight the need for randomized evidence. However, the small number of selenium trials and their relatively small sample size resulted in wide CIs; therefore, beneficial or harmful cardiovascular effects could not be ruled out. In addition, selenium was often used in combination with other vitamins or minerals, which makes it impossible to isolate the specific effects of selenium or of different selenium forms in those trials.
Several trials of selenium supplementation conducted in Chinese populations with low intakes of a variety of vitamins and minerals, including selenium, could not be included in this meta-analysis. Three of these trials reported only cancer outcomes (72–74). Two other trials conducted in Linxian, China, reported cerebrovascular disease but not coronary heart disease or total cardiovascular disease. In these trials, the relative risks of cerebrovascular disease mortality in a comparison of participants receiving 50 μg selenium/d in combination with vitamin E and β-carotene with participants receiving placebo were 0.90 (95% CI: 0.76, 1.07) in healthy participants (75) and 0.62 (0.37, 1.06) in participants with esophageal dysplasia at baseline (76). The relevance of these findings to the effects of selenium in coronary heart disease prevention in Western populations is uncertain. Finally, a randomized trial conducted in institutionalized elderly patients in France evaluated the efficacy of 100 μg selenium/d in combination with zinc in improving immune function and lowering the rate of infections (77). Although coronary heart disease endpoints were not available, the relative risk of total mortality after a 2-y follow-up in participants receiving selenium supplements compared with those receiving placebo was 1.14 (95% CI: 0.91, 1.37).
In conclusion, observational studies showed an inverse association between selenium concentrations and coronary heart disease incidence, but the validity of this evidence is uncertain. Randomized trials, on the other hand, are still inconclusive with respect to the effect of selenium supplementation. The ongoing Selenium and Vitamin E Cancer Prevention Trial, a placebo controlled trial that is testing the effects of 200 μg selenium/d in 32 400 men in the United States and Canada (78), will provide more definitive evidence. The results of this trial are scheduled to appear in 2013. Until then, the observational evidence that low selenium concentrations are a cardiovascular risk factor should be treated as suggestive but not definitive. Furthermore, the public should be warned against the use of selenium supplements for cardiovascular disease prevention. The benefits of selenium supplementation are uncertain, and their indiscriminate use carries a risk of toxicity.
APPENDIX A
See Appendix Table in Figures and Tables section.
Appendix Table.
Quality criteria for evaluating the design and data analysis of observational studies on selenium and coronary heart disease1
| Prospective cohort studies
(reference number) |
Case-control studies (reference
number) |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 38 | 40 | 41 | 42 | 52 | 43 | 53 | 44 | 45 | 46 | 47 | |
| All observational studies | |||||||||||||||||||||||||
| Exposure was assessed at the individual level | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Outcomes were based on objective tests or standard criteria in ≥90% of study Participants | ▪ | ▪ | ▪ | □ | ▪ | □ | ▪ | ▪ | ▪ | □ | □ | ▪ | ▪ | □ | ▪ | □ | ▪ | ▪ | ▪ | ▪ | ▪ | □ | □ | ▪ | ▪ |
| The authors presented internal comparisons within study participants | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| The authors controlled for potential confounding risk factors in addition to age | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | □ | □ | □ | ▪ | ▪ | ▪ | □ | ▪ | □ | □ | □ | □ |
| Prospective cohort studies | |||||||||||||||||||||||||
| Loss to follow-up was independent of exposure | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | — | — | — | — | — | — | — | — | — | — | — |
| The intensity of search of disease was independent of exposure status | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | — | — | — | — | — | — | — | — | — | — | — |
| Case-control studies | |||||||||||||||||||||||||
| Data were collected in a similar manner for all Participants | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | □ | ▪ | ▪ |
| The same exclusion criteria were applied to all Participants | — | — | — | — | — | — | — | — | — | — | — | — | — | — | □ | □ | ▪ | ▪ | ▪ | □ | ▪ | ▪ | □ | □ | □ |
| The selection process for noncases was described | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ▪ | □ | ▪ | ▪ | ▪ | □ | ▪ | □ | □ | □ | □ |
| Samples were collected ≤24 h after the onset of symptoms for all cases | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ▪ | □ | □ | □ | ▪ | ▪ | ▪ | □ | □ | ▪ | ▪ |
| The study was based on incident cases of disease | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ▪ | ▪ | □ | ▪ | ▪ | ▪ | ▪ | □ | □ | ▪ | ▪ |
| Noncases were persons who would have been excluded if they had developed coronary heart disease | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ▪ | ▪ | ▪ | ▪ | □ | □ | ▪ | □ | □ | □ | □ |
Quality criteria were adapted from Longnecker et al (36). ▪ Indicates the criterion was fulfilled; □ indicates the criterion was not fulfilled; — indicates the criterion was not applicable.
Footnotes
EG, AN-A, and GF-M conceived the idea for the study and developed the search strategy. GF-M and AN-A abstracted the data and conducted data analyses. RP-B conducted statistical analyses for and graphical display of the dose-response meta-analysis. All authors contributed to data and analyses verification and to the writing and revision of the manuscript. The authors have no conflict of interest to declare.
From the Departments of Epidemiology (GF-M, AN-A, and EG) and Environmental Health Sciences (AN-A), Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD; the Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, MD (GF-M, AN-A, and EG); the Department of Preventive Medicine, Bellvitge University Hospital, L’Hospitalet de Llobre-gat, Barcelona, Spain (GF-M); and the Division of Biostatistics, National Center for Epidemiology, Instituto de Salud Carlos III, Madrid, Spain (RP-B).
Supported by grants 1 R01 ES012673-01 from the National Institute of Environmental Health Sciences and 0230232N from the American Heart Association.
Reprints not available. Address correspondence to A Navas-Acien, Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, 615 N Wolfe Street, Room W7033B, Baltimore, MD 21205. E-mail: anavas@jhsph.edu.
References
- 1.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–41. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 3.Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–42. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 4.Shamberger RJ. Selenium in the drinking water and cardiovascular disease. J Environ Pathol Toxicol. 1980;4:305–8. [PubMed] [Google Scholar]
- 5.Masironi R. Geochemistry and cardiovascular diseases. Philos Trans R Soc Lond B Biol Sci. 1979;288:193–203. doi: 10.1098/rstb.1979.0101. [DOI] [PubMed] [Google Scholar]
- 6.Hunter DJ, Morris JS, Chute CG, et al. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132:114–22. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
- 7.Satia JA, King IB, Morris JS, Stratton K, White E. Toenail and plasma levels as biomarkers of selenium exposure. Ann Epidemiol. 2005;16:53–8. doi: 10.1016/j.annepidem.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Longnecker MP, Stram DO, Taylor PR, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7:384–90. doi: 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Ovaskainen ML, Virtamo J, Alfthan G, et al. Toenail selenium as an indicator of selenium intake among middle-aged men in an area with low soil selenium. Am J Clin Nutr. 1993;57:662–5. doi: 10.1093/ajcn/57.5.662. [DOI] [PubMed] [Google Scholar]
- 10.Monget AL, Richard MJ, Cournot MP, et al. Effect of 6 month supplementation with different combinations of an association of antioxidant nutrients on biochemical parameters and markers of the antioxidant defence system in the elderly. The Geriatrie/Min.Vit. Aox Network. Eur J Clin Nutr. 1996;50:443–9. [PubMed] [Google Scholar]
- 11.Luoma PV, Sotaniemi EA, Korpela H, Kumpulainen J. Serum selenium, glutathione peroxidase activity and high-density lipoprotein cholesterol–effect of selenium supplementation. Res Commun Chem Pathol Pharmacol. 1984;46:469–72. [PubMed] [Google Scholar]
- 12.Salonen JT, Salonen R, Seppanen K, et al. Effects of antioxidant supplementation on platelet function: a randomized pair-matched, placebo-controlled, double-blind trial in men with low antioxidant status. Am J Clin Nutr. 1991;53:1222–9. doi: 10.1093/ajcn/53.5.1222. [DOI] [PubMed] [Google Scholar]
- 13.Nyyssonen K, Porkkala E, Salonen R, Korpela H, Salonen JT. Increase in oxidation resistance of atherogenic serum lipoproteins following antioxidant supplementation: a randomized double-blind placebo-controlled clinical trial. Eur J Clin Nutr. 1994;48:633–42. [PubMed] [Google Scholar]
- 14.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 15.Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet. 1982;2:175–9. doi: 10.1016/s0140-6736(82)91028-5. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen TA, Alfthan G, Huttunen JK, et al. Serum selenium concentration related to myocardial infarction and fatty acid content of serum lipids. Br Med J (Clin Res Ed) 1983;287:517–9. doi: 10.1136/bmj.287.6391.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virtamo J, Valkeila E, Alfthan G, Punsar S, Huttunen JK, Karvonen MJ. Serum selenium and the risk of coronary heart disease and stroke. Am J Epidemiol. 1985;122:276–82. doi: 10.1093/oxfordjournals.aje.a114099. [DOI] [PubMed] [Google Scholar]
- 18.Salonen JT, Salonen R, Penttila I, et al. Serum fatty acids, apolipoproteins, selenium and vitamin antioxidants and the risk of death from coronary artery disease. Am J Cardiol. 1985;56:226–31. doi: 10.1016/0002-9149(85)90839-2. [DOI] [PubMed] [Google Scholar]
- 19.Ringstad J, Thelle D. Risk of myocardial infarction in relation to serum concentrations of selenium. Acta Pharmacol Toxicol (Copenh) 1986;59(suppl):336–9. doi: 10.1111/j.1600-0773.1986.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 20.Kok FJ, de Bruijn AM, Hofman A, Valkenburg HA. Selenium status and chronic disease mortality: Dutch epidemiological findings. Int J Epidemiol. 1987;16:329–32. doi: 10.1093/ije/16.2.329. [DOI] [PubMed] [Google Scholar]
- 21.Ringstad J, Jacobsen BK, Thomassen Y, Thelle DS. The Tromso Heart Study: serum selenium and risk of myocardial infarction a nested case-control study. J Epidemiol Community Health. 1987;41:329–32. doi: 10.1136/jech.41.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suadicani P, Hein HO, Gyntelberg F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis. 1992;96:33–42. doi: 10.1016/0021-9150(92)90035-f. [DOI] [PubMed] [Google Scholar]
- 23.Salvini S, Hennekens CH, Morris JS, Willett WC, Stampfer MJ. Plasma levels of the antioxidant selenium and risk of myocardial infarction among U.S. physicians Am J Cardiol. 1995;76:1218–21. doi: 10.1016/s0002-9149(99)80344-0. [DOI] [PubMed] [Google Scholar]
- 24.Marniemi J, Jarvisalo J, Toikka T, Raiha I, Ahotupa M, Sourander L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol. 1998;27:799–807. doi: 10.1093/ije/27.5.799. [DOI] [PubMed] [Google Scholar]
- 25.Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol. 2001;30:1119–26. doi: 10.1093/ije/30.5.1119. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa K, Ascherio A, Morris JS, et al. Prospective study of selenium levels in toenails and risk of coronary heart disease in men. Am J Epidemiol. 2003;158:852–60. doi: 10.1093/aje/kwg052. [DOI] [PubMed] [Google Scholar]
- 27.Wei WQ, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79:80–5. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 28.Akbaraly NT, Arnaud J, Hininger-Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005;51:2117–23. doi: 10.1373/clinchem.2005.055301. [DOI] [PubMed] [Google Scholar]
- 29.Korpela H, Kumpulainen J, Jussila E, et al. Effect of selenium supplementation after acute myocardial infarction. Res Commun Chem Pathol Pharmacol. 1989;65:249–52. [PubMed] [Google Scholar]
- 30.Kuklinski B, Weissenbacher E, Fahnrich A. Coenzyme Q10 and anti-oxidants in acute myocardial infarction. Mol Aspects Med. 1994;15(suppl):S143–7. doi: 10.1016/0098-2997(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 31.You WC, Chang YS, Heinrich J, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S-allyl cysteine levels, and toxicity. Eur J Cancer Prev. 2001;10:257–63. doi: 10.1097/00008469-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Hercberg S, Galan P, Preziosi P, et al. The SU.VI. MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 33.Stranges S, Marshall JR, Trevisan M, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163:694–9. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 34.Ringdal O, Andersen KJ, Svendsen E, Julshamn K. Trace elements and myocardial infarction, an autopsy study from western Norway. Acta Pharmacol Toxicol (Copenh) 1986;59(suppl):358–60. doi: 10.1111/j.1600-0773.1986.tb02779.x. [DOI] [PubMed] [Google Scholar]
- 35.Rajpathak S, Rimm E, Morris JS, Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J Am Coll Nutr. 2005;24:250–6. doi: 10.1080/07315724.2005.10719472. [DOI] [PubMed] [Google Scholar]
- 36.Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 1988;260:652–6. [PubMed] [Google Scholar]
- 37.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 38.Oster O, Drexler M, Schenk J, et al. The serum selenium concentration of patients with acute myocardial infarction. Ann Clin Res. 1986;18:36–42. [PubMed] [Google Scholar]
- 39.Akesson B, Steen B. Plasma selenium and glutathione peroxidase in relation to cancer, angina pectoris and short-term mortality in 68-year-old men. Compr Gerontol [A] 1987;1:61–4. [PubMed] [Google Scholar]
- 40.Auzepy P, Blondeau M, Richard C, Pradeau D, Therond P, Thuong T. Serum selenium deficiency in myocardial infarction and congestive cardiomyopathy. Acta Cardiol. 1987;42:161–6. [PubMed] [Google Scholar]
- 41.Salonen JT, Salonen R, Seppanen K, et al. Relationship of serum selenium and antioxidants to plasma lipoproteins, platelet aggregability and prevalent ischaemic heart disease in Eastern Finnish men. Atherosclerosis. 1988;70:155–60. doi: 10.1016/0021-9150(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 42.Kok FJ, Hofman A, Witteman JC, et al. Decreased selenium levels in acute myocardial infarction. JAMA. 1989;261:1161–4. [PubMed] [Google Scholar]
- 43.Thiele R, Schuffenhauer M, Winnefeld K, Dawcynski H, Pleissner J, Pfeifer R. [Selenium level in patients with acute myocardial infarct and in patients with severe angina pectoris without myocardial infarct] Med Klin (Munich) 1995;90(suppl):45–8. (in German). [PubMed] [Google Scholar]
- 44.Coudray C, Roussel AM, Mainard F, Arnaud J, Favier A. Lipid peroxidation level and antioxidant micronutrient status in a pre-aging population; correlation with chronic disease prevalence in a French epidemiological study (Nantes, France) J Am Coll Nutr. 1997;16:584–91. [PubMed] [Google Scholar]
- 45.Navarro-Alarcon M, Lopez-Garcia de la Serrana, Perez-Valero V, Lopez-Martinez C. Serum and urine selenium concentrations in patients with cardiovascular diseases and relationship to other nutritional indexes. Ann Nutr Metab. 1999;43:30–6. doi: 10.1159/000012764. [DOI] [PubMed] [Google Scholar]
- 46.Bor MV, Cevik C, Uslu I, Guneral F, Duzgun E. Selenium levels and glutathione peroxidase activities in patients with acute myocardial infarction. Acta Cardiol. 1999;54:271–6. [PubMed] [Google Scholar]
- 47.Zachara BA, Ukleja-Adamowicz M, Nartowicz E, Lecka J. Increased plasma glutathione peroxidase activity in patients with acute myocardial infarction. Med Sci Monit. 2001;7:415–20. [PubMed] [Google Scholar]
- 48.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 50.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 51.Egger M, Davey Smith G, Altman DG. Systematic reviews in health care: meta-analysis in context. London, United Kingdom: BMJ Books; 2001. [Google Scholar]
- 52.Beaglehole R, Jackson R, Watkinson J, Scragg R, Yee RL. Decreased blood selenium and risk of myocardial infarction. Int J Epidemiol. 1990;19:918–22. doi: 10.1093/ije/19.4.918. [DOI] [PubMed] [Google Scholar]
- 53.Kardinaal AF, Kok FJ, Kohlmeier L, et al. Association between toenail selenium and risk of acute myocardial infarction in European men. The EURAMIC Study. European Antioxidant Myocardial Infarction and Breast Cancer. Am J Epidemiol. 1997;145:373–9. doi: 10.1093/oxfordjournals.aje.a009115. [DOI] [PubMed] [Google Scholar]
- 54.Gail MH, You WC, Chang YS, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Control Clin Trials. 1998;19:352–69. doi: 10.1016/s0197-2456(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 55.Davey SG, Ebrahim S. Folate supplementation and cardiovascular disease. Lancet. 2005;366:1679–81. doi: 10.1016/S0140-6736(05)67676-3. [DOI] [PubMed] [Google Scholar]
- 56.Neve J. Selenium as a risk factor for cardiovascular diseases. J Cardiovasc Risk. 1996;3:42–7. [PubMed] [Google Scholar]
- 57.Brigelius-Flohe R, Banning A, Schnurr K. Selenium-dependent enzymes in endothelial cell function. Antioxid Redox Signal. 2003;5:205–15. doi: 10.1089/152308603764816569. [DOI] [PubMed] [Google Scholar]
- 58.Arteel GE, Briviba K, Sies H. Protection against peroxynitrite. FEBS Lett. 1999;445:226–30. doi: 10.1016/s0014-5793(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 59.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–35. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 60.Trankmann P, Thiele R, Winnefeld K, Seliger K. [Effect of administration of selenium and vitamin E on heart failure and ventricular arrhythmias in patients with acute myocardial infarct] Med Klin (Munich) 1999;94(suppl):78–80. doi: 10.1007/BF03042199. (in German). [DOI] [PubMed] [Google Scholar]
- 61.Huang K, Liu H, Chen Z, Xu H. Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis. 2002;162:137–44. doi: 10.1016/s0021-9150(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 62.Van Dokkum W, Van der Torre HW, Schaafsma G, Kistemaker C, Ockhuizen T. Supplementation with selenium-rich bread does not influence platelet aggregation in healthy volunteers. Eur J Clin Nutr. 1992;46:445–50. [PubMed] [Google Scholar]
- 63.Mark SD, Wang W, Fraumeni JF, Jr, et al. Do nutritional supplements lower the risk of stroke or hypertension? Epidemiology. 1998;9:9–15. [PubMed] [Google Scholar]
- 64.Feroci G, Badiello R, Fini A. Interactions between different selenium compounds and zinc, cadmium and mercury. J Trace Elem Med Biol. 2005;18:227–34. doi: 10.1016/j.jtemb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Lin SM, Yang MH. Arsenic, selenium, and zinc in patients with Black-foot disease. Biol Trace Elem Res. 1988;15:213–21. doi: 10.1007/BF02990138. [DOI] [PubMed] [Google Scholar]
- 66.Cheng YY, Qian PC. The effect of selenium-fortified table salt in the prevention of Keshan disease on a population of 1.05 million. Biomed Environ Sci. 1990;3:422–8. [PubMed] [Google Scholar]
- 67.Alissa EM, Bahijri SM, Ferns GA. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med Sci Monit. 2003;9:RA9–18. [PubMed] [Google Scholar]
- 68.Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors. 2001;14:107–14. doi: 10.1002/biof.5520140115. [DOI] [PubMed] [Google Scholar]
- 69.Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004;58:391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- 70.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15:804–10. doi: 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 71.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 72.Li W, Zhu Y, Yan X, et al. [The prevention of primary liver cancer by selenium in high risk populations] Zhonghua Yu Fang Yi Xue Za Zhi. 2000;34:336–8. (in Chinese). [PubMed] [Google Scholar]
- 73.Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56:117–24. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 74.Yu SY, Zhu YJ, Li WG, et al. A preliminary report on the intervention trials of primary liver cancer in high-risk populations with nutritional supplementation of selenium in China. Biol Trace Elem Res. 1991;29:289–94. doi: 10.1007/BF03032685. [DOI] [PubMed] [Google Scholar]
- 75.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 76.Li JY, Taylor PR, Li B, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85:1492–8. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 77.Girodon F, Galan P, Monget AL, et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX geriatric network. Arch Intern Med. 1999;159:748–54. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]
- 78.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]


