Summary
Purpose
To study the relation between the spike frequency during intraoperative electrocorticography (ECoG) under general anesthesia with isoflurane and that during extraoperative ECoG monitoring in children with intractable neocortical epilepsy.
Methods
Twenty-one children (age, 1–16 years; 15 boys and six girls) who underwent intraoperative and extraoperative ECoG monitoring with subdural electrode arrays were studied. The spike frequency and the spatial pattern of spike frequency were compared between intraoperative and extraoperative ECoGs for each patient (by using Wilcoxon signed-ranks and Spearman’s rank correlation, respectively).
Results
In 15 of 21 patients, the spike frequency was significantly lower during intraoperative than during extraoperative ECoG (mean z = −6.3; p < 0.001). In four of 21 patients, no significant difference was found in the spike frequency between intraoperative and extraoperative recordings. In two of 21 patients, spike frequency reached one spike/min neither during intraoperative nor extraoperative recording; therefore appropriate comparison of spike frequency was not possible. A significant positive correlation in the spike-frequency pattern was seen between intraoperative and extraoperative recordings in nine of nine cases who had ≥10 spikes/min during intraoperative ECoG (mean rho =0.62; p < 0.01), in five of six cases with one to nine spikes/min (mean rho =0.50; p < 0.01), and in none of five cases with less than one spike/min (mean rho =0.13).
Conclusions
General anesthesia often decreases the spike frequency in children with neocortical epilepsy, yet intraoperative ECoG can reliably reflect the awake interictal spiking pattern when spike frequency exceeds one spike/min during intraoperative ECoG recording.
Keywords: Clinical neurophysiology, Pediatric epilepsy surgery, Quantitative interictal intracranial electroencephalography, Focal cortical dysplasia, Tuberous sclerosis complex
Cortical resection is an effective treatment for selected children with medically intractable partial epilepsy of neo-cortical origin (1). To delineate the resection margin in patients with neocortical epilepsy, prolonged extraoperative and/or intraoperative electrocorticography (ECoG) recordings are frequently used (2,3). Despite its common use, reliability of intraoperative ECoG is uncertain in children with neocortical epilepsy, because the relation between intraoperative and extraoperative spike frequency or the spatial pattern of spike frequency has not been well documented (2). The potential effect of anesthetic agents on the degree and pattern of interictal epileptiform activity is one of the unresolved issues (2). The inhalation anesthetic isoflurane is routinely applied during intraoperative ECoG recording in many epilepsy centers, including our institute (4). In adults, interictal spike frequency on ECoG has been reported to decrease at 0.5 to 1.5% concentration of isoflurane (5), to increase at 1.7% (6), and to be stable at 0.25 to 1.25% (4). No similar data are available in children. In the present study of children with neocortical epilepsy, we determined the spike frequency on ECoG by using an automatic spike-detection program, and compared the interictal spike frequency and the spatial pattern of spike frequency between intraoperative recording under the effect of isoflurane and extraoperative recording under nonsedated conditions.
METHODS
Patients
Twenty-one children (ages, 1–16 years; 15 boys and six girls) from a consecutive series of 22 children with drug-resistant neocortical focal seizures studied between March 2002 and May 2003 were included in the study. One child in whom both intraoperative and extraoperative ECoG recordings showed a diffuse burst-suppression pattern was excluded from the present study, because appropriate spike counting could not be performed. The subjects underwent scalp video-EEG monitoring, magnetic resonance imaging (MRI), glucose metabolism positron emission tomography (PET), as well as intraoperative and extraoperative ECoG monitoring with subdural electrodes as part of their presurgical evaluation. On MRI, six children showed cortical tubers, two children each showed a brain tumor, polymicrogyria, and old cerebral infarction due to meningitis, and one child each had subcortical heterotopias, pachygyria, and evidence of previous resection without a specific pathologic diagnosis. In the remaining six children, MRI was normal, but glucose metabolism PET scan showed focal hypometabolic regions in the epileptic hemisphere in five children and focal hypermetabolic regions in the other child. Seven children had a history of secondarily generalized tonic–clonic seizures in addition to partial seizures. Nine children had a history of epileptic spasms in addition to partial seizures.
Location of cortical resection for each patient is shown in Table 1. In all patients, cortical resection included the presumed epileptogenic zone, which consisted of the brain regions showing ictal onset, early spread, persistently and independently spiking bursts, or imaging abnormalities (1), whereas eloquent areas were spared, based on results of functional mapping in eight children (patients 1, 3, 7, 8, 10, 14, 17, and 18). After surgery (follow-up, 9–23 months; mean, 16.3 months), 13 patients had a seizure-free outcome. Patient 19 had rare isolated seizures, six patients (patients 3, 6, 8–10, and 14) had ≥90% reduction of seizure frequency, and patient 7 had 50% reduction of seizure frequency. Histopathologic analyses of the resected tissues were consistent with MR findings in patients who showed structural lesions such as malformations of cortical development, brain tumors, old infarction, and fibrotic scars associated with previous resection. Microscopic cortical dysgenesis was seen in three (patients 7, 11, and 13) of the six patients who had normal MRIs. Gliosis without definite cortical dysplasia was seen in three patients (10, 12, and 18).
TABLE 1.
Patient data
| Patient | Age (yr)/Gender | Scalp ictal EEG onset | MRI | Cortical resection |
|---|---|---|---|---|
| 1 | 1/M | L P-C | Multiple cortical tubers | L P-F |
| 2 | 1/F | R T | Multiple cortical tubers | R T-P-O |
| 3 | 5/M | R T-F | Multiple cortical tubers | R T-P |
| 4 | 5/M | L posterior | Pachygyria | L O-T-P |
| 5 | 5/M | L T | Multiple cortical tubers | L T-O-P |
| 6 | 6/M | Not available | Multiple cortical tubers | R T-O-P |
| 7 | 7/F | L C | Normal | L P-T |
| 8 | 7/M | L C-P | Multiple cortical tubers | L T-P-O |
| 9 | 7/M | L hemisphere | Previous resection | L subtotal hemispherectomy |
| 10 | 7/F | R hemisphere | Normal | R subtotal hemispherectomy |
| 11 | 8/F | R F-C | Normal | L F-T-P |
| 12 | 8/M | L T-C | Normal | L subtotal hemispherectomy |
| 13 | 9/M | L F | Normal | L F |
| 14 | 10/F | R F-C | Subcortical heterotopias | L F-P |
| 15 | 11/M | Not detectable | Brain tumor | Lesionectomy at L precentral gyrus |
| 16 | 11/M | R T | Brain tumor | R T-O |
| 17 | 11/F | L C | Polymicrogyria | L subtotal hemispherectomy |
| 18 | 14/M | R T-F | Normal | R T-P |
| 19 | 15/M | R T | Polymicrogyria | R T-P |
| 20 | 15/M | L T-C | Old infarction | L T-P |
| 21 | 16/M | R hemisphere | Old infarction | R subtotal hemispherectomy |
Four patients (9, 10, 12, and 17) underwent a subtotal hemispherectomy sparing the primary sensorimotor cortex as well as the medial occipital cortex. Patient 21, who preoperatively had a significant contralateral hemiparesis, underwent a subtotal hemispherectomy including the primary sensorimotor cortex but sparing the medial occipital cortex.
F, female; M, male; R, right; L, left; F, frontal, T, temporal; C, central; P, parietal; O, occipital.
Subdural electrode placement
For ECoG recording, platinum grid electrodes (10-mm intercontact distance) were surgically implanted. The total number of electrode contacts in each subject ranged from 56 to 112. The placement of intracranial electrodes was guided by the results of ictal scalp EEG recording, seizure semiology, MRI, and interictal glucose metabolism PET. The primary sensorimotor cortex also was covered with grid electrodes for subsequent functional mapping, if the abnormalities appeared to be close to this region. Electrode plates were stitched to dura mater and to adjacent plates if present, to prevent movement of subdural electrodes after placement. In addition, digital images were obtained in-traoperatively when electrodes were placed, and they were compared with images taken when the brain surface was exposed again after the prolonged extraoperative ECoG monitoring.
Intraoperative ECoG recording
Intraoperative ECoG recordings were obtained by using a 128-channel Nicolet Biomedical BMSI 5000 digital system (sampling rate, 200 Hz; Nicolet Biomedical Inc., Madison, WI, U.S.A.). Medications were discontinued on the morning of the electrode-placement procedure. At the time of craniotomy, anesthesia was induced with 8% sevoflurane in 100% oxygen, fentanyl, 1 μg/kg, and vecuronium, 0.1 mg/kg. Subsequently, anesthesia was maintained with 0.5 to 1.25% isoflurane in an air/oxygen mixture (fraction of inspired oxygen, 0.5). Nitrous oxide was not used. End-tidal carbon dioxide was kept in a hypocarbic range. Fentanyl and vecuronium were added as necessary during maintenance of anesthesia. At least 30 min before the initiation of intraoperative ECoG recording, isoflurane concentration was set between 0.5 and 1.0%. Intraoperative ECoG for the entire set of grid electrodes was continuously and simultaneously recorded for 10 min. The ECoG data were subsequently imported into a 128-channel Stellate HARMONIE 5.0 digital system (sampling rate, 200 Hz; Stellate Systems Inc., Montreal, Quebec, Canada).
Prolonged extraoperative ECoG recording
Extraoperative ECoG recordings were obtained by using a 128-channel Stellate HARMONIE 5.0 digital system for 2 to 5 days. Antiepileptic medications (AEDs) were held until a sufficient number of habitual seizures were captured. For quantitative interictal extraoperative ECoG analysis, three distinct 10-min segments of the continuous awake ECoG were selected based on the following criteria: (a) at least a 3-h interval between segments; and (b) ≥2 h after a partial seizure and ≥8 h after a secondarily generalized tonic–clonic seizure. We have validated this approach and have shown high agreement in spike-frequency pattern among the three 10-min segments in a separate group of children (7).
Visual analysis of ictal ECoG data
Ictal ECoG recordings during prolonged extraoperative monitoring were visually reviewed in the referential and bipolar montages by two of the three electroencephalographers (E.A., A.S., and J.S.), who obtained a consensus for each electrode to determine whether it should be classified as being part of the seizure-onset zone. Seizure onset was defined as a sustained rhythmic change in the ECoG accompanied by subsequent clinically typical seizure activity, not explained by level of arousal, and clearly distinguished from background ECoG and interictal activity (8,9). Brief bursts of spikes and periodic spikes at a frequency of <2 Hz before seizures were not considered part of seizure onset for this analysis, based on a report that brief bursts of spikes and slow periodic spikes are often seen interictally without clinical symptoms and do not necessarily indicate epileptic seizures (9). In cases with this type of initial ECoG changes, we defined the ictalonset electrodes based on the subsequently evolving rhythmic discharge (9).
Quantitative analysis of intraoperative and extraoperative ECoG
Quantitative analysis of intraoperative and extraoperative ECoG data was performed by using the Stellate SENSA 5.0 software. The SENSA software includes the spike-detection module developed at the Montreal Neurological Institute (10–12). The software determines a spike (including sharp wave) based on a preset threshold and a 5-s background time segment immediately preceding the analyzed spike. To exclude spikes from the background, the software identifies potential spikes, disregards a 0.64-s time segment around the spike (0.32 s before and 0.32 s after), and adds 0.64 s at the beginning of the 5-s background time segment. The spike-detection procedure was applied to ECoG data in the referential montage. If the extracranial reference was artifactual, the most inactive intracranial electrode was used as reference. The results of the automatic detection procedure were visually reviewed, and false-positive spikes such as movement artifacts, background fluctuation (13), mu rhythm (14), high-amplitude fast-wave oscillation (15), and mittens (16) were removed. Conversely, the automatic spike-detection program missed true spikes when they occurred after long-lasting high-amplitude polymorphic slow-wave activity or a cluster of spike–wave activity, because the software assumes normal background in a 5-s segment immediately preceding the analyzed spike (7). In these cases, we visually counted all nondetected spikes if their amplitudes were similar or higher compared with adjacent spikes that were detected. Subsequently, the software summed all confirmed spikes in each individual channel for each 10-min segment, yielding a spike-frequency pattern for the subdural electrodes. In cases in which extremely frequent spikes (>30 spikes/min in an electrode) were seen and spike distribution visually appeared consistent, a spike frequency for a 5-min segment instead of a 10-min segment was determined. The spike frequency for three interictal segments was averaged for each electrode.
Statistical analysis
The Wilcoxon signed-ranks test was applied to the spike-frequency data for each patient, to compare the spike frequency during intraoperative ECoG recording with that during extraoperative ECoG recording. Subsequently, the Spearman’s rank correlation was applied to the spike-frequency data for each patient, to determine whether the spatial pattern of spike frequency was similar between intraoperative and extraoperative ECoG recordings. Thereby, the raw spike-frequency values (not spike-frequency categories described later) were used for each statistical analysis. Electrodes showing consistent artifacts during either intraoperative or extraoperative ECoG were excluded from statistical analyses, and a total of 1,751 electrode channels was included in statistical analyses. Interictal spike frequency was subsequently classified into four categories: “frequent” (≥10 spikes/min), “occasional” (<10 but ≥1 spikes/min), “rare” (<1 spike/min), and “zero.” This classification is similar to that used in previous studies (17,18).
A receiver operating characteristics (ROC) analysis was performed to determine whether the spike frequency during intraoperative and extraoperative ECoG could predict seizure-onset electrodes, regardless of the number of multifocal ictal onset foci within the same hemisphere. Because the magnitude of spiking differed widely among patients, spike frequency was normalized to the maximal spike frequency (percentage of the maximal spike frequency) within each individual patient. ROC analyses were performed with the normalized spike frequency as the predictor and “onset” electrodes as the gold standard characterizing true positive (TP, “onset” electrode) and true negative (TN, “nononset” electrodes) cases. Sensitivity and specificity were computed as follows:
where PC represents all positive cases, and NC, all negative cases. The ROC analysis was performed for each individual patient by using normalized spike frequency during intraoperative ECoG recording, and repeated by using normalized spike frequency during extraoperative ECoG recording. The ROC analysis was performed by using the intraoperative and extraoperative ECoG data derived from the 11 individuals (patients 2, 5, 7, 8, 10, 14, 16, 17–19, and 21) who had at least three ictal ECoG recordings available and at least “occasional” spikes during intraoperative ECoG recording. These inclusion criteria were used in our previous study of interictal spike activity in children with neocortical epilepsy (7). Ten patients were excluded from the ROC analysis according to the criteria. Appropriate ROC curves cannot be delineated if only rare spikes occurred. Therefore six patients with at most “rare” spikes during intraoperative ECoG recording were excluded from the ROC analysis. An additional four patients (patients 6, 9, 12, and 20) were excluded from the ROC analysis because of an insufficient number of seizures. No epileptic seizures were captured during extraoperative ECoG monitoring in two of these four patients (patients 6 and 9), who had a resection based on the interictal ECoG findings and neuroimaging abnormalities. Patient 12 revealed continuous spike–waves during slow sleep (in other words, electrical status epilepticus during slow sleep) involving the left frontoparietotemporal region in addition to a single clinical seizure arising from the left parietotemporal region. It is controversial whether widespread brain regions showing continuous spike–waves during slow sleep should be classified as “onset” electrodes. Another patient (patient 20) with a massive old cerebral infarction had only a single clinical seizure during extraoperative ECoG recording, but it remained unclear whether that single seizure was indeed representative of the entire ictal-onset zone in this child.
RESULTS
Spike frequency for intraoperative and extraoperative ECoG
The relation between the spike frequency during intraoperative and extraoperative ECoG is shown in Fig. 1. “Frequent,” “occasional,” “rare,” and “zero” interictal spike discharges were seen at the electrode showing the maximal frequency in nine of 21, six of 21, five of 21, and one of 21 patients during intraoperative recording, and 16 of 21, three of 21, one of 21, and one of 21 patients during extraoperative recording, respectively. In 15 (71%) of 21 patients, the spike frequency was significantly lower during intraoperative ECoG recording compared with extraoperative recording (Fig. 1A; mean z, −6.3; p < 0.001). In four of 21 patients, no significant difference in the spike frequency between intraoperative and extra-operative ECoG recordings was found. In the remaining two patients, the spike frequency neither during intraoperative nor extraoperative recording reached 1 spike/min; therefore appropriate comparison of spike frequency was not possible.
FIG. 1.
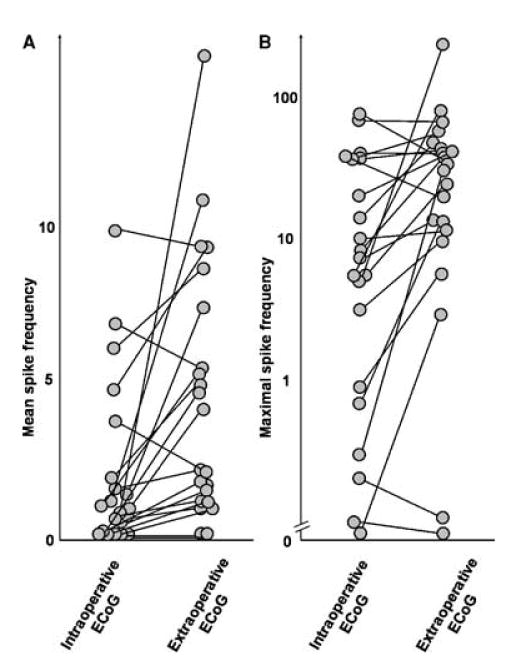
Interictal spike frequency during intraoperative and extra-operative electrocorticography recording in 21 children with neo-cortical epilepsy. Each circle represents mean spike frequency (A) and maximal spike frequency (B) for each patient.
No significant difference in spike frequency was noted during intraoperative ECoG between the subjects with MRI abnormalities [mean spikes/min (standard deviation) = 21 (27)] and those with normal MRI [mean spikes/min, 13 (15) (Mann–Whitney U test; p = 0.8].
Spike-frequency pattern for intraoperative and extraoperative ECoG
A representative case showing the relation between the spike-frequency patterns for intraoperative and extraoperative ECoG is shown in Figure 2. Using the Spearman’s rank correlation, a significant similarity in the spike-frequency pattern was demonstrated between intraoperative and extraoperative recordings in nine of nine cases who had “frequent” spikes (mean rho =0.62; p < 0.01), in five of six cases who had “occasional” spikes (mean rho =0.50; p < 0.01), and in none of five cases who had only “rare” spikes (mean rho =0.13) during intraoperative ECoG recording. The electrodes showing maximal spike frequency during intraoperative and extraoperative ECoG recordings were identical in five of the 15 cases who had at least “occasional” spikes during intraoperative ECoG.
FIG. 2.
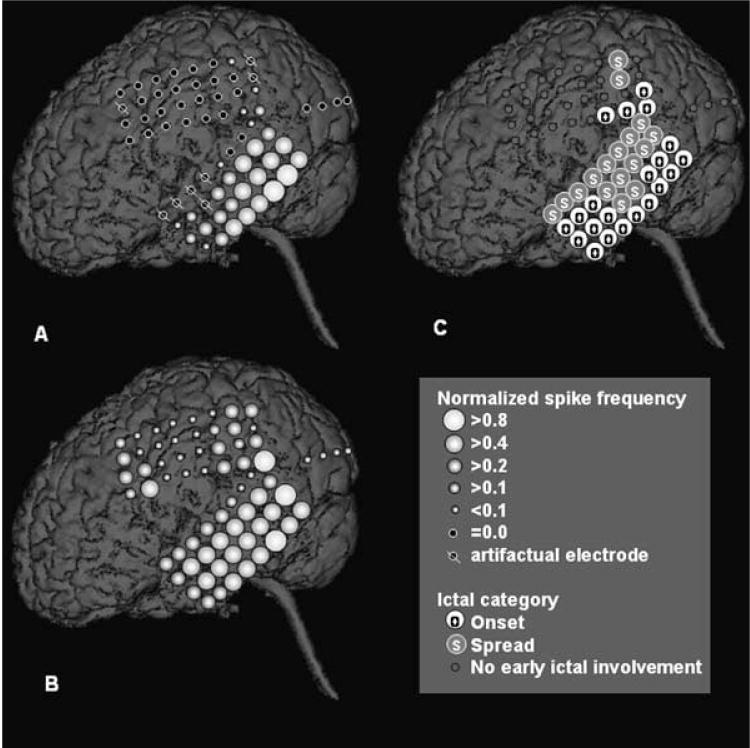
A 5-year-old boy with a diagnosis of tuberous sclerosis complex and uncontrolled seizures. A: Normalized spike frequency during intraoperative electrocorticography (ECoG) is shown. The maximal spike frequency (8.3 spikes/min) was seen in the left posterior temporal region. B: Normalized spike frequency during extraoperative ECoG is shown. The maximal spike frequency (37.4 spikes/min) was also seen in the posterior temporal region. Although the left frontal region also showed frequent interictal spike activity, none of the frontal spikes appeared independent of the spikes in the left temporal region. Both spike topography mappings showed a similar gradient of spike frequency, being highest in the posterior temporal region, intermediate in the anterior temporal region and the parietal region, and lowest in the primary sensorimotor cortex and the lateral occipital region. The Spearman’s rank correlation demonstrated a significant positive correlation in the spike-frequency pattern between intraoperative and extraoperative ECoG recordings (rho = 0.62; p < 0.001). C: Ictal ECoG data are shown. Ictal onset involved the left temporal neocortex and a portion of the inferior parietal region. Ictal discharges propagated to the entire temporal region and a portion of the parietal region within 10 s from the seizure onset.
Correlation between interictal spike frequency and ictal onset
Nine patients (patients 1, 4, 12–16, 19, and 20) had a single ictal-onset focus. Six patients (patients 3, 5, 10, 11, 18, and 21) had two distinct ictal-onset foci, and three patients (patients 2, 7, and 8) had three distinct foci, whereas a single patient (patient 17) had four distinct foci within the same hemisphere.
The spatial relation between interictal spike frequency and ictal-onset zones was initially assessed in the five patients who had “rare” interictal spike activity during intra-operative ECoG recording and at least three ictal recordings available. In these five patients, the brain region showing the maximal spike frequency was ≥3 cm away from “onset” electrodes and was not considered as a part of the presumed epileptogenic zone described earlier (1). Conversely, a single patient (patient 13) had “rare” interictal spike activity during extraoperative ECoG recording. In this patient, the brain region showing the maximal spike frequency was outside of the lobe showing ictal onset and was not surgically resected, although the patient became seizure free. The spatial relation between interictal spike frequency and ictal-onset zones was subsequently assessed in the 11 patients who had at least three ictal ECoG recordings available and at least “occasional” spikes during both intraoperative and extraoperative recordings. The electrode showing the maximal spike frequency during intraoperative ECoG was within “onset” zones in seven of 11 patients, within 2 cm from “onset” electrodes and associated with neuroimaging abnormalities in two of 11 patients, and >2 cm away from “onset” electrodes but associated with neuroimaging abnormalities, as well as persistently and rhythmically spiking bursts (Fig. 3), in two of 11 patients. Similarly, the electrode showing the maximal spike frequency during extraoperative ECoG was within “onset” zones in seven of 11 patients, within 2 cm from “onset” electrodes and associated with neuroimaging abnormalities in one of 11 patients, and >2 cm away from “onset” zones but associated with persistently and rhythmically spiking bursts in three of 11 patients.
FIG. 3.
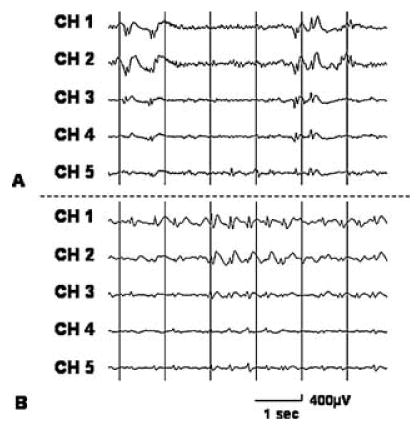
Independent repetitive spike–wave bursts on intraoperative and extraoperative electrocorticography (ECoG) in a 10-year-old girl with uncontrolled seizures. Both (A) intraoperative and (B) extraoperative ECoG showed frequent independent spike–wave bursts in the right subfrontal region. Ictal ECoG showed rhythmic fast-wave bursts in the right middle and superior frontal regions, and the subfrontal regions did not receive early ictal propagation. Brain magnetic resonance imaging showed a subcortical hetero-topia in the right subfrontal region, and glucose positron emission tomography scan showed glucose hypometabolism in the cortex overlying the heterotopia.
Four of the six patients showing “rare” interictal spike activity during intraoperative ECoG recording had at least “occasional” spikes during extraoperative ECoG recording. The brain regions showing the maximal spike frequency during extraoperative ECoG recording were within 2 cm of “onset” electrodes and associated with neuroimaging abnormalities in three patients and within “onset” zones in the remaining patient; the regions were considered to be a part of the presumed epileptogenic zones in these four patients.
The ROC analysis was performed by using the 11 patients who had at least “occasional” spikes during both intraoperative and extraoperative ECoG recordings (Fig. 4). Eight of the 11 patients (73%) had at least two distinct ictal-onset foci. The ROC analysis demonstrated that electrodes showing >20% of the maximum of the normalized spike frequency during intraoperative recording had a mean specificity of 0.87 and a mean sensitivity of 0.30 for concordance with “onset” electrodes as the “gold standard.” Similarly, electrodes showing >20% of the maximal spike frequency during extraoperative ECoG had a mean specificity of 0.84 and a mean sensitivity of 0.45.
FIG. 4.
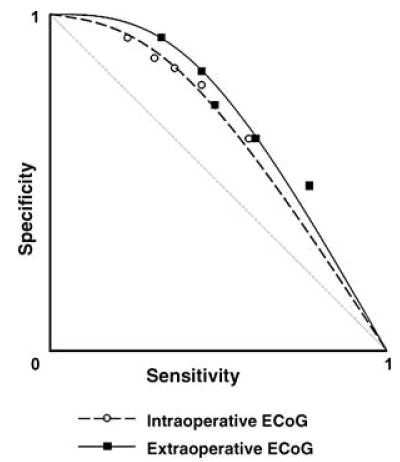
Receiver operating characteristics (ROC) curve. Cutoff thresholds of 40%, 20%, 15%, 10%, and 5% of the maximal spike frequency during intraoperative electrocorticography (ECoG) resulted in mean sensitivity of 0.22, 0.30, 0.35, 0.44, and 0.57, as well as mean specificity of 0.94, 0.87, 0.85, 0.79, and 0.63 for detection of “onset” electrodes, respectively. Similarly, cutoff thresholds of 40%, 20%, 15%, 10%, and 5% of the maximal spike frequency during extraoperative ECoG resulted in mean sensitivity of 0.32, 0.45, 0.47, 0.59, and 0.75, as well as mean specificity of 0.93, 0.84, 0.74, 0.64, and 0.50 for detection of “onset” electrodes. Sensitivity and specificity for each cutoff threshold are shown by white circles (for intraoperative ECoG) and black squares (for extraoperative ECoG).
The brain regions showing maximal spike frequency were surgically resected in 14 of the 15 patients who showed at least “occasional” interictal spike activity during intraoperative ECoG recording. In a single patient (patient 7), the primary sensory leg area, which showed the maximal spike frequency during intraoperative and extraoperative recordings, was not surgically resected, and the patient had 50% reduction of seizure frequency after surgery.
DISCUSSION
The present study demonstrates that general anesthesia with isoflurane often decreases interictal spike frequency in children with neocortical epilepsy, but that the spike-frequency pattern during intraoperative ECoG is similar to that during extraoperative ECoG in 14 of 15 cases, in which more than one spike/min occurred during intraoperative recording. The results in the present study also suggest that intraoperative ECoG showing only “rare” (<1 spike/min) spike activity is a poor indicator of the epileptogenic zone. The spike data during intraoperative recording seemed to be less reliable than those during extraoperative recording in the four patients showing “rare” spikes during intraoperative ECoG but at least “occasional” spikes during extraoperative ECoG recording. The data from the present study did not prove a specific mechanism of suppressive effect of isoflurane on spike activity.
Brain regions showing the maximal spike frequency more than one spike/min were often concordant with a portion of the visually defined “onset” zones in the present study. This finding may warrant the additional placement of subdural grid electrodes on the adjacent cortex before dural closure, when frequent interictal spike activity is seen in the edge of the grid during intraoperative ECoG recording. The result is consistent with our previous study in a separate group of children (7), which reported that brain regions showing the highest spike frequency were found to be a part of the “onset” zones in all of the 13 children with neocortical epilepsy and that electrodes showing >18% of the maximal spike frequency had a mean sensitivity of 0.71 for detecting “onset” electrodes. Conversely, sensitivity for detecting “onset” electrodes was considerably lower in the present study, compared with the previous study (7). In the present study, electrodes showing >20% of the maximum of the normalized spike frequency during intraoperative and extraoperative ECoG had a mean sensitivity of 0.30 and 0.45 for concordance with “onset” electrodes, respectively. Twelve of the 13 subjects in the previous study had a single ictal-onset focus (7), whereas eight of the 11 subjects applied to the ROC analysis in the present study had at least two distinct ictal-onset zones. Thus the discrepancy in sensitivity between the present and previous studies may be due to the number of spatially distinct ictal-onset foci. The findings suggest that interictal spike activity on ECoG, regardless of intraoperative or extraoperative recording, may not predict every distinct focus, but a subset of the seizure foci in children with multifocal neocortical epilepsy.
The interpretation of this study requires consideration of several methodologic issues. First, the results of the automatic spike-detection procedure were visually reviewed, and false-positive spikes were removed in the present study. However, it was not possible on intracranial ECoG recordings to differentiate “small sharp spikes” (14) from pathologic epileptiform activity. “Small sharp spikes” are monophasic low-amplitude sharply contoured waveforms on scalp EEG recordings and do not have a specific clinical significance. The waveform of “small sharp spikes” on intracranial ECoG recording has not been reported previously. We hypothesize that sharply contoured waves with uncertain clinical significance, such as “small sharp spikes,” could be incidentally seen in any location on both intraoperative and extraoperative ECoG recordings. Such sharply contoured waves are difficult to differentiate from pathologic epileptiform activity and may affect the spike-frequency patterns considerably when only “rare” inter-ictal epileptiform activity is observed. In contrast, the effects of such “rare” nonepileptic sharply contoured waves on the spike-frequency pattern would be negligible if interictal spike frequency exceeds one spike/min, regardless of intraoperative or extraoperative ECoG recording.
Factors that may affect interictal spike frequency include inhalation anesthetic agents, opioids, AEDs, postictal state, and sleep. Some of these issues were addressed in our previous study (7). Inhalation anesthetic agents such as nitrous oxide and sevoflurane have been reported to decrease interictal spike frequency in patients with temporal lobe epilepsy (19,20). None of these inhalational agents were used during intraoperative ECoG recording in the present study. Several reports suggest that fentanyl may decrease (21) or increase (22) spike frequency during intraoperative ECoG in patients with temporal lobe epilepsy. Morphine has been reported to increase spike frequency at high doses but to decrease spike frequency at lower doses in rats (23). The effect of opioids on interictal spiking cannot be eliminated in the present study, because fentanyl and morphine were intravenously administered for pain control as necessary during intraoperative and extraoperative ECoG recording, respectively. Previous scalp EEG and long-term extraoperative ECoG studies in adults with partial epilepsy found that interictal spike frequency was increased during the 24-h period after a secondarily generalized tonic–clonic seizure or partial seizures but not simply after a decrease in medication (24,25). In those studies, the subjects had scalp EEG and prolonged ECoG monitoring for >10 days, and the seizure frequency generally ranged from weekly to monthly. Conversely, the present study included 21 children, who underwent extraoperative ECoG monitoring for ≤5 days and whose seizure frequency ranged from daily to weekly, irrespective of medication. To maximize the amount of extra-operative ECoG segments eligible for the statistical analysis and to minimize the potential effect of seizures on interictal spike frequency, we selected awake ECoG segments ≥8 h after a secondarily generalized tonic–clonic seizure and ≥2 h after a partial seizure for the analysis of interictal spike frequency. In patients with frequent seizures, which are common in children with intractable epilepsy, the effect of seizures on interictal spiking cannot be completely eliminated. In addition, sleep was reported to precipitate interictal spike activity (26) on scalp EEG. Few studies have attempted to determine whether increase or decrease of spike frequency due to medications, postictal state, or sleep is confined to the region of seizure onset in children with neocortical epilepsy. To determine the spatial relation between spike frequencies during intraoperative and extraoperative ECoG recording in the present study, a nonparametric (rank order) test was applied to diminish such effects by removing the effect of global increases or decreases of absolute spike frequency on the results.
The relation between spike frequency and surgical outcomes was not fully assessed in the present study because of insufficient follow-up periods. The spike frequency was categorized quite roughly as “frequent,” “occasional,” “rare,” and “zero,” because of the limited number of patients, when the relation between spike frequency and ictal onset zones was examined. Therefore further follow-up studies on surgical outcome using much larger numbers of patients and raw spike-frequency values may be warranted to determine the optimal cutoff value of interictal spike variables to be used for tailoring the margins of the resection. Studies using intraoperative ECoG showed that long-lasting repetitive spike activity is frequently observed in dysplastic cortex, whereas less epileptiform activity is observed in the normal-appearing adjacent cortex (27,28). Complete resection of regions showing such repetitive spike activity was related to favorable surgical outcome (27). In the present study, intraoperative and extraoperative ECoG recordings showed persistently and rhythmically spiking bursts >2 cm away from “onset” electrodes in a subset of patients (see Fig. 3). Regardless of ictal involvement, they were surgically resected in the present study, unless such brain regions were within the eloquent cortex.
Acknowledgments
This work was supported by NIH grants NS47550, NS34488, and NS38324. We are grateful to Craig Watson, M.D., Ph.D., Judy Ahn-Ewing, BA, R EEG/EP T, CNIM, Howard L. Wolfe, R.EEG T., Carol Pawlak, R.EEG T., Ann Atto, R.EEG/EP T., Monica Adams, B.S.N., M.S.A., and the staff of the Divisions of Electroneurodiagnostics as well as Anesthesiology at Children’s Hospital of Michigan, Wayne State University, for the assistance in performing the studies described.
References
- 1.Luders HO, Engel J, Jr, Munari C. General Principles. In: Engel J Jr, editor. Surgical treatment of epilepsies. 1993. 2nd ed. New York: Raven Press; pp. 137–53. [Google Scholar]
- 2.Keene DL, Whiting S, Ventureyra EC. Electrocorticography. Epileptic Disord. 2000;2:57–63. [PubMed] [Google Scholar]
- 3.Onal C, Otsubo H, Araki T, et al. Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg. 2003;98:1017–26. doi: 10.3171/jns.2003.98.5.1017. [DOI] [PubMed] [Google Scholar]
- 4.Fiol ME, Boening JA, Cruz-Rodriguez R, et al. Effect of isoflurane (Forane) on intraoperative electrocorticogram. Epilepsia. 1993;34:897–900. doi: 10.1111/j.1528-1157.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 5.Ito BM, Sato S, Kufta CV, et al. Effect of isoflurane and enflurane on the electrocorticogram of epileptic patients. Neurology. 1988;38:924–8. doi: 10.1212/wnl.38.6.924. [DOI] [PubMed] [Google Scholar]
- 6.Watts AD, Herrick IA, McLachlan RS, et al. The effect of sevoflurane and isoflurane anesthesia on interictal spike activity among patients with refractory epilepsy. Anesth Analg. 1999;89:1275–81. [PubMed] [Google Scholar]
- 7.Asano E, Muzik O, Shah A, et al. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. 2003;44:425–34. doi: 10.1046/j.1528-1157.2003.38902.x. [DOI] [PubMed] [Google Scholar]
- 8.Spencer SS, Guimaraes P, Katz A, et al. Morphological patterns of seizures recorded intracranially. Epilepsia. 1992;33:537–45. doi: 10.1111/j.1528-1157.1992.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 10.Gotman J, Gloor P. Automatic recognition and quantification of interictal epileptic activity in the human scalp EEG. Electroencephalogr Clin Neurophysiol. 1976;41:513–29. doi: 10.1016/0013-4694(76)90063-8. [DOI] [PubMed] [Google Scholar]
- 11.Gotman J. Automatic recognition of epileptic seizures in the EEG. Electroencephalogr Clin Neurophysiol. 1982;54:530–40. doi: 10.1016/0013-4694(82)90038-4. [DOI] [PubMed] [Google Scholar]
- 12.Gotman J. The use of computer in analysis and display of EEG data. In: Daly D, Pedley T, editors. Current practice of clinical EEG. 2nd ed. New York: Raven Press; 1990. pp. 51–83. [Google Scholar]
- 13.Benbadis SR, Tatum WO. Overintepretation of EEGs and misdiagnosis of epilepsy. J Clin Neurophysiol. 2003;20:42–4. doi: 10.1097/00004691-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mizrahi EM. Avoiding the pitfalls of EEG interpretation in childhood epilepsy. Epilepsia. 1996;37(suppl 1):S41–51. doi: 10.1111/j.1528-1157.1996.tb06021.x. [DOI] [PubMed] [Google Scholar]
- 15.Aoki F, Fetz EE, Shupe L, et al. Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor tasks. Clin Neurophysiol. 1999;110:524–37. doi: 10.1016/s1388-2457(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 16.Misulis KE, Head TC. Normal electroencephalographic patterns. In: Misulis KE, Head TC, editors. Essentials of clinical neurophysiology. 3rd ed. Burlington, MA: Butterworth Heinemann; 2003. pp. 65–84. [Google Scholar]
- 17.Kanazawa O, Blume WT, Girvin JP. Significance of spikes at temporal lobe electrocorticography. Epilepsia. 1996;37:50–5. doi: 10.1111/j.1528-1157.1996.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 18.Fedi M, Reutens D, Okazawa H, et al. Localizing value of alpha-methyl-L-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57:1629–36. doi: 10.1212/wnl.57.9.1629. [DOI] [PubMed] [Google Scholar]
- 19.Sato Y, Sato K, Shamoto H, et al. Effect of nitrous oxide on spike activity during epilepsy surgery. Acta Neurochir. 2001;143:1213–5. doi: 10.1007/s007010100016. [DOI] [PubMed] [Google Scholar]
- 20.Endo T, Sato K, Shamoto H, et al. Effects of sevoflurane on electro-corticography in patients with intractable temporal lobe epilepsy. J Neurosurg Anesthesiol. 2002;14:59–62. doi: 10.1097/00008506-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Koyama S, Makino Y, Tanaka K, et al. Fentanyl administration during sevoflurane anesthesia suppresses spike waves from epileptic focus on electrocorticogram. Masui. 2002;51:755–8. [PubMed] [Google Scholar]
- 22.Manninen PH, Burke SJ, Wennberg R, et al. Intraoperative localization of an epileptogenic focus with alfentanil and fentanyl. Anesth Analg. 1999;88:1101–6. doi: 10.1097/00000539-199905000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Massotti M, Gale K. Electroencephalographic evidence for a dose-related biphasic effect of morphine on bicuculline-induced seizures in the rat. Epilepsy Res. 1989;4:81–9. doi: 10.1016/0920-1211(89)90012-0. [DOI] [PubMed] [Google Scholar]
- 24.Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- 25.Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroencephalogr Clin Neurophysiol. 1989;72:7–15. doi: 10.1016/0013-4694(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Minecan D, Natarajan A, Marzec M, et al. Relationship of epileptic seizures to sleep stage and sleep depth. Sleep. 2002;25:899–904. [PubMed] [Google Scholar]
- 27.Palmini A, Gambardella A, Andermann F, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–87. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- 28.Morioka T, Nishio S, Ishibashi H, et al. Intrinsic epileptogenicity of focal cortical dysplasia as revealed by magnetoencephalography and electrocorticography. Epilepsy Res. 1999;33:177–87. doi: 10.1016/s0920-1211(98)00096-5. [DOI] [PubMed] [Google Scholar]


