Abstract
Background
Aging is associated with decreased manual dexterity. Recent findings have identified changes in multi-finger synergies in elderly individuals. The purpose of current work was to study age-related changes in adjustments of multi-finger synergies in preparation to a quick targeted force pulse production task.
Methods
Right-handed elderly and young subjects produced force pulses by pressing on individual force sensors with the four fingers of the right hand. Prior to the force pulse, the subjects produced a constant low level of the total force. An index of multi-finger synergies was computed across trials for each time sample for each subject and each condition.
Results
During steady-state force production, subjects showed co-variation of commands to fingers that stabilized the total force. An index of this co-variation started to decrease prior to the initiation of the force pulse (anticipatory synergy adjustment). Anticipatory synergy adjustments in young subjects started earlier and were larger than in elderly subjects. In particular, young and elderly subjects showed significant anticipatory synergy adjustments starting about 150 ms and about 50 ms prior to the force pulse initiation, respectively. There were no significant differences between the two groups in other indices of performance such as reaction time, time to peak force, and magnitude of the peak force.
Interpretation
We conclude that healthy aging is associated with decreased feed-forward adjustments of multi-finger synergies in preparation to action. This may contribute to the age-related decline in the hand function. Based on similarities in age-related changes in anticipatory postural adjustments and anticipatory synergy adjustments we suggest a hypothesis that the two phenomena may share common mechanisms.
Keywords: synergy, finger, force production, aging, anticipation
Introduction
Aging is associated with a general decline in the hand function, which interferes with activities of daily living (Francis & Spirduso, 2000; Giampaoli et al., 1999; Hughes et al., 1997; Rantanen et al., 1999). These changes may receive contributions from the changes in the number of motor units, muscle composition, and muscle strength (Bemben, 1998; Campell et al., 1973; Kirkendall & Garrett, 1998; Owings & Grabiner, 1998; Winegard et al., 1997). In recent studies, changes in multi-digit synergies have been documented in elderly that can also potentially contribute to decreased performance in everyday prehensile tasks (Shim et al., 2004; Shinohara et al., 2003, 2004). Those studies used a definition of a synergy as a neural organization of elemental variables that stabilizes an important performance variable over repetitive trials at a task (reviewed in Latash et al., 2002). In different studies, elemental variables were associated either with forces and moments of forces produced by individual digits on a hand-held object (Shim et al., 2003; Zatsiorsky & Latash, 2004) or with hypothetical commands to fingers (finger modes, Danion et al., 2003; Latash et al., 2001). Multi-digit synergies stabilizing such performance variables as the total force and the total moment produced on an external object have been shown to be weaker in elderly as compared to young persons (Shim et al., 2004; Shinohara et al., 2003).
In this study, we use the idea of multi-digit synergies to address the following question: Is aging associated with a decreased modulation of multi-finger synergies in a feed-forward manner in preparation to a quick action? This question is based on a recent series of studies that have demonstrated a novel phenomenon termed anticipatory covariation (ACV) or anticipatory synergy adjustment (ASA) (Olafsdottir et al., 2005; Shim et al., 2005). Those studies have shown that an index of a multi-finger synergy stabilizing the total force produced by a set of fingers shows a decline 100-150 ms prior to the initiation of a quick force pulse. Such changes were not seen when the subjects were required to produce similar force pulses under a simple reaction time instruction (Olafsdottir et al., 2005). The functional importance of ASAs has been assumed to turn off synergies that would otherwise counteract a planned quick action.
There is another phenomenon that resembles ASAs, anticipatory postural adjustments (APAs, reviewed in Massion 1992). APAs are seen, in particular, in standing subjects as changes in the activity of postural muscles about 100 ms prior to an action by the subject that is associated with a postural perturbation. APAs have been interpreted as reflections of a feed-forward control mechanism with the purpose to generate forces and moments of forces that act against expected perturbing forces and torques (Bouisset & Zattara, 1990; Cordo & Nashner, 1982). APAs have been reported to be delayed and reduced in magnitude in elderly persons (Inglin & Woollacott, 1988; Rogers et al., 1982; Woollacott et al., 1988).
In an earlier paper (Olafsdottir et al., 2005), we have speculated that ASAs and APAs could be phenomena of a common nature: Both reflect feed-forward changes in multi-muscle synergies related to a planned adjustment of a steady-state (postural) task. Based on this idea, we hypothesized that elderly persons would show a reduced ability to produce ASAs, similar to their documented reduction in APA generating abilities. Testing this hypothesis is important for two reasons. First, if supported, this hypothesis would imply a basic problem with feed-forward control of synergies in elderly, a problem that may need to be addressed with special exercise. Second, proving this hypothesis would provide additional evidence for the commonality of the phenomena of ASAs and APAs – a result that would mean reconsideration of the nature and role of APAs in postural control.
Methods
Subjects
Ten young (on average, 27 years old with the standard deviation, SD=4) and ten elderly (77 years old, SD=4) subjects volunteered to participate in the study. Both groups consisted of five males and five females. The average height and mass were 172 (SD=12.1) cm and 66.1 (SD=13.0) kg for the young subjects, and 165.4 (SD=10.5) cm and 72.9 (SD=14) kg for the elderly subjects. All subjects were healthy and right-handed, according to their preferential hand use during writing and eating. The elderly subjects were recruited from a local retirement community and passed a screening process that involved a cognition test (mini-mental status exam ≥24 points), a depression test (Beck depression inventory ≤ 20 points), a quantitative sensory test (monofilaments ≤ 3.22) and a general neurological examination. We purposefully selected for the study elderly subjects who exercised regularly and were in a generally good physically shape (self-reported). All subjects gave informed consent according to the procedures approved by the Office for Research Protection of The Pennsylvania State University.
Apparatus
Four piezoelectric sensors (Model 208A03, PBC Piezotronics Inc., Depew, NY, USA) amplified by AC/DC conditioners (M482M66, PBC Piezotronics, Inc., Depew, NY, USA) were used to measure the forces generated by the fingers. Cotton pads were attached to the surface of the sensors to increase friction and prevent possible effects of skin temperature. The sensors were placed in a metal frame sitting in a grove on a wooden board. The sensors were medio-laterally spaced 30 mm apart and could be adjusted in the forward-backward direction within 60 mm to fit each subject's hand anatomy. Once the appropriate position of the sensors had been determined, double sided tape was placed under the bases of the sensors to prevent them from moving from that position.
During the experiment, the subjects sat in a chair facing the testing table with the right shoulder at approximately 45° of abduction and flexion, and the elbow flexed about 135°. Metacarpophalangeal joints were flexed about 20° and all interphalangeal joints were slightly flexed such that the hand formed a dome. A wooden piece, shaped to fit comfortably under the subject's palm, helped maintain a constant configuration of the hand and fingers. The forearm was attached to the board with Velcro straps. A 17“ computer monitor, located about 0.8 m away from the subject, displayed the task (see later) and the actual total force produced by all four fingers. In reaction time trials, imperative auditory signals were delivered through the headphones. Figure 1 displays the experimental setup. A LabVIEW-based program (National Instruments, Austin, TX, USA) was used for data acquisition. The data were collected at 1000 Hz with a 12-bit resolution.
Figure 1.
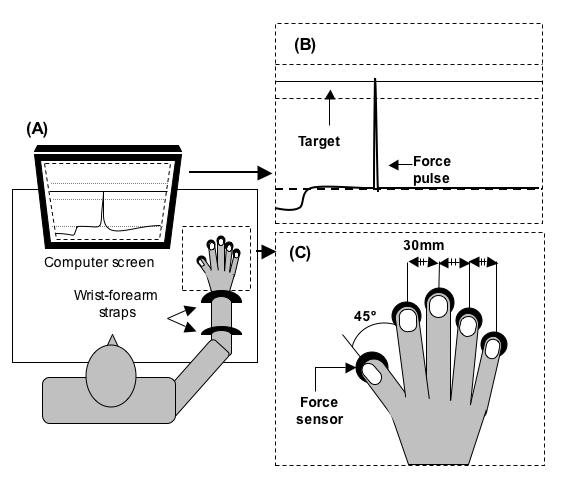
A. Schematic illustration of the experimental setup. B. The experimental task shown on the computer monitor. C. The configuration of force sensors and fingers.
Procedure
Prior to each trial the subject sat relaxed with the fingers of the right hand resting on the sensors. The computer generated two beeps (get ready) and a cursor showing the total force generated by all four fingers started to move across the screen. There were six control trials and two main series. First, maximal voluntary contraction (MVC) force was measured. In these trials, the subjects were required to press “as hard as possible“, with all four fingers. Subjects were given an interval of 3 s to reach peak force. Two attempts at the MVC task were recorded, and the attempt with the higher peak force was used to set up other tasks. Following the MVC task, subjects were asked to produce ramp patterns of force from 0 to 10% of MVC over 5 s by pressing down with one finger at a time in separate trials. They were instructed not to pay attention to possible force production by other fingers of the hand and not to lift any finger off its sensor at any time. An oblique red line was shown on the screen, and the participant's task was to trace this line in time with the cursor representing the force of the task finger. These data were used to generate linear estimates of the relations between changes in individual finger forces and change in the total force during multi-finger tasks (the Jacobian; Scholz et al., 2002). These relations are non-trivial because of the phenomenon of enslaving, i.e. unintended force production by fingers when other fingers of the hand produce force (Kilbreath & Gandevia, 1994; Zatsiorsky et al., 2000).
There were two main series of trials, reaction time (RT) and self-paced (SP). In these series, horizontal lines representing a background force (FBG, 5% of MVC) and a target force (FT, 25% of the MVC with 5% error margins) were displayed on the screen. A vertical line indicated the time of 3 s after the initiation of each trial. Each trial lasted 7 s. At the trial initiation, the subject sat relaxed with the right hand fingers positioned on the sensors. After a get ready signal, a cursor showing the total force started moving over the screen at a constant speed. The subject was asked to press on the sensors such that the total force trace followed the FBG line, wait until the cursor crossed the vertical line, and then produce a very quick force pulse to FT. All the subjects were able to stabilize total force by the time 3 s; this was confirmed by visual observation. We also performed linear regression analysis on the force data from four randomly selected subjects within the time window from 3 s to 3.3 s. The largest slope of the regression line was 0.0005 N/s suggesting that the subjects did indeed achieve steady-state force production by that time. Hence, the interval from crossing the vertical line to the force pulse inititation is referred to as “steady-state”.
In SP trials, the subject was free to initiate the force pulse at any time after the vertical line but was specifically instructed not to produce the pulse immediately after the cursor crossed the vertical line. In RT trials, the subjects produced the force pulse as quickly as possible in response to a brief (100 ms) “chirp” sound, delivered via headphones unpredictably within a 300 ms time interval starting 300 ms after the force trace crossed the vertical line on the screen. Young subjects performed 15 trials within each series with 8 s intervals between the trials and 3 min intervals between the series. Elderly subjects performed 20 trials within each series. More trials were collected for elderly subjects because we expected more rejected trials for this group (see later). The trials within each series were presented in blocks. The order of series was balanced across subjects. Three practice trials were given prior to each series.
Data analysis
The data were processed off-line using MatLab-based software. The force data were low-pass filtered at 80 Hz using a 2nd-order, zero-lag Butterworth filter prior to computation of the derivative of the force with respect to time (dF/dt). Unfiltered data were used for the analysis of force variance components. The following time indices were calculated for the SP and RT series. Time of the initiation of change in the total force (tF) during the force pulse was defined as the time when dF/dt reached 5% of its peak value in that particular trial. Reaction time (tRT) was defined as the time from the beginning of the auditory signal to tF. Trials in the RT series with reaction times shorter than 100 ms and longer than 300 ms as well as trials within both RT and SP series that showed multiple force peaks were rejected from further analysis. On average, for the young subjects, 0.7 trials were rejected in the RT series and 2.1 trials in the SP series while respectively 4.9 and 3.4 trials were rejected for the elderly subjects. Trials with the peak landing outside the error margins were rejected and repeated during the experiment. For both groups, on average, four trials were repeated per subject. Time to peak force (tPF) was defined as the time from tF to the time when peak force occurred. Prior to further analysis, all the trials within each series were aligned by tF.
For further analyses, we used the framework of the uncontrolled manifold (UCM) hypothesis (reviewed in Latash et al., 2002, Scholz & Schöner, 1999). The hypothesis assumes that the controller organizes covariation among elemental variables to stabilize a certain value of a performance variable (total force in our study). Individual finger forces cannot be considered independent elemental variables because of the phenomenon of enslaving (Kilbreath & Gandevia, 1994; Zatsiorsky et al., 2000). Hence, the first step was to convert the data sets from time series of finger forces to time series of elemental variables, force modes.
Force modes were defined similarly to previous studies (Latash et al., 2001; Scholz et al., 2002). Briefly, single-finger force ramp trials were used to compute the enslaving matrix E for each subject. The entries of the E matrix were computed as the ratios of the change in the force of each finger to the change in the total force over the ramp duration. The E matrix was used to compute changes in the vector of hypothetical independent commands to fingers (force modes, m) based on force changes.
Further analysis was done across repetitive trials performed by a subject at different time slices over the duration of the task. According to the UCM hypothesis, more variance in the m space per dimension is expected within a manifold (UCM) corresponding to a constant value of the total force than in an orthogonal complement to that manifold. For each time, ti, the average vector mAV was computed. Then, for each trial j, deviations (Δmj) between mj and mAV were computed. Variance of the Δmj data set was then computed along a direction orthogonal to the UCM computed for an average value of the total force observed across trials at that particular time slice. We will refer to this index as VORT. This was done using the Raleigh fraction (13):
| (1) |
where J is the Jacobian matrix relating small changes in modes (Jm) or forces (J) to changes in the total force, cov(m) is the covariance matrix in the mode space, cov(f) is the covariance matrix in the finger force space, and T is the sign of transpose. For total force stabilization analysis, J = [1, 1, 1, 1], Jm can be computed as: Jm = JE-1T.
VORT reflects the amount of mode variance in the data set that corresponds to a change in the total force. The difference between the total amount of variance (VTOT) and VORT corresponds to variance that does not affect the average value of the performance variable, the total force. We will address this variance as VUCM (variance within the UCM, cf. Latash et al. 2002): VUCM = VTOT − VORT. Note that the finger mode space is four-dimensional, VORT lies along a one-dimensional sub-space corresponding to a change in the total force, while VUCM lies in a three-dimensional null-space, where the total force is constant. Therefore, to compare the amounts of variance per dimension, the following index was used:
| (2) |
Normalization by the total amount of variance per dimension (VTOT/4) was used to compare the data across subjects who could show different amounts of the total variance. Note that positive values of ΔV correspond to proportionally more variance within the UCM, i.e. they correspond to a predominantly negative co-variation among changes in finger modes and may be interpreted as a synergy stabilizing a constant value of the total force. If ΔV = 0, this means that the amount of variance per dimension is the same in directions that correspond to a change in the total force and along directions that keep the force unchanged. ΔV < 0 may be interpreted as a reflection of a predominantly positive co-variation among changes in finger modes contributing to a change in the total force or destabilizing it.
Statistics
The data in the text are presented as group means and standard deviations. Since the experiment was focused on effects of aging, the data were pooled across the genders. For statistical analysis, we used non-parametric tools because of the small number of subjects. Non-parametric Mann-Whitney test was used to compare maximal total force in the four-finger MVC task, peak force (FPEAK), time to peak force (tPF) and reaction time (tRT) within and between the age groups.
To compare ΔV time profiles between the two tasks (RT and SP), a time interval from 300 ms before tF to the onset of force pulse (tF) was selected. Average ΔV indices were computed for each subject and each task over six 50 ms time windows within that time interval. Non-parametric Friedmang's test with factors Condition (RT and SP) and Time was used to test for main effects on ΔV. The level of significance was set at p = 0.05. For post-hoc comparisons, Mann-Whitney tests were used with the p-value adjusted for multiple comparisons (p = 0.0083).
Results
The two subject groups did not differ in most of their performance indices. In particular, the elderly subjects were not significantly weaker and not significantly slower than the young subjects. The only significant difference between the two groups was in the pattern of the ΔV index of force stabilization by multi-finger synergies.
The elderly subject group produced, on average, 86.2 (32.0) N in the four-finger MVC task, which was not significantly different from the 96.5 (29.2) N produced, on average, by the younger subjects. Figure 2 shows a typical performance by a young (solid line) and elderly (dashed line) subject in a reaction-time (RT) trial. Elderly subjects produced force pulses that, on average, reached a peak of 21.0 (7.9) N in RT trials and 21.5 (7.5) N in SP trials. These indices were not significantly different from 24.0 (7.3) N and 24.3 (7.5) N peak forces produced by the younger subjects in respective tasks (p > 0.05; Mann-Whitney test).
Figure 2.
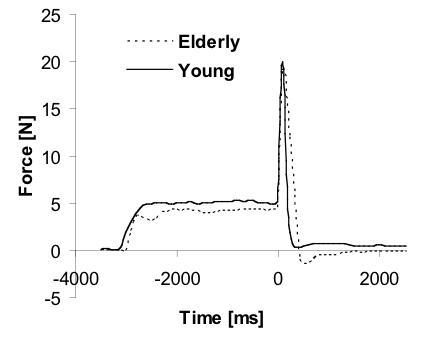
Typical time profile of the total finger force produced by a young (thick line) and an elderly (dashed line) subject in a reaction time trial. Time zero marks the onset of the force pulse (tF) and the time scale is ms to and from that point in time.
The time (tPF) from the onset of the force pulse (tF, defined as the time when the rate of force change reached 5% of its peak value in that trial) to the force peak was approximately 15 ms shorter in the RT trials (young subjects: 115.4 ms, SD=36.0; elderly subjects: 115.4 ms, SD=21.0) than in the SP trials (young subjects: 130.7 ms, SD=29.6 ms; elderly subjects: 131.0 ms, SD=23.8) in both age groups. This difference was statistically significant (p < 0.05) but no difference was found between the age groups within the RT and SP conditions (p> 0.05). The reaction time was, on average, 194.3 (SD=25.3) ms for the elderly and 206.6 (SD=27.0) ms for the young subjects, this difference was not statistically significant (p > 0.05).
To analyze changes in covariation patterns of hypothetical independent commands to fingers (finger modes), we used an index ΔV computed within the framework of the UCM hypothesis (see the Methods). During steady-state force production, ΔV was consistently positive in all subjects corresponding to covariation of finger modes that stabilized the total force value across trials. During the steady-state phase, the average value of ΔV was 1.03 (0.03) in RT trials and 0.99 (0.03) in SP trials for the young subjects while ΔV was respectively 0.67 (0.04) and 0.84 (0.03) for the elderly subjects. The difference between the young and elderly subjects in ΔV value was significant for RT trials (p<0.05, Mann-Whitney test) but did not reach significance level for SP trials. Not that the index ΔV reflects the relative amount of “good variability” (variance within the UCM) in the total variability of commands to fingers (modes); hence higher ΔV in one subject group does not by itself imply that that group was more or less accurate than the other subject group.
Prior to the initiation of a force pulse, ΔV in SP trials started to drift down, whereas in RT trials no such drift was observed. This was true for both elderly and young subjects, but in young subjects this early drift was larger and started earlier. Since subjects could show significantly different baseline ΔV values during steady-state force production, to compare changes in ΔV across subjects, an index (ΔΔV) was computed reflecting changes in ΔV as compared to its value 300 ms prior to tF. Figure 3 illustrates ΔΔV time profiles averaged across subjects within each group, for the SP (solid lines) and RT (dashed lines) conditions separately. For statistical comparisons, ΔΔV values were averaged over 50 ms time windows starting 300 ms prior to tF to tF. A Friedman test showed for both age groups significant effects of both factors, condition (two levels, RT and SP) and time (six levels) (p < 0.05) on the ΔΔV. Mann-Whitney test has confirmed that, in young subjects, ΔΔV for the SP trials became smaller than for the RT trials 150 ms prior to tF. (p < 0.008). In contrast, elderly subjects showed a significant difference between the two conditions only 50 ms prior to tF (p < 0.008). The early drop in ΔΔV, estimated from 300 ms prior to tF to tF, was more than twice as large in the young subjects compared to the elderly subjects (0.24 vs. 0.09) but this difference did not reach significance (p > 0.05 , Mann-Whitney test).
Figure 3.

The average (across subjects) time profiles of changes in ΔV (ΔΔV) for young subjects (A) and elderly subjects (B). The thick solid and dashed lines show averages for the SP and RT tasks, respectively, and thin lines show their standard error. Time zero marks the onset of the force pulse (tF) and the time scale is ms to and from that time point.
During the force pulse, all subjects showed a drop in ΔV, commonly into negative values (Figure 3). This was quantified by calculating the difference between the baseline value 300 ms before tF and the minimum value of ΔV after tF. On average, young subjects had a maximal drop of about 2.2 (1.0) in RT trials and 2.0 (1.0) in SP trials whereas elderly subjects showed on average a maximal drop of about 1.4 (0.8) in RT trials and 1.7 (1.0) in SP trials. This difference was not significant in any of the comparisons (p > 0.05; Mann-Whitney).
Discussion
The main result of the current experiment is the demonstration of significant differences between young and elderly persons in the processes of preparation to a quick action. Both groups were able to modify multi-digit force-stabilizing synergies in preparation to a quick force pulse. However, such preparation started later in elderly persons and led to smaller changes in the synergy index. This finding supports our hypothesis on an age-related decrease in the anticipatory modifications of multi-element synergies during preparation to an action. Taken together with earlier reports on delayed and decreased anticipatory postural adjustments (APAs) in elderly (Inglin & Woollacott, 1988; Rogers et al., 1982; Woollacott et al., 1988), the findings suggest that advanced age leads to a generally decreased use of feed-forward control in preparation to self-initiated actions.
We would like to emphasize that our elderly subjects were purposefully selected to match the young controls in their level of performance. They did not show significantly longer reaction times (cf. Stelmach et al., 1987; Welford, 1984), slower force development (cf. Owings & Grabiner, 1998), or lower force producing abilities (cf. Narici et al., 1991; Shinohara et al., 2003; Winegard et al., 1997) as compared to the younger subjects. Therefore, the observed age-related differences in the synergy index changes were not related to the difference in characteristics of the prepared actions. The contrast between the basically unchanged performance and significantly changed processes of preparation is the strongest reason to claim that the changed indices of preparation of multi-finger synergies is a sign of aging that may be unrelated to the mentioned, well documented changes such as weakening and slowing down.
Force-stabilizing synergies in multi-digit action
A number of studies have documented multi-digit force-stabilizing synergies during steady-state force production tasks and slow changes in the total force (Latash et al., 2001; Scholz et al., 2002; Shim et al. 2003, 2004). However, several studies showed that fast changes in the total force could be associated with co-variation of signals to fingers that could potentially destabilize the total force (Latash et al., 2001, 2002; Scholz et al., 2002). In particular, it was shown that the amount of finger variance within the UCM (VUCM) changed in parallel with the force level while the amount of variance orthogonal to the UCM (VORT) changed in parallel with the first derivative of force. A recent modeling study based on experiments with fast multi-finger force production has confirmed these observations by showing that VORT could exceed VUCM during fast force changes (Goodman et al., 2005).
Our task involved both steady-state and quick force pulse components. During the steady-state force production, both young and elderly persons showed multi-finger synergies stabilizing the total force (positive ΔV values). These results are similar to those reported in earlier studies (Shinohara et al., 2003, 2004). In one of the earlier studies, it has been shown that the index of synergy stabilization is smaller in elderly persons than in younger persons (Shinohara et al., 2004). Our subjects showed a similar trend with smaller ΔV values observed in the elderly subjects. This difference was significant for the steady-state values observed in the RT trials and was under the level of significance in the SP trials.
During the quick force pulse production, both subject groups showed a rapid drop in the synergy index leading sometimes to its negative values that correspond to destabilization of the total force. These results are in line with the mentioned experimental and modeling studies (Goodman et al., 2005; Latash et al., 2001; Scholz et al., 2002).
Aging effects on feed-forward control
A number of studies reported age-related deficits in the production of adequate adjustments in preparation to action. In particular, elderly subjects use excessive grip forces and smaller grip force modulation prior to lifting an object (Cole, 1991; Cole et al., 1999; Kinoshita & Francis, 1996; although see Gilles & Wing, 2003). They also show smaller and delayed APAs (Inglin & Woollacott, 1988; Rogers et al., 1982; Woollacott et al., 1988).
All the mentioned studies analyzed outputs of particular motor elements (muscles and digits), not patterns of their coordination with respect to important task-specific performance variables. In contrast, our study focused on an index of covariation of commands to fingers related to the production of a particular time profile of the total force. The demonstration of delayed and decreased anticipatory synergy adjustments (ASAs) allows to speculate on the relations between ASAs and APAs as well as on general effects of aging on feed-forward control.
ASAs and APAs show the following similarities (De Wolf et al., 1998; Lee et al., 1987; Massion, 1992; Olafsdottir et al., 2005; Shim et al., 2005). First, they are both observed 100-150 ms prior to an action. Second, they are both delayed, i.e., emerge closer to the time of action initiation under the simple reaction time instruction. Third, they are both decreased and delayed in elderly (the current study). This seems to us too much to consider these similarities as coincidental. In contrast, we would like to suggest that APAs, or at least some of the typical changes in muscle activity described as APAs, may represent a particular example of ASAs. In turn, ASAs represent a particular example of feed-forward changes in control signals, an example of changes that are not necessarily reflected in the explicit performance.
Taken together, our results and previously published data suggest that aging is associated with decreased feed-forward control of both explicit performance variables (such as grip force adjustments in Cole et al., 1999; Kinoshita & Francis, 1996) and multi-element synergies (such as those quantified in the current study).
Concluding comments
Our study did not show any differences in the performance of the task by the young and elderly subjects. A natural question emerges: What is the functional role of ASAs if they are not reflected in the task performance? We can only offer tentative suggestions that are beyond the scope of the present study and require further investigation.
First, not all young subjects demonstrate equally clear ASAs (Olafsdottir et al., 2005). Some young subjects show minimal ΔV changes in preparation to action; the mentioned earlier study has suggested that using larger ΔV changes might help subjects avoid excessive destabilization of the total force during the force pulse production. This was supported by more negative values of ΔV during the force pulse in those subjects who showed smaller ASAs. However, in our study, no significant differences were found in the minimal ΔV values during the pulse production.
Another recent study (Kim et al., 2006) investigated ASAs during multi-finger constant force production tasks in preparation to self-triggered and unexpected perturbations applied to one of the fingers. In self-triggered conditions, ASAs were seen starting about 150 ms prior to the perturbation. When similar perturbations were applied unexpectedly, no ASAs were observed. After the perturbation, the subjects restored the force-stabilizing synergy significantly quicker in trials with ASAs. So, one can tentatively conclude that ASAs are not an obligatory mechanism for quick actions or reactions but they may help stabilize the performance variable after the action. In the current study, the subjects were instructed to relax after the force pulse; therefore, we cannot compare the rate of synergy restoration after the force pulse across the subject groups.
All these hypothesis create room for new experimental studies that will hopefully shed more light on the nature and function of ASAs and their changes with age.
Acknowledgments
The screening process of the elderly subjects was conducted at the General Clinical Research Center (The Pennsylvania State University). Special thanks are given to Dr. Simon Goodman for his invaluable help with the UCM analysis.
Footnotes
Grants
The study was in part supported by NIH grants AG-018751, NS-035032, AR-048563, and M01 RR-10732.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bemben MG. Age-related alterations in muscular endurance. Sports Med. 1998;25:259–269. doi: 10.2165/00007256-199825040-00004. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Segmental movement as a perturbation to balance? Facts and concepts. In: Winters JM, Woo SL-Y, editors. Multiple Muscle Systems. Biomechanics and Movement Organization. Springer-Verlag; New York: 1990. pp. 498–506. [Google Scholar]
- Campbell MJ, McComas AJ, Petito F. Physiological changes in aging muscles. J. Neurol. Neurosurg. Psychiat. 1973;36:174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KJ. Grasp force control in older adults. J. Mot. Behav. 1991;23:251–258. doi: 10.1080/00222895.1991.9942036. [DOI] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL, Harper JG. Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J. Neurosci. 1999;19:3238–3247. doi: 10.1523/JNEUROSCI.19-08-03238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J. Neurophysiol. 1982;47:1888–1905. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Danion F, Schöner G, Latash ML, Li S, Scholz JP, Zatsiorsky VM. A force mode hypothesis for finger interaction during multi-finger force production tasks. Biol. Cybern. 2003;88:91–98. doi: 10.1007/s00422-002-0336-z. [DOI] [PubMed] [Google Scholar]
- De Wolf S, Slijper H, Latash ML. Anticipatory postural adjustments during self-paced and reaction-time movements. Exp. Brain Res. 1998;121:7–19. doi: 10.1007/s002210050431. [DOI] [PubMed] [Google Scholar]
- Francis KL, Spirduso WW. Age differences in the expression of manual asymmetry. Exp. Aging Res. 2000;26:169–180. doi: 10.1080/036107300243632. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gibbs J, Dunlop D, Edelman P, Singer R, Chang RW. Predictors of decline in manual performance of older adults. J. Am. Geriatr. Soc. 1997;45:905–910. doi: 10.1111/j.1532-5415.1997.tb02957.x. [DOI] [PubMed] [Google Scholar]
- Giampaoli S, Ferrucci L, Cecchi F, Lo Noce C, Poce A, Santaquilani A, Vescio MF, Menotti A. Hand-grip strength predicts insident disability in non-disabled older men. Age Aging. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- Gilles MA, Wing AM. Age-related changes in grip force and dynamics of hand movement. J. Mot. Behav. 2003;35:79–85. doi: 10.1080/00222890309602123. [DOI] [PubMed] [Google Scholar]
- Goodman SR, Shim JK, Zatsiorsky VM, Latash ML. Motor variability within a multi-effector system: Experimental and analytical studies of multi-finger production of quick force pulses. Exp. Brain Res. 2005;163:75–85. doi: 10.1007/s00221-004-2147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglin B, Woollacott MH. Anticipatory postural adjustments associated with reaction time arm movements: a comparison between young and old. J. Gerontol. 1988;43:M105–M113. doi: 10.1093/geronj/43.4.m105. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J. Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Shim JK, Zatsiorsky VM, Latash ML. Anticipatory adjustments in multi-finger synergies in preparation to a self-triggered perturbation. Exp. Brain Res. 2006 doi: 10.1007/s00221-006-0505-8. in press. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Francis PR. A comparison of prehension force control in young and elderly individuals. Eur. J. Appl. Physiol. Occup. Physiol. 1996;74:450–460. doi: 10.1007/BF02337726. [DOI] [PubMed] [Google Scholar]
- Kirkendall DT, Garrett WE., Jr. The effects of aging and training on skeletal muscle. Amer. J. Sports. Med. 1998;26:598–602. doi: 10.1177/03635465980260042401. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JF, Danion F, Schöner G. Structure of motor variability in marginally redundant multi-finger force production tasks. Exp. Brain Res. 2001;141:153–165. doi: 10.1007/s002210100861. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schöner G. Motor control strategies revealed in the structure of motor variability. Exer. Sport. Sci. Rev. 2002;30:26–31. doi: 10.1097/00003677-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Lee WA, Buchanan TS, Rogers MW. Effects of arm acceleration and behavioral conditions on the organization of postural adjustments during arm flexion. Exp. Brain Res. 1987;66:257–270. doi: 10.1007/BF00243303. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J. Appl. Physiol. 1991;71:1277–1281. doi: 10.1152/jappl.1991.71.4.1277. [DOI] [PubMed] [Google Scholar]
- Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML. Anticipatory covariation of finger forces during self-paced and reaction time force production. Neurosci. Lett. 2005;381:92–96. doi: 10.1016/j.neulet.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Normally aging older adults demonstrate the bilateral deficit during ramp and hold contractions. J. Gerontol. Ser. A., Biol. Sci. & Med. Sci. 1998;53:B425–B429. doi: 10.1093/gerona/53a.6.b425. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Soderberg GL. Age-related changes in postural responses preceding rapid self-paced and reaction time arm movements. J, Gerontol. 1982;47:M159–M165. doi: 10.1093/geronj/47.5.m159. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Danion F, Latash ML, Schöner G. Understanding finger coordination through analysis of the structure of force variability. Biol. Cybern. 2002;86:29–39. doi: 10.1007/s004220100279. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G. The uncontrolled manifold concept: Identifying control variables for a functional task. Exp. Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Shim JK, Latash ML, Zatsiorsky VM. Prehension synergies: Trial-to-trial variability and hierarchical organization of stable performance. Exp. Brain Res. 2003;152:173–184. doi: 10.1007/s00221-003-1527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Lay B, Zatsiorsky VM, Latash ML. Age-related changes in finger coordination in static prehension tasks. J. Appl. Physiol. 2004;97:213–224. doi: 10.1152/japplphysiol.00045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Olafsdottir H, Zatsiorsky VM, Latash ML. The emergence and disappearance of multi-digit synergies during force production tasks. Exp. Brain Res. 2005;164:260–270. doi: 10.1007/s00221-005-2248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force production by the intrinsic and extrinsic hand muscles and finger interaction during maximal contraction tasks. J. Appl. Physiol. 2003;95:1361–1369. doi: 10.1152/japplphysiol.00070.2003. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Scholz JP, Zatsiorsky VM, Latash ML. Finger interaction during accurate multi-finger force production tasks in young and elderly persons. Exp. Brain Res. 2004;156:282–292. doi: 10.1007/s00221-003-1786-9. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Goggin NL, Garcia-Colera A. Movement specification time with age. Exp. Aging Res. 1987;13:39–46. doi: 10.1080/03610738708259298. [DOI] [PubMed] [Google Scholar]
- Welford AT. Psychomotor performance. Ann. Rev. Gerontol. Geriatr. 1984;4:237–273. [PubMed] [Google Scholar]
- Winegard KJ, Hicks AL, Vandervoort AA. An evaluation of the length-tension relationship in elderly human plantarflexor muscles. J. Gerontol. Ser. A., Biol. Sci. Med. Sci. 1997;52:B337–B343. doi: 10.1093/gerona/52a.6.b337. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Inglin B, Manchester D. Response preparation and posture control. Neuromuscular changes in the older adult. Ann. New York Acad. Sci. 1988;515:42–53. doi: 10.1111/j.1749-6632.1988.tb32964.x. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Latash ML. Prehension synergies. Exer. Sport Sci. Rev. 2004;32:75–80. doi: 10.1097/00003677-200404000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li Z-M, Latash ML. Enslaving effects in multi-finger force production. Exp. Brain Res. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]


