Abstract
5-Aminolevulinic acid (ALA) and carnosine have important physiological and pathophysiological roles in the CNS. Both are substrates for the proton-coupled oligopeptide transporter PEPT2. The purpose of the current study was to determine the importance of PEPT2 in the uptake of ALA and carnosine in rat and mouse (PEPT2+/+ and PEPT2−/−) cultured neonatal astrocytes. Although neonatal astrocytes are known to express PEPT2, its quantitative importance in the transport of these compounds is not known. [14C]ALA uptake in neonatal rat astrocytes was inhibited by dipeptides, an α-amino containing cephalosporin (which is a PEPT2 substrate) but was not affected by a non-amino containing cephalosporin (which is not a PEPT2 substrate). Uptake was pH sensitive as expected from a proton-coupled transporter and was saturable (Vmax = 715 ± 29 pmol/mg/min, Km = 606 ± 14 μM). [3H]Carnosine uptake in neonatal rat astrocytes was inhibited by dipeptides but not by histidine (a substrate for the peptide/histidine transporters PHT1 and PHT2), and also showed saturable transport (Vmax 447 ± 23 pmol/mg/min, Km 43± 5.5 μM). Neonatal astrocytes from PEPT2−/− mice had a 62% reduction in [14C]ALA uptake and a 92% reduction in [3H]carnosine uptake compared to PEPT2+/+ mice. These results demonstrate that PEPT2 is the primary transporter responsible for the astrocytic uptake of ALA and carnosine.
Keywords: 5-aminolevulinic acid, carnosine, astrocytes, knockout mouse, uptake, PEPT2
1. Introduction
PEPT2, one of four members of the mammalian proton-coupled oligopeptide transporter family (i.e., PEPT1, PEPT2, PHT1, PHT2) (Herrera-Ruiz and Knipp, 2003), is an electrogenic, sodium-independent transporter that translocates di- and tripeptides across biologic membranes using an inwardly-directed electrochemical gradient for protons. PEPT2 is present and functionally active in the brain (Berger and Hediger, 1999,Novotny et al., 2000,Ocheltree et al., 2004,Ocheltree et al., 2004,Shen et al., 2005,Shen et al., 2004,Teuscher et al., 2000,Xiang et al., 2006) (e.g. choroid plexus and astrocytes) where a wide range of small peptides acts as neurotransmitters or neuromodulators (Mains, 1999).
5-Aminolevulinic acid (ALA), an endogenous peptidomimetic PEPT2 substrate (Doring et al., 1998,Novotny et al., 2000), is a precursor for porphyrins. Orally delivered ALA results in an accumulation of porphyrins in gliomas as compared to adjacent normal tissue, and the porphyrins can be detected by fluorescence microscopy during surgery. It is undergoing clinical trial to demarcate gliomas (Stummer et al., 2003). ALA and its derivatives have been used as photosensitizers for photodynamic therapy (Fukuda et al., 2005). ALA is also important in some CNS disease states. An accumulation of the porphyrin precursors, ALA and/or porphobilinogen, has been suggested as the cause of the neuropsychiatric symptoms that occur in hereditary hepatic porphyrias (Stummer et al., 1998). Little is known about the transport of ALA in the CNS. Our earlier studies demonstrated that PEPT2 is the main transporter responsible for ALA uptake in isolated choroid plexus (Ocheltree et al., 2004), and that PEPT2 is the major transporter for GlySar (a synthetic dipeptide) into cultured neonatal astrocytes (Xiang et al., 2006). However, although many have shown that PEPT2 is expressed in cultured neonatal astrocytes (Dieck et al., 1999,Dringen et al., 1998,Wada et al., 2005), the quantitative importance of PEPT2 in astrocytic ALA transport is unknown.
Carnosine (β-alanyl-L-histidine) is present in muscle and the CNS. Many putative roles have been assigned to this dipeptide. In this regard, it may act as a neuroprotective agent scavenging free radicals and inhibiting protein glycosylation by reactive aldehydes (Boldyrev et al., 1997,Hipkiss, 1998,Stvolinsky et al., 1999). Carnosine may also reduce the toxicity of β-amyloid, the primary neurotoxin in Alzheimer’s disease and prevent β-amyloid aggregation (Hipkiss, 1998,Preston et al., 1998). Moreover, it may protect against metal-induced cell toxicity (Horning et al., 2000). Finally, carnosine may serve as a neurotransmitter in the olfactory bulb area where it is present in high concentrations (Marchis et al., 2000,Margolis, 1974). Previous studies indicate that PEPT2 is the sole transporter for the uptake of carnosine in choroid plexus (Teuscher et al., 2004). Cultured astrocytes take up carnosine (Hoffmann et al., 1996). However, as with ALA, the quantitative importance of PEPT2 in that uptake is undefined.
Given the significance of ALA and carnosine in CNS physiology and disease, the objective of this study was to determine the importance of PEPT2 in the uptake of ALA and carnosine in cultured neonatal astrocytes. The studies employed both rat and mouse astrocytes. The latter allowed comparison between cells with (PEPT2+/+) or without (PEPT2−/−) the transport protein.
2. Results
2.1. [14C]ALA Uptake
The uptake of [14C]ALA in rat neonatal astrocytes in the presence or absence of inhibitors is shown in Fig. 1. Uptake was inhibited at 4°C and by dipeptides (GlyGly, GlySar and carnosine) and the α-amino containing cephalosporin, cefadroxil, but not by cephalothin, a cephalosporin without an α-amino group. In addition, pH sensitivity studies demonstrated a marked impact. In this regard, [14C]ALA uptake was 95% and 24% higher at pH 6.5 and pH 7.0, respectively, compared to pH 7.4, as expected for a proton coupled-transporter. The concentration-dependent uptake of [14C]ALA (10 to 1000 μM) demonstrated saturable transport with a Vmax of 714 ± 28 pmol/mg protein/min, a Km of 606 ± 14 μM and a nonsaturable Kd of 0.028 ± 0.001 μl/mg protein/min (Fig. 2).
Figure 1.
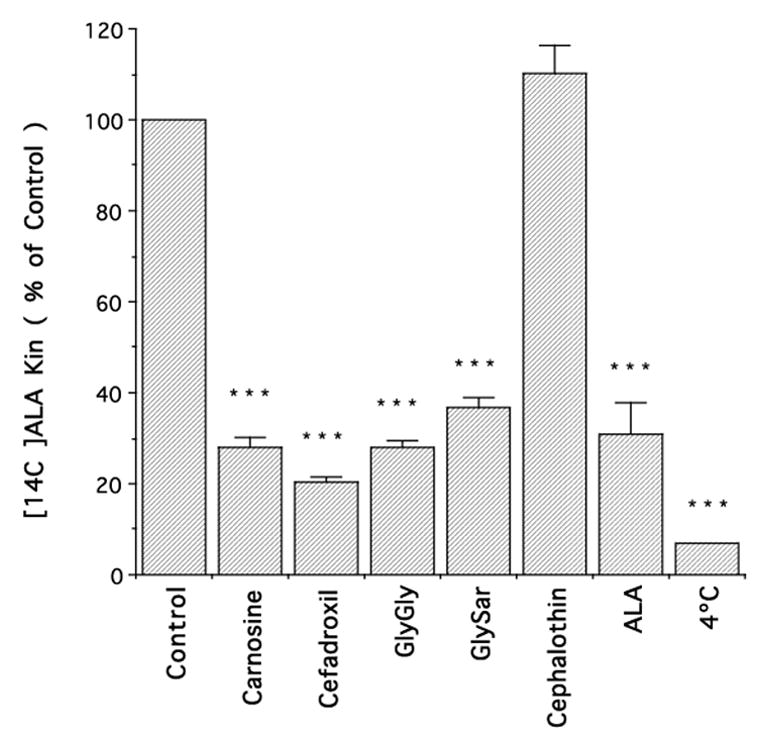
Influx rate constant (Kin) for [14C]ALA uptake into rat neonatal astrocytes (4 μM ALA in the external media) in the presence of different potential inhibitors (1 mM). All data are expressed as mean ± S.E. (n=3 preparations, each performed in triplicate); *** p<0.001, as compared to control.
Figure 2.
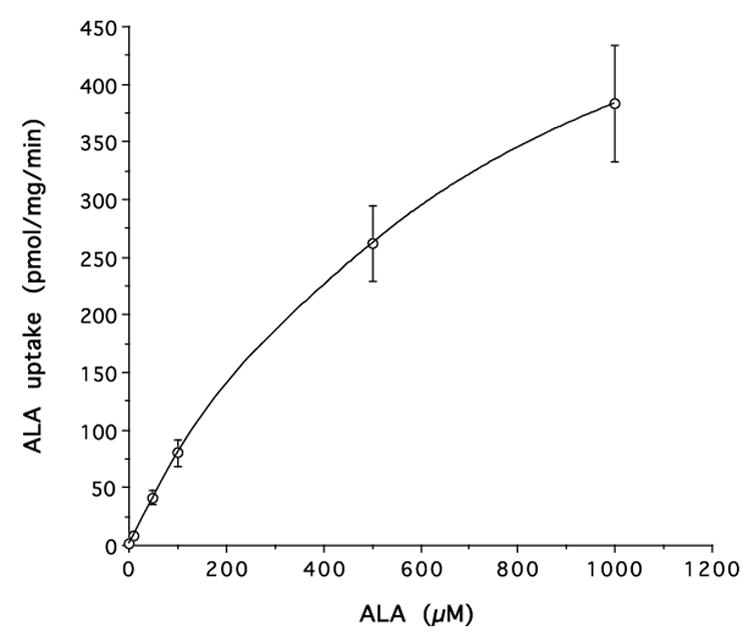
Concentration-dependent uptake of [14C]ALA in rat neonatal astrocytes in the presence of 10–1000 μM ALA. The uptake was fitted by nonlinear regression in which Vmax = 714 pmol/mg protein/min, Km = 606 μM and Kd = 0.028 μl/mg protein/min (r2=0.999).
Experiments on PEPT2 null mice showed an approximate 62% reduction in [14C]ALA uptake as compared to PEPT2+/+ mice (Fig. 3A). This difference was absent when studies were performed in the presence of a saturating concentration of ALA (10 mM).
Figure 3.
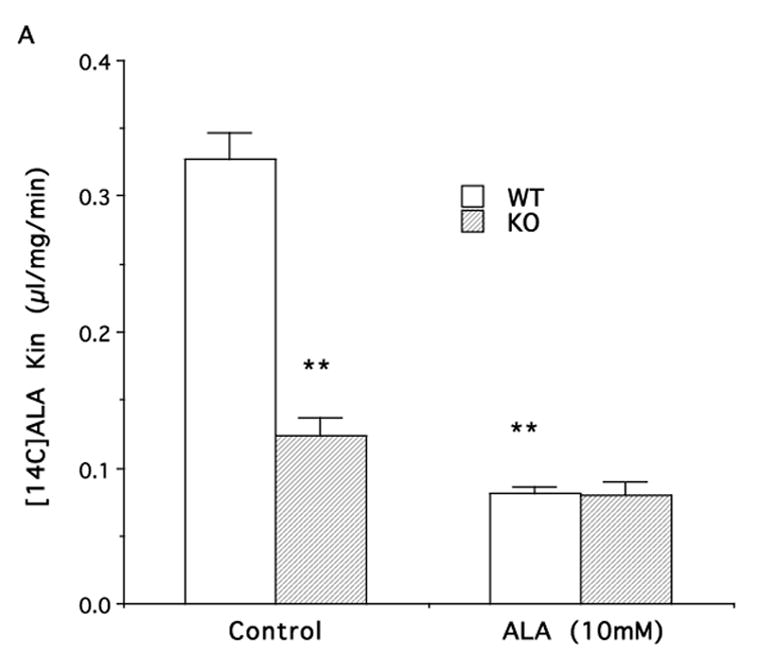
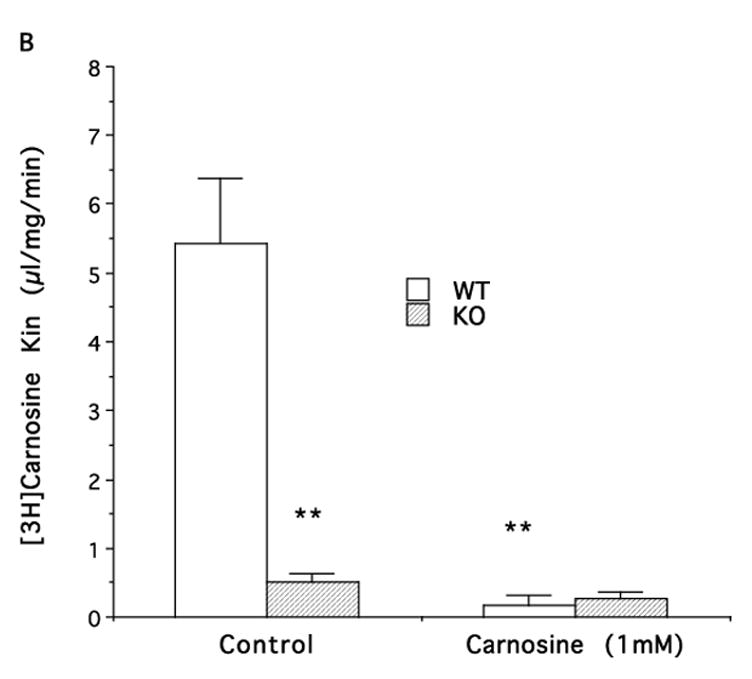
Influx rate constants (Kin) for [14C]ALA (A) and [3H]carnosine (B) uptake into neonatal astrocytes from wild-type (WT) or PEPT2 knock-out (KO) mice. Values are given as mean ± S.E. (n=3–6 preparations, with each performed in triplicate). ** p<0.01, as compared to WT control.
2.2. [3H]Carnosine Uptake
In rat neonatal astrocytes, the Kin for [3H]carnosine was much higher than [14C]ALA (7.78 ± 0.68 vs. 0.82 ± 0.16 μl/mg/min, respectively). The uptake of [3H]carnosine was greatly reduced by 1 mM GlySar (93%) and 1 mM unlabeled carnosine (92%) but not by 1 mM histidine, a substrate of PHT1 and PHT2 (Fig. 4). The uptake kinetics of carnosine were evaluated over the concentration range of 2 to 100 μM. As shown in Fig. 5, there was saturable transport uptake with a Vmax of 447 ± 23 pmol/mg protein/min, a Km of 43±5.5 μM.
Figure 4.
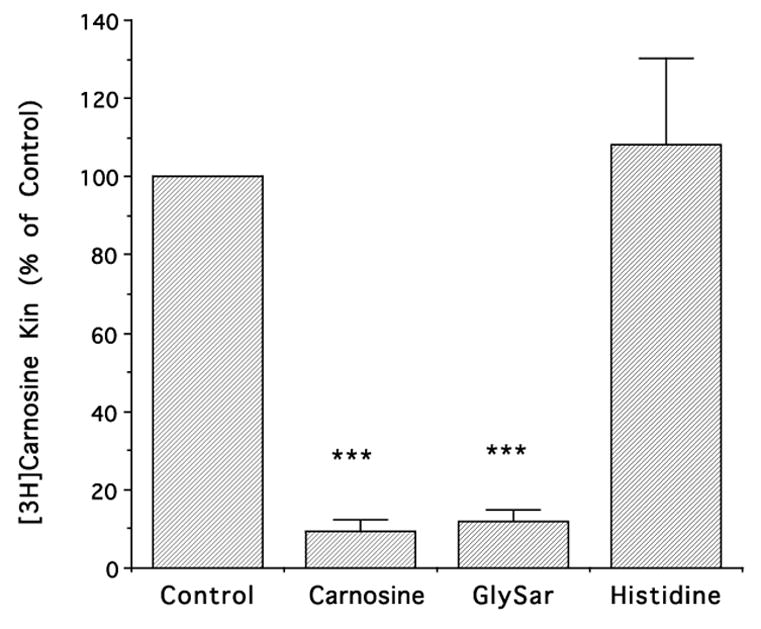
Effect of different potential inhibitors (at 1 mM) on the influx rate constant (Kin) for [3H]carnosine uptake into rat neonatal astrocytes. Carnosine was present at 0.02 μM. All data are expressed as mean ± S.E. (n=3 preparations, each performed in triplicate); *** p<0.001, as compared to control.
Figure 5.
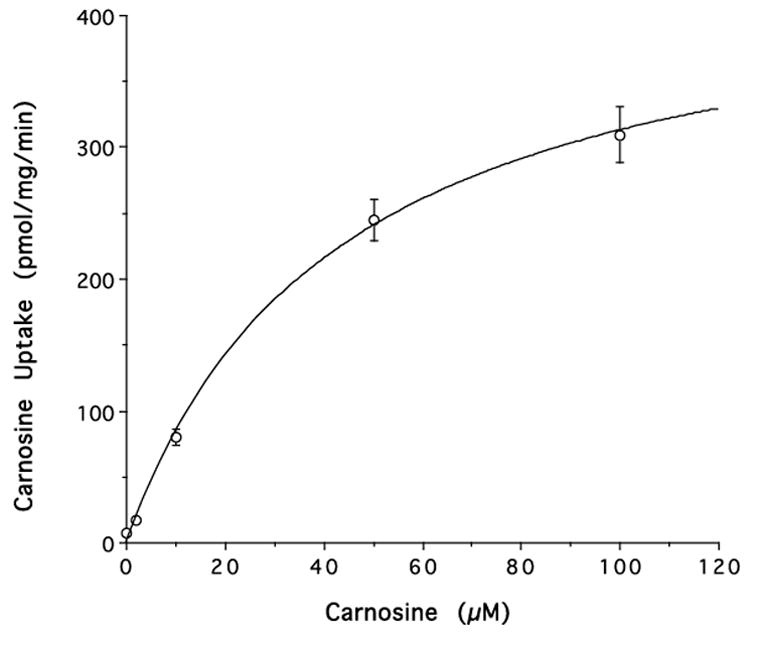
Concentration-dependent uptake of [3H]carnosine into rat neonatal astrocytes in the presence of 2–100 μM carnosine. The uptake was fitted by nonlinear regression in which Vmax = 447 pmol/mg protein/min and Km = 43 μM (r2=0.999).
Astrocytes from PEPT2−/− mice showed a marked reduction in [3H]carnosine uptake (96%) compared to PEPT2+/+ mice (Fig. 3B). Similar to the results with ALA, this difference was absent when studies were performed in the presence of saturating concentrations of carnosine (1 mM).
3. Discussion
PEPT2 transports a wide range of di- and tripeptides and peptidomimetics (Daniel and Herget, 1997,Herrera-Ruiz and Knipp, 2003,Shen et al., 2003,Shu et al., 2002,Teuscher et al., 2001,Teuscher et al., 2000). Mammals express at least four members of the proton-coupled oligopeptide transporter family (i.e., PEPT1, PEPT2, PHT1, PHT2) and their overlapping substrate specificity makes it difficult to define the role of PEPT2 in transport in a particular cell type. The current study examined the role of PEPT2 in the transport of carnosine and ALA, an endogenous peptide and peptidomimetic, respectively. While others have shown that PEPT2 is present in cultured neonatal astrocytes, the current study used both pharmacological and genetic (knockout) approaches to demonstrate that PEPT2 is the predominant (and perhaps only) transporter for both of these compounds in cultured astrocytes.
There is evidence at both the mRNA and protein level that PEPT2 is present in neonatal astrocytes (Berger and Hediger, 1999,Shen et al., 2004,Xiang et al., 2006). Data from this study in rat neonatal astrocytes indicated that the uptake of the endogenous peptidomimetic ALA (at 4 μM) was: (a) inhibited ~65–80% by dipeptides, (b) inhibited by an α-amino containing cephalosporin, cefadroxil, but not by cephalothin, a cephalosporin without an α-amino group, and (c) pH dependent. All of these properties are consistent with PEPT2-mediated transport (Novotny et al., 2000,Ocheltree et al., 2004,Teuscher et al., 2004,Xiang et al., 2006). The Km for ALA uptake was about 600 μM. This is somewhat higher than the 260 μM Km we have measured for ALA in rat choroid plexus tissue (Novotny et al., 2000). Both numbers suggest that ALA has a lower affinity for PEPT2 than dipeptides such as GlySar and carnosine(Smith et al., 2004).
Astrocytes from PEPT2 knockout mice were also used to better quantify the role of this transporter in ALA transport. [14C]ALA uptake was markedly reduced in PEPT2−/− mouse astrocytes as compared to cells from PEPT2+/+ mice (~62% reduction). However, the difference in uptake between wild type and KO mouse astrocytes dissappeared in the presence of saturating concentrations of ALA (10 mM). In PEPT2−/− mouse astrocytes, there was a tendency for [14C]ALA uptake to be reduced with addition of 10 mM ALA, suggesting that their might be a second saturable transporter in addition to PEPT2. However, the reduction did not reach statistical significance and the magnitude of this change was small (~15% of wild type total uptake at 4 μM ALA). The [14C]ALA uptake that was not saturated by 10 mM ALA was about 0.08 μl/mg protein/min in both PEPT2 null and wild type astrocytes. This represented ~24% of the total uptake of 4 μM ALA in wild type astrocytes.
Carnosine, an endogenous dipeptide, is present in brain and other tissues. Previous findings (Teuscher et al., 2004) showed that PEPT2 accounts for over 90% of carnosine uptake in choroid plexus. The data from this study with neonatal astrocytes had very similar findings. The carnosine uptake was virtually eliminated in PEPT2−/− mice compared to that in PEPT2+/+ mice (a 96% reduction in uptake). The kinetic study demonstrated that carnosine uptake in rat astrocytes was via a high affinity transporter (Km ~ 40 μM) which is consistent with PEPT2. The inhibitor study in rat astrocytes was also consistent with carnosine uptake being by PEPT2 but not by PHT1 or PHT2. Thus, although both PHT1 and PHT2 have both been identified in brain and astrocytes (Sakata et al., 2001,Wada et al., 2005,Yamashita et al., 1997), carnosine uptake was not affected by L-histidine, a PHT1 and PHT2 substrate (Sakata et al., 2001,Yamashita et al., 1997), but was inhibited by the dipeptide GlySar.
These results suggest that astrocytic PEPT2 (at least early in development) has a prominent role in the clearance of endogenous small peptides and peptidomimetics from the brain extracellular fluid and potentially in regulating the activity of neuroactive peptides in that space. In addition to endogenous peptides/peptidomimetics, PEPT2 has been shown to transport a number of pharmacological agents including β-lactam antibiotics (e.g., α-amino containing penicillins and cephalosprins), angiotensin converting enzyme inhibitors and nucleoside analog prodrugs (Rubio-Aliaga and Daniel, 2002). Thus, astrocytic PEPT2 may also influence drug distribution within the brain. This may be important in the treatment of meningitis where non-α-amino containing cephalosprins (which are not PEPT2 substrates) would not be cleared from the extracellular space by PEPT2, but α-amino containing cephalosprins (which are PEPT2 substrates) would be cleared.
The effect of PEPT2 on the transport of the porphyrin/heme precursor, ALA, suggests that this transporter may have a role in regulating porphyrin and heme synthesis. Knowledge of the mechanisms by which ALA is taken up into cells within the CNS may be important in understanding the potential role of ALA in porphyrias. In addition, such knowledge may be helpful in understanding and improving photodynamic therapy or delineating tumors with this agent. The expression of PEPT2 in astrocytes appears to decrease with age (Shen et al., 2004), raising the question of whether the ability of ALA to delineate gliomas (Stummer et al., 2003,Stummer et al., 1998) also varies with age. ALA ester derivatives have also been used in tumor treatment (Fukuda et al., 2005). Whether these are PEPT2 substrates has yet to be investigated.
It should be noted that ALA uptake was only reduced about 62% in PEPT2 KO astrocytes in contrast to an approximate 92% reduction in carnosine uptake. The reason for this difference appears to be that under non-saturating conditions, the uptake of ALA in wild-type astrocytes is 10–15 fold less than that for carnosine (this was true for mouse and rat astrocytes). Thus, although the amount of non-saturable (apparently non-transporter mediated) uptake was similar for both compounds, this represents a much larger proportion of total uptake for ALA. Thus, we also found that saturating concentrations of ALA only reduced [14C]ALA uptake by ~70%, whereas there was close to a 95% reduction in [3H]carnosine uptake in the presence of 1 mM carnosine.
As with all in vitro studies, care should be taken extrapolating the current results to in vivo situation. Culture may affect transporter expression. In addition, with PEPT2, there are apparent developmental changes in expression (Shen et al., 2004). Thus, in vivo experiments are required to confirm the importance of astrocytic PEPT2 in the distribution of neuropeptides and peptidomimetics in the brain.
In conclusion, this study has characterized the functional significance of PEPT2 in the transport of ALA and carnosine in neonatal astrocytes. The results suggest an important role for PEPT2 in CNS neuropeptide homeostasis and in the distribution of small peptide/peptidomimetic therapeutics within the brain.
4. Experimental Procedures
Late gestation Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI) and PEPT2-knockout mice were generated on a C57BL/6 mouse background and genotyped by PCR, as described by Shen et al. (Shen et al., 2003). [3H]Carnosine (9 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA,), [14C]mannitol (53 mCi/mmol) was from American Radiolabeled Chemicals (St Louis, MO) and [14C]ALA (51.5 mCi/mmol) from New England Nuclear Life Science Products (Boston, MA). ALA, carnosine, glycylglycine (GlyGly), glycylsarcosine (GlySar), cefadroxil, cephalothin and histidine were purchased from Sigma (St Louis, MO). All other reagents for cell culture were purchased from Invitrogen Corporation (Grand Island, NY).
Neonatal astrocyte cultures were prepared from 1–2 day old mice and rats according to the method of Hertz et al. (Hertz et al., 1982) with slight modifications (Stamatovic et al., 2005,Xiang et al., 2006). Mice were either PEPT2−/− (null) or PEPT2+/+ (wild type). Brain tissue was mechanically dissociated in Ca2+/Mg2+-free Hanks balanced salt solution (HBSS) and then digested with 0.25% Trypsin-EDTA and 10 U/μl DNAse I at 37°C for 20 min. Trypsin digestion was stopped by adding DMEM containing 10% fetal bovine serum (FBS), followed by low speed centrifugation to remove debris. To obtain single cell suspensions, the brain tissue was triturated with a solution of Ca2+/Mg2+-free HBSS supplemented with 10 U/μl DNAse I and 3.8% (w/w) MgSO4. Cells were then washed twice in HBSS, resuspended in complete astrocyte media (DMEM, 10% inactivated fetal bovine serum, 1x glutamine, 1x antibiotic/antimycotic) and seeded on 12 well plates. Monolayers were grown under an atmosphere of 5% CO2/95% air at 37°C. Two weeks af ter initial plating, the cells were shaken at 220 rpm for 2 h at 4°C. After this time, the supernatant, containing mainly microglia, was removed. The remaining cells were cultured for 2 days and then used for uptake experiments.
[14C]ALA and [3H]carnosine uptake was measured in neonatal mouse and rat astrocyte cultures. Experiments were performed in triplicate on each individual preparation. Preliminary experiments showed that [14C]ALA uptake was linear over 240 min. All the experiments with [14C]ALA employed an uptake time of 60 min with the exception of pH experiments, where there was concern over pH equilibration. For those experiments, 10-min uptake times were used. [3H]Carnosine uptake was linear over 60 min and 10-min uptake times were used in these studies. At the start of the experiment, cells were normally transferred to artificial CSF containing (in mM) 127 NaCl, 20 NaHCO3, 2.4 KCl, 0.5 KH2PO4, 1.1 CaCl2, 0.85 MgCl2, 0.5 Na2SO4 and 5.0 glucose (pH 7.4), bubbled with 5% CO2 and 95% O2. After 30 sec at 37°C, the buffer was removed and fresh uptake buffer containing [14C]ALA and [3H]mannitol (0.2 μCi/ml of each) or [3H]carnosine and [14C]mannitol (0.2 and 0.1 μCi/ml, respectively) were added to initiate uptake. Transport was measured at 37°C. At the end of the experiment, the medium was aspirated and the cells rapidly washed four times with ice-cold uptake buffer. The cells were solubilized in methylbenzethonium hydroxide and counted in a liquid scintillation counter. The protein content of the solublized cell monolayers was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The volume of distribution (VD) for [14C]ALA (corrected for extracellular contamination) was determined as:
where ALAtiss and Manntiss are the dpm per mg protein for [14C]ALA and [3H]mannitol, R is the ratio of [14C] ALA to [3H]mannitol radioactivity in the media, and ALAmedia is the dpm per μl of media. Dividing VD (μl/mg) by the duration of uptake equals the influx rate constant (Kin). The same formula was used to calculate the VD and Kin for carnosine uptake.
For kinetic studies, the concentration-dependent uptake of ALA and carnosine was best fit to a Michaelis-Menten relationship with a nonsaturable component:
Where Vmax is the maximal rate of saturable uptake, Km is the Michaelis constant, Kd is the rate constant for nonsaturable processes and C is the substrate concentration.
All data are reported as the mean ± standard error of the mean (S.E.). Statistical differences were evaluated by analysis of variance (ANOVA) with Scheffe’s or Dunnett’s post-hoc tests for multiple groups.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01 GM035498 to D.E.S.; R01 NS034709 and P01 HL018575 to R.F.K.).
Abbreviations
- PEPT2
peptide transporter 2
- ALA
5-Aminolevulinic acid
- PHT1
peptide, histine transporter 1
- PHT2
peptide, histine transporter 2
- GlySar
glycylsarcosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berger UV, Hediger MA. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat Embryol (Berl) 1999;199:439–49. doi: 10.1007/s004290050242. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Stvolinsky SL, Tyulina OV, Koshelev VB, Hori N, Carpenter DO. Biochemical and physiological evidence that carnosine is an endogenous neuroprotector against free radicals. Cell Mol Neurobiol. 1997;17:259–71. doi: 10.1023/A:1026374114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Herget M. Cellular and molecular mechanisms of renal peptide transport. Am J Physiol. 1997;273:F1–8. doi: 10.1152/ajprenal.1997.273.1.F1. [DOI] [PubMed] [Google Scholar]
- Dieck ST, Heuer H, Ehrchen J, Otto C, Bauer K. The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative beta-Ala-Lys-Nepsilon-AMCA in astrocytes. Glia. 1999;25:10–20. doi: 10.1002/(sici)1098-1136(19990101)25:1<10::aid-glia2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Doring F, Walter J, Will J, Focking M, Boll M, Amasheh S, Clauss W, Daniel H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest. 1998;101:2761–7. doi: 10.1172/JCI1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Hamprecht B, Broer S. The peptide transporter PepT2 mediates the uptake of the glutathione precursor CysGly in astroglia-rich primary cultures. J Neurochem. 1998;71:388–93. doi: 10.1046/j.1471-4159.1998.71010388.x. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Casas A, Batlle A. Aminolevulinic acid: from its unique biological function to its star role in photodynamic therapy. Int J Biochem Cell Biol. 2005;37:272–6. doi: 10.1016/j.biocel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J Pharm Sci. 2003;92:691–714. doi: 10.1002/jps.10303. [DOI] [PubMed] [Google Scholar]
- Hertz L, Juurilink BHL, Fosmark H, Schouboe A. Astrocytes in the primary culture. In: Pfeiffer SE, editor. Neuroscience Approached Through Cell Culture. Vol. 1. CRL Press; Boca Raton: 1982. pp. 175–186. [Google Scholar]
- Hipkiss AR. Carnosine, a protective, anti-ageing peptide? Int J Biochem Cell Biol. 1998;30:863–8. doi: 10.1016/s1357-2725(98)00060-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann AM, Bakardjiev A, Bauer K. Carnosine-synthesis in cultures of rat glial cells is restricted to oligodendrocytes and carnosine uptake to astrocytes. Neurosci Lett. 1996;215:29–32. doi: 10.1016/s0304-3940(96)12937-2. [DOI] [PubMed] [Google Scholar]
- Horning MS, Blakemore LJ, Trombley PQ. Endogenous mechanisms of neuroprotection: role of zinc, copper, and carnosine. Brain Res. 2000;852:56–61. doi: 10.1016/s0006-8993(99)02215-5. [DOI] [PubMed] [Google Scholar]
- Mains RE. Peptides. In: Siegel BWAGJ, Albers RW, Fisher SK, editors. In Basic Neurochemistry. 6. Lippincott-Rave; Philadelpia: 1999. pp. 363–382. MD. [Google Scholar]
- Marchis SD, Modena C, Peretto P, Migheli A, Margolis FL, Fasolo A. Carnosine-related dipeptides in neurons and glia. Biochemistry (Mosc) 2000;65:824–33. [PubMed] [Google Scholar]
- Margolis FL. Carnosine in the primary olfactory pathway. Science. 1974;184:909–11. doi: 10.1126/science.184.4139.909. [DOI] [PubMed] [Google Scholar]
- Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep RF. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J Neurochem. 2000;75:321–8. doi: 10.1046/j.1471-4159.2000.0750321.x. [DOI] [PubMed] [Google Scholar]
- Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. Mechanisms of cefadroxil uptake in the choroid plexus: studies in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2004;308:462–7. doi: 10.1124/jpet.103.060400. [DOI] [PubMed] [Google Scholar]
- Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. Role of PEPT2 in the choroid plexus uptake of glycylsarcosine and 5-aminolevulinic acid: studies in wild-type and null mice. Pharm Res. 2004;21:1680–5. doi: 10.1023/b:pham.0000041465.89254.05. [DOI] [PubMed] [Google Scholar]
- Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN. Toxic effects of beta-amyloid(25–35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and beta-alanine. Neurosci Lett. 1998;242:105–8. doi: 10.1016/s0304-3940(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci. 2002;23:434–40. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- Sakata K, Yamashita T, Maeda M, Moriyama Y, Shimada S, Tohyama M. Cloning of a lymphatic peptide/histidine transporter. Biochem J. 2001;356:53–60. doi: 10.1042/0264-6021:3560053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Keep RF, Hu Y, Smith DE. PEPT2 (Slc15a2)-mediated unidirectional transport of cefadroxil from cerebrospinal fluid into choroid plexus. J Pharmacol Exp Ther. 2005;315:1101–8. doi: 10.1124/jpet.105.090654. [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Keep RF, Brosius FC., 3rd Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol Pharm. 2004;1:248–56. doi: 10.1021/mp049944b. [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Keep RF, Xiang J, Brosius FC., 3rd Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J Biol Chem. 2003;278:4786–91. doi: 10.1074/jbc.M207397200. [DOI] [PubMed] [Google Scholar]
- Shu C, Shen H, Teuscher NS, Lorenzi PJ, Keep RF, Smith DE. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther. 2002;301:820–9. doi: 10.1124/jpet.301.3.820. [DOI] [PubMed] [Google Scholar]
- Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56:1765–91. doi: 10.1016/j.addr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stummer W, Reulen HJ, Novotny A, Stepp H, Tonn JC. Fluorescence-guided resections of malignant gliomas--an overview. Acta Neurochir Suppl. 2003;88:9–12. doi: 10.1007/978-3-7091-6090-9_3. [DOI] [PubMed] [Google Scholar]
- Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, Reulen HJ. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien) 1998;140:995–1000. doi: 10.1007/s007010050206. [DOI] [PubMed] [Google Scholar]
- Stvolinsky SL, Kukley ML, Dobrota D, Matejovicova M, Tkac I, Boldyrev AA. Carnosine: an endogenous neuroprotector in the ischemic brain. Cell Mol Neurobiol. 1999;19:45–56. doi: 10.1023/A:1006960407008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher NS, Keep RF, Smith DE. PEPT2-mediated uptake of neuropeptides in rat choroid plexus. Pharm Res. 2001;18:807–13. doi: 10.1023/a:1011088413043. [DOI] [PubMed] [Google Scholar]
- Teuscher NS, Novotny A, Keep RF, Smith DE. Functional evidence for presence of PEPT2 in rat choroid plexus: studies with glycylsarcosine. J Pharmacol Exp Ther. 2000;294:494–9. [PubMed] [Google Scholar]
- Teuscher NS, Shen H, Shu C, Xiang J, Keep RF, Smith DE. Carnosine uptake in rat choroid plexus primary cell cultures and choroid plexus whole tissue from PEPT2 null mice. J Neurochem. 2004;89:375–82. doi: 10.1111/j.1471-4159.2004.02333.x. [DOI] [PubMed] [Google Scholar]
- Wada M, Miyakawa S, Shimada A, Okada N, Yamamoto A, Fujita T. Functional linkage of H+/peptide transporter PEPT2 and Na+/H+ exchanger in primary cultures of astrocytes from mouse cerebral cortex. Brain Res. 2005;1044:33–41. doi: 10.1016/j.brainres.2005.02.064. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chiang PP, Hu Y, Smith DE, Keep RF. Role of PEPT2 in glycylsarcosine transport in astrocyte and glioma cultures. Neurosci Lett. 2006;396:225–9. doi: 10.1016/j.neulet.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T, Takagi T, Tohyama M. Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem. 1997;272:10205–11. doi: 10.1074/jbc.272.15.10205. [DOI] [PubMed] [Google Scholar]


