Abstract
The σ subunit of bacterial RNA polymerase (RNAP) is required for promoter-specific transcription initiation and can also participate in downstream events. Several functionally important intersubunit interactions between Escherichia coli σ70 and the core enzyme (α2ββ′ω) have been defined. These include an interaction between conserved region 2 of σ70 (σ2) and the coiled-coil domain of β′ (β′ coiled-coil) that is required for sequence-specific interaction between σ2 and the DNA during both promoter open complex formation and σ70-dependent early elongation pausing. Here, we describe a previously uncharacterized interaction between a region of σ70 adjacent to σ2 called the nonconserved region (σ70 NCR) and a region in the N-terminal portion of β′ that appears to functionally antagonize the σ2/β′ coiled-coil interaction. Specifically, we show that the σ70 NCR/β′ interaction facilitates promoter escape and hinders early elongation pausing, in contrast to the σ2/β′ coiled-coil interaction, which has opposite effects. We also demonstrate that removal of the σ70 NCR results in a severe growth defect; we suggest that its importance for growth may reflect its role in promoter escape.
Keywords: promoter escape, RNA polymerase, transcription pausing, λQ, σ70
Introduction
Multisubunit DNA-dependent RNA polymerases are structurally conserved throughout all domains of life (Ebright, 2000). The bacterial RNA polymerase (RNAP) core enzyme (subunit composition α2ββ′ω) is catalytically active for RNA chain elongation during transcription. However, to recognize promoter sequences and initiate transcription, the core enzyme must associate with a σ factor, forming the RNAP holoenzyme (Gross et al, 1998). The primary σ factor of Escherichia coli, σ70, directs transcription from promoters typically defined by two conserved sequence elements, the −10 and −35 elements that are separated by ∼17 base pairs (bp) (Gross et al, 1998). All primary σ factors share four regions of conserved sequence (regions 1–4) that are connected by intervening sequences of variable size (Lonetto et al, 1992), and regions 2 and 4 contain DNA-binding domains that recognize the −10 and −35 elements, respectively (Murakami and Darst, 2003). At least two other regions of σ (regions 1.2 and 3.0) can make sequence-dependent contact with auxiliary promoter elements (Bown et al, 1997; Feklistov et al, 2006; Haugen et al, 2006).
The transcription process can be divided into a number of distinct steps (deHaseth et al, 1998). First, the RNAP holoenzyme binds to duplex promoter DNA to form the closed RNAP–promoter complex. Next, a series of conformational changes leads to the formation of the initiation-competent open complex in which the DNA is locally melted (between −11 and +1) to expose the transcription start site. RNAP can then initiate transcription, typically synthesizing short abortive RNA products that are repetitively released and resynthesized before RNAP breaks its contacts with the promoter and escapes into productive elongation (Hsu, 2002). During the course of elongation, the transcription complex may encounter pause sites and potential arrest sites at which the nascent RNA remains stably bound to the enzyme (Artsimovitch and Landick, 2000). Finally, upon reaching a termination site, RNAP releases the RNA transcript and dissociates from the DNA.
In the context of the RNAP holoenzyme, σ70 forms extensive contacts with the core enzyme that involve each of the four conserved regions of σ70 (Sharp et al, 1999; Murakami et al, 2002; Vassylyev et al, 2002), including regions 2 and 4, which contact the coiled-coil domain of the β′ subunit (Arthur and Burgess, 1998; Young et al, 2001) and the flexible flap domain of the β subunit (Kuznedelov et al, 2002; Geszvain et al, 2004; Nickels et al, 2005), respectively. Both of these interactions have important implications for the functional properties of the holoenzyme at multiple stages of the transcription cycle. The interaction between σ70 region 2 (σ2) and the β′ coiled-coil, in particular, which is essential for holoenzyme formation, is also required for functional interaction between σ70 and the promoter −10 element, allowing σ2 to make sequence-specific contacts with bases of the nontemplate strand in the context of the open promoter complex (Marr and Roberts, 1997; Young et al, 2001).
σ70 can also play functional roles during the elongation phase of transcription (Mooney et al, 2005). The most well-characterized example involves the regulation of late gene transcription from the bacteriophage λ promoter PR′, where σ70 mediates an early elongation pause that is essential for the function of the Q antiterminator protein (λQ), a regulator of late gene expression that enables RNAP to read through specific transcription terminators located within the late gene operon (Roberts et al, 1998) (Figure 1A). Although λQ induces terminator readthrough as a stable component of the transcription elongation complex (Deighan and Hochschild, 2007), it must first engage the RNAP holoenzyme during the early elongation pause to gain access to the elongation complex (Roberts et al, 1998). This engagement process depends on two DNA sequence elements, a Q binding element (QBE) that is located between the PR′ −10 and −35 elements (Yarnell and Roberts, 1992) and a pause-inducing element that is located in the initial transcribed region (Ring et al, 1996) (Figure 1A). The requirements of the engagement process—that DNA-bound λQ interacts with the paused elongation complex—ensure that λQ specifically targets late gene transcription complexes.
Figure 1.
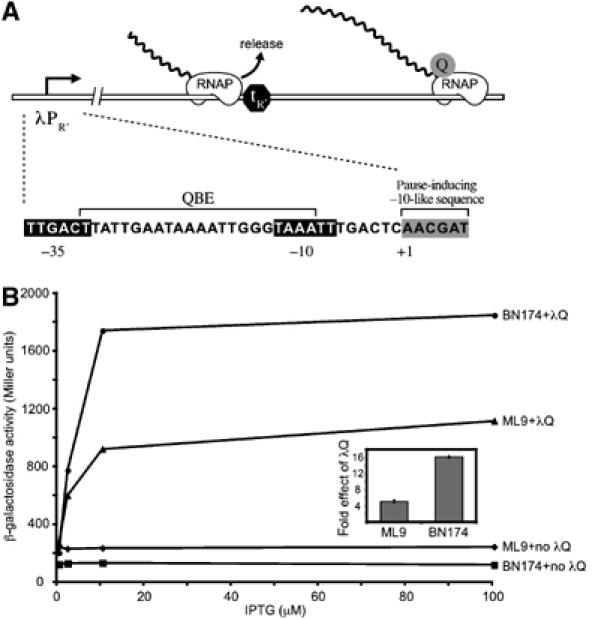
The σ70 L402F substitution impairs λQ-mediated antitermination in vivo. (A) Presence of λQ allows RNAP that has initiated from PR′ to read through transcription terminator tR′. Blow-up depicts functionally important elements at PR′, including the promoter −10 and −35 elements, the QBE, and the pause-inducing −10-like element. (B) Effect of substitution L402F in chromosomally encoded σ70 on λQ-dependent lacZ expression from a PR′-lacZ reporter in vivo. β-Galactosidase assays were performed to quantify lacZ expression levels in BN174 (WT) or ML9 (L402F) cells containing either a plasmid that directed the synthesis of λQ under the control of an IPTG-inducible promoter or a control plasmid that did not encode λQ. Note that the basal level of lacZ expression is ∼2-fold higher in ML9 cells. Inset shows ‘fold effect of λQ' values for cells grown in the presence of 100 μM IPTG; these values were determined by dividing the β-galactosidase activity in the presence of λQ by the β-galactosidase activity in the absence of λQ. Shown are the averages of three independent sets of measurements (and standard deviations).
The PR′ pause-inducing element resembles a promoter −10 element and pausing, which manifests itself in complexes containing nascent RNAs of 16 or 17 nucleotides (nt), is mediated by a protein–DNA interaction between σ2 and the nontemplate strand of the −10-like element (Ring et al, 1996). Moreover, as during open complex formation, establishment of sequence-specific contacts between σ2 and the −10-like pause element depends on the interaction between σ2 and the β′ coiled-coil. Thus, σ70 substitutions that weaken the σ2/β′ coiled-coil interaction (including σ70 substitution L402F) reduce pausing at PR′, resulting in impaired λQ antitermination function both in vitro and in vivo (Ko et al, 1998; Sharp et al, 1999). These σ70 substitutions also destabilize open complexes (Ko et al, 1998), increasing promoter escape (Chan and Gross, 2001).
Here, we describe the results of a genetic screen for σ70 mutations that suppress the PR′ pause defect caused by σ70 substitution L402F. Designed to further probe the σ2/β′ coiled-coil interface, this screen unexpectedly uncovered amino-acid substitutions in the nonconserved region (NCR) of σ70, a region located between conserved regions 1.2 and 2.1 that we show to be important for cell growth. The analysis of these substitutions led to the identification of a previously uncharacterized interaction between the σ70 NCR and a region of β′ located near its N-terminus. We show that this interaction inhibits σ70-dependent pausing during early elongation and also facilitates promoter escape. In contrast, the σ2/β′ coiled-coil interaction is required for σ70-dependent pausing (Ko et al, 1998) and impedes promoter escape (Chan and Gross, 2001). Our results suggest that the interaction between the σ70 NCR and the β′ N-terminal region may functionally antagonize the σ2/β′ coiled-coil interaction during specific stages of the transcription cycle. Furthermore, our finding that the σ70 NCR/β′ interaction facilitates promoter escape suggests a possible explanation for the important role of the σ70 NCR in vivo.
Results
Substitutions in the σ70 NCR that promote λQ-mediated antitermination
As a strategy to functionally probe the σ70–core interface, we performed a genetic screen to identify second-site substitutions in σ70 that restore the ability of RNAP containing σ70 L402F (Eσ70 L402F) to engage the promoter-proximal pause site at λPR′. We designed this screen by taking advantage of the fact that the pause defect of Eσ70 L402F manifests itself as a defect in λQ-mediated antitermination both in vitro and in vivo (Ko et al, 1998). To assay λQ antitermination function at PR′ in vivo, we used a PR′-lacZ fusion, encompassing PR′ sequence from −109 to +232, which includes terminator tR′ (Figure 1A) (Nickels et al, 2002). The level of lacZ expression from this construct reports on the ability of plasmid-encoded λQ to function as an antiterminator for transcripts initiating from PR′. We introduced the PR′-lacZ reporter in single copy on an F′ episome into a strain containing the L402F mutation in the rpoD gene (encoding σ70) or an otherwise isogenic strain containing wild-type (WT) rpoD. Plasmids directing the synthesis of λQ or no λQ under the control of an isopropyl-β-D-thiogalactoside (IPTG)-inducible promoter were introduced into these reporter strains. At maximal induction, λQ increased expression from the test promoter ∼16-fold in cells containing σ70 WT (strain BN174) and only ∼5 fold in cells containing σ70 L402F (strain ML9) (Figure 1B). Western blotting revealed similar levels of λQ in the two strains (data not shown).
To identify second-site substitutions in σ70 that suppress the effect of the L402F substitution on early elongation pausing at PR′, we used error-prone PCR to introduce random mutations into the complete σ70 coding sequence present on plasmid pBRσ70 L402F, which directs the synthesis of σ70 L402F. The resulting expression libraries encoding σ70 L402F with other random substitutions were introduced into reporter strain cells bearing the L402F mutation in rpoD and already containing λQ (see Materials and methods). Clones expressing lacZ at increased levels were identified on indicator medium and the σ70-encoding plasmids were isolated and their effects on lacZ transcription reconfirmed. A total of 17 suppressor substitutions at 15 amino-acid positions were uncovered that increased lacZ transcription specifically in the presence of λQ (Figure 2). Western blotting indicated that in general these second-site substitutions did not enhance lacZ expression by causing increases in λQ protein levels; the only exception was substitution A370V, which caused the λQ protein level to increase by ∼50–100% (data not shown). Surprisingly, the screen did not yield any new substitutions in σ2, although true revertants and other substitutions were frequently observed at residue 402. Rather, all of the second-site substitutions isolated in the screen mapped to the σ70 NCR, a domain located between conserved regions 1.2 and 2.1 that varies in both length and sequence among primary σ factors (Lonetto et al, 1992). We located the amino-acid residues affected by the suppressor mutations in a crystal structure of a σ70 fragment extending from region 1.2 to 2.4 (Malhotra et al, 1996). The substitutions affecting surface-exposed residues (R281C, E284K, R285H, K299E, F306S, K359E, I367F, A370V, and K371E), which define a semicontinuous surface (Figure 3), were selected for further study; we note that five of these nine residues are positively charged.
Figure 2.
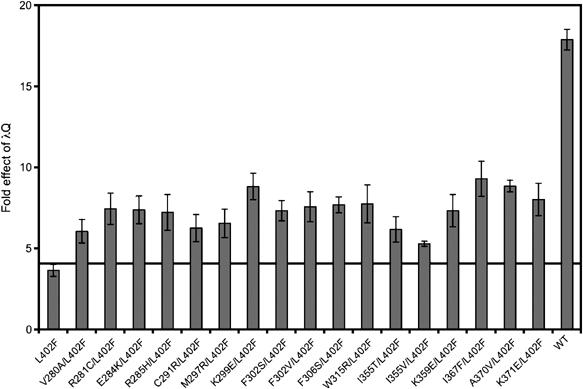
Substitutions in the σ70 NCR suppress the defect in λQ-mediated antitermination caused by the L402F substitution in σ70. ML9 cells encoding σ70 L402F at the chromosomal rpoD locus and harboring the PR′-lacZ fusion on an F′ episome were cotransformed with compatible plasmids directing the synthesis of the indicated σ70 protein and directing the synthesis of either λQ or no λQ. Cells were grown in the presence of 100 μM IPTG and assayed for β-galactosidase activity. ‘Fold effect of λQ' values were calculated as described for Figure 1. Shown are the averages of three independent sets of measurements (and standard deviations).
Figure 3.
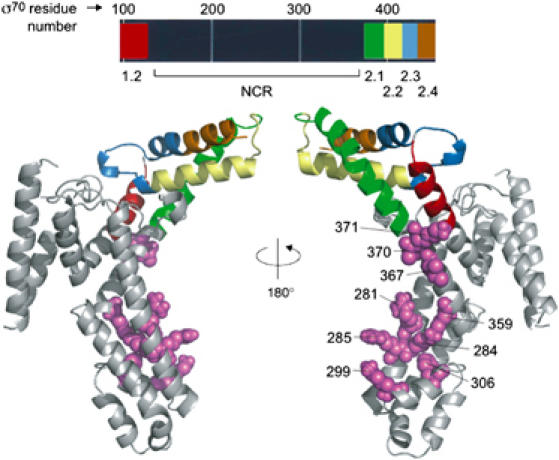
Substitutions in the σ70 NCR form a semicontinuous surface. Top: linear representation of the crystallized σ70 fragment, with conserved regions color-coded. Bottom: σ70 fragment structure, with conserved regions colored as above (adapted from Malhotra et al, 1996). The NCR is shown in gray and residues identified by suppressor substitutions are colored violet and labeled.
To confirm that the effects of the σ70 substitutions on transcription from PR′ are specific to the antitermination function of λQ, we examined PR′ transcription in vitro in the presence of λQ. After purifying the WT and mutant σ70 proteins, we assayed λQ function in the presence of the corresponding reconstituted holoenzymes by performing single-round transcription assays using a PR′ template containing terminator tR′. When λQ is present, it modifies RNAP so that it can read through the terminator, producing a full-length run-off transcript. Therefore, the percentage of full-length transcripts (% readthrough) is a measure of λQ function. Under our conditions, 33 and 5.5% readthrough values were observed for Eσ70 WT and Eσ70 L402F, respectively, in the presence of λQ. We found that eight out of nine of the suppressor substitutions partially restored readthrough (to between 9 and 19%; data not shown) when tested in the context of Eσ70 L402F. The only exception was substitution A370V, which did not increase terminator readthrough above the experimental error; nevertheless, this substitution had effects on other functional properties of RNAP that were similar to those of the other substitutions (see below). None of the substitutions significantly increased the intrinsic ability of RNAP to read through tR′ in the absence of λQ (data not shown).
Substitutions in the σ70 NCR enhance promoter-proximal pausing in vitro
To determine whether the substitutions in the σ70 NCR, like the L402F substitution, affect λQ antitermination function indirectly by affecting promoter-proximal pausing, we performed in vitro transcription time courses under single-round conditions using a λ PR′ template, monitoring the RNA content of each reaction at various time points after the initiation of transcription. As observed previously (Grayhack et al, 1985; Yarnell and Roberts, 1992; Ko et al, 1998), the 16- and 17-nt RNA species appeared early in the time course and decayed over time, whereas the full-length transcript accumulated throughout the time course (Figure 4A, left panel). Furthermore, as expected, the L402F substitution in σ70 caused a marked reduction in the 16- and 17-nt pause species (Figure 4A, left panel) (Ko et al, 1998).
Figure 4.
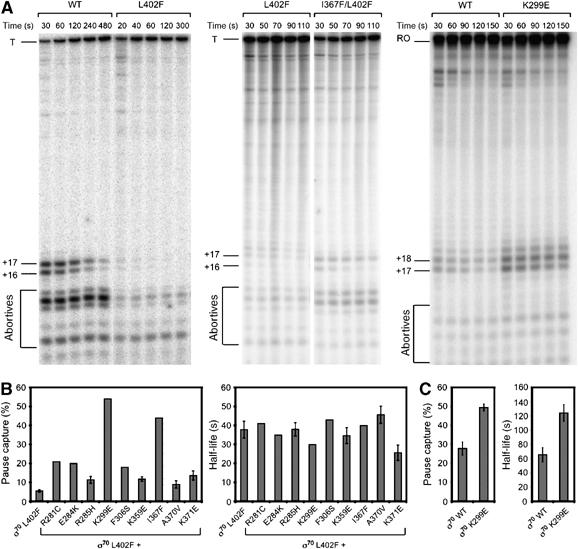
Substitutions in the σ70 NCR enhance pausing at λPR′ and at placUV5. (A) Single-round in vitro transcription time-course assays using a PR′ template (left and middle panels) or a placUV5 template (right panel) and RNAP reconstituted with the indicated σ70 proteins. Aliquots of single reactions were removed and stopped at the indicated time points after transcription was initiated. The RNA was labeled internally with [α-32P]UTP (middle and right panels) or end-labeled with [γ-32P]ATP (left panel). +16 and +17, 16- and 17-nt RNA species, respectively, produced from the λPR′ template; T, 194-nt terminated transcript produced from the λPR′ template; +17 and +18, 17- and 18-nt RNA species, respectively, produced from the placUV5 template; RO, 96-nt runoff transcript produced from the placUV5 template. A faint 18-nt RNA species was also observed during time courses with the λPR′ template when the RNA was internally labeled. This is likely the result of nucleotide deprivation at U19 under conditions where the UTP concentration was reduced to improve incorporation of [α-32P]UTP, and has been observed previously under similar reaction conditions (Ko et al, 1998). (B, C) Effects of substitutions on pause capture and pause half-life. The percentage of elongation complexes paused (100 (16-nt+17-nt)/(16-nt+17-nt+T) or 100 (17-nt+18-nt)/(17-nt+18-nt+T)) was approximated at each time point, plotted, and fit to the exponential equation Y=Y0e−kt (Supplementary Figure S1). Exponential equations were solved to obtain pause capture (left panels) and half-life (right panels) values for each holoenzyme. Pause capture was approximated by extrapolating the equations to t=0. Error bars represent standard deviations from at least three separate experiments. In cases where error bars are not shown, the mutants were assayed twice, with similar results.
Compared to Eσ70 L402F, RNAP holoenzymes reconstituted with each of the double mutant σ70 proteins produced a relative increase in the amount of 16- and 17-nt RNA species (Figure 4A, middle panel, and Supplementary Figure S1). To quantify the effects of the suppressor substitutions on pausing, we calculated pause capture (i.e. the percentage of transcription complexes that functionally engage the pause element) and pause half-life (Ko et al, 1998) (see legend of Figure 4). Under our experimental conditions, we calculated pause capture values of 71 and 5.4% for Eσ70 WT and Eσ70 L402F, respectively. The pause capture values for RNAPs reconstituted with the double mutant σ70 proteins ranged from 9% for Eσ70 A370V/L402F to 54% for Eσ70 K299E/L402F (Figure 4B and Supplementary Figure S1). In contrast to the effects on pause capture, we did not detect significant effects of the suppressor substitutions on pause half-life (Figure 4B and Supplementary Figure S1). We conclude, therefore, that the σ70 NCR suppressor substitutions enhance λQ antitermination function by increasing the percentage of RNAP holoenzymes that functionally engage the pause element during early elongation at λPR′.
The suppressor substitutions also enhanced pausing at λPR′ in the context of otherwise WT σ70, but these effects were more subtle (data not shown), presumably because the pause capture for Eσ70 WT is already quite high. To determine whether the suppressor substitutions could produce significant effects on σ70-dependent promoter-proximal pausing even in the context of WT σ70, we took advantage of a promoter (placUV5) that contains a promoter-proximal pause element that functions less efficiently than that of PR′ (Nickels et al, 2004). We assayed transcription in vitro from a placUV5 template using Eσ70 WT and Eσ70 K299E (Figure 4A, right panel) and found that substitution K299E increased both pause capture (from 28 to 50%) and pause half-life (∼2-fold) (Figure 4C and Supplementary Figure S1). In addition, we found that substitution K299E increased utilization of the plac pause element in vivo (Supplementary Figure S1). We conclude that the σ70 NCR suppressor substitutions enhance σ70-dependent early elongation pausing both in the presence and absence of substitution L402F.
Substitutions in the σ70 NCR disrupt an interaction with the β′ subunit
Because the L402F substitution specifically weakens the interaction between σ2 and the β′ coiled-coil, we sought to determine whether the substitutions in the σ70 NCR restore early elongation pausing by strengthening the σ2/β′ coiled-coil interaction. To do this, we employed a transcription-based bacterial two-hybrid assay (Dove et al, 1997; Dove and Hochschild, 2004). In this assay, interaction between a protein domain X fused to a subunit of RNAP and a partner domain Y fused to a DNA-binding protein activates transcription from a test promoter bearing a recognition site for the DNA-binding protein in the upstream region. This two-hybrid assay enabled us to detect an interaction between σ70 residues 94–448 (specifying the structurally characterized σ70 fragment comprising region 1.2, the NCR, and region 2) (Malhotra et al, 1996) and β′ residues 262–309 (specifying the coiled coil) (Young et al, 2001). Specifically, the σ70 fragment was fused to the CI protein of bacteriophage λ (λCI) and the β′ coiled-coil fragment was fused to the N-terminal domain of the RNAP α subunit in place of its C-terminal domain (Figure 5A). Introduction of plasmids encoding these two fusion proteins into strain FW102 F′OL2–62, which bears test promoter placOL2–62 linked to a lacZ reporter gene, results in the activation of lacZ transcription (up to ∼13-fold) (Figure 5B). As expected, introduction of the L402F substitution into the σ70 moiety of the λCI-σ70 fusion protein weakened this interaction substantially (Figure 5C). However, the interaction was neither weakened nor significantly strengthened by any of the suppressor substitutions (which were individually introduced into the otherwise WT σ70 moiety) (Figure 5C).
Figure 5.
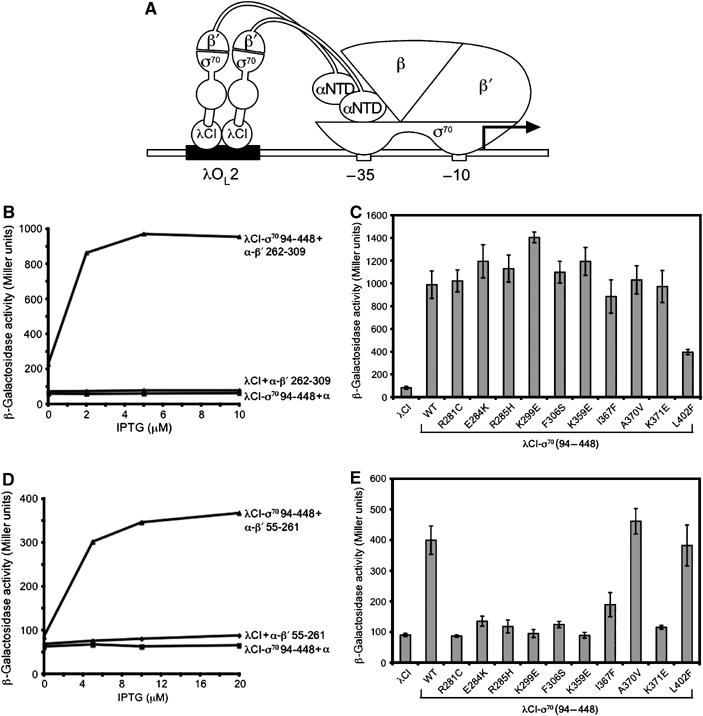
Substitutions in the σ70 NCR specifically disrupt an interaction with β′ 55–261. (A) Schematic of bacterial two-hybrid assay used to detect protein–protein interactions between fragments of σ70 and β′. (B) λCI-σ70 94–448 activates transcription from the test promoter in cells containing the α–β′ 262–309 fusion protein. Strain FW102 F′OL2–62 cells containing compatible plasmids directing the synthesis of the indicated proteins were grown in the presence of the indicated concentrations of IPTG and assayed for β-galactosidase activity. (C) Effects of substitutions in the σ70 NCR on the interaction between the σ moiety of the λCI-σ70 94–448 fusion protein and the β′ moiety of the α–β′ 262–309 fusion protein. Cells were grown in the presence of 10 μM IPTG and assayed for β-galactosidase activity. (D) λCI-σ70 94–448 activates transcription from the test promoter in cells containing the α–β′ 55–261 fusion protein. Strain FW102 F′OL2–62 cells containing compatible plasmids directing the synthesis of the indicated proteins were grown in the presence of the indicated concentrations of IPTG and assayed for β-galactosidase activity. (E) Effects of substitutions in the σ70 NCR on the interaction between the σ moiety of the λCI-σ70 94–448 fusion protein and the β′ moiety of the α–β′ 55–261 fusion protein. Cells were grown in the presence of 20 μM IPTG and assayed for β-galactosidase activity. (C, E) Assays were performed three times in duplicate on separate occasions; shown are the average values from all trials with standard deviations.
Using the two-hybrid assay, we also detected an interaction between the same σ70 (94–448) fragment and a more N-terminal region of β′, present within a fragment extending from residue 55 to 261 (Figure 5D). In contrast to the σ70/β′ coiled-coil interaction, this second interaction was unaffected by the σ70 L402F substitution, but was disrupted by all but one (A370V) of the suppressor substitutions (Figure 5E). That the substitutions in the σ70 NCR did not affect the σ70/β′ coiled-coil interaction suggests that their disruptive effects on the σ70/β′ 55–261 interaction are specific and not the result of defects in the synthesis, stability, or folding of the mutant λCI-σ70 fusion proteins.
We conclude from the results of these two-hybrid assays that the substitutions in the σ70 NCR isolated in our genetic screen specifically weaken an interaction with a region of β′ contained within the 55–261 fragment that we call the sigma NCR interaction domain (SNCRID). Furthermore, our results suggest that, in contrast to the interaction between σ2 and the β′ coiled-coil, the interaction between the σ70 NCR and the β′ SNCRID inhibits early elongation pausing (because substitutions that disrupt the interaction enhance pausing). Consistent with this idea, we found that strengthening the σ70 NCR/β′ SNCRID interaction decreased early elongation pausing (Supplementary Figure S2).
The results of a previous study revealed that the σ70 NCR is not required for transcription in vitro, at least from some promoters, but raised the possibility that it is essential for viability (Kumar et al, 1995). We sought to revisit this issue by replacing the σ70 NCR with a seven-residue linker that connects conserved regions 1.2 and 2.1 in the stationary phase σ factor σ38 (see Supplementary Results). We found that although this chimeric σ factor was stably produced, cells containing the chimera in the absence of WT σ70 were severely compromised for growth (Supplementary Figure S3).
Substitutions in the β′ subunit that enhance early elongation pausing
The hypothesis that an interaction between the σ70 NCR and the β′ SNCRID inhibits σ70-dependent early elongation pausing suggests that it should be possible to isolate substitutions on the interacting surface of β′ that disrupt its interaction with the σ70 NCR and enhance early elongation pausing. To identify such substitutions, we screened for β′ mutants that enhanced λQ-mediated antitermination in cells containing the L402F mutation in rpoD. After random PCR mutagenesis of a fragment encoding β′ residues 1–258 (present on plasmid pBRβ′), we identified eight substitutions at five amino-acid positions (E148, E162, E170, E171, and E175—all negatively charged residues) that suppressed the defect in λQ-mediated antitermination caused by σ70 substitution L402F. At maximal induction, λQ stimulated lacZ transcription from the λPR′-lacZ test promoter 8.2- to 9.7-fold in ML9 (rpoD L402F) cells directing the synthesis of the mutant β′ proteins from pBRβ′, compared with only 4.8-fold in ML9 cells directing the synthesis of WT β′ from pBRβ′ (Figure 6A).
Figure 6.
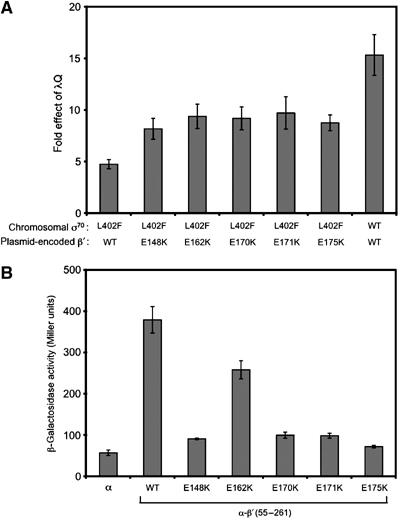
Substitutions in β′ suppress the defect in λQ-mediated antitermination caused by the σ70 L402F substitution and weaken the interaction between the β′ SNCRID and the σ70 NCR. (A) Cells encoding either σ70 L402F or WT σ70 at the chromosomal rpoD locus and harboring the PR′-lacZ fusion on an F′ episome were cotransformed with compatible plasmids, one directing the synthesis of the indicated β′ protein (in addition to chromosomally encoded WT β′) and the other directing the synthesis of either λQ or no λQ. Cells were grown in the presence of 100 μM IPTG and assayed for β-galactosidase activity. ‘Fold effect of λQ' values were calculated as described for Figure 1. Shown are the averages of three independent sets of measurements (and standard deviations). (B) Effects of substitutions in the β′SNCRID on the interaction between the β′ moiety of the α–β′55–261 fusion protein and the σ moiety of the λCI-σ70 94–448 fusion protein. Strain FW102 F′OL2–62 cells containing compatible plasmids directing the synthesis of the λCI-σ70 fusion protein and either α or the indicated α–β′ fusion protein were grown in the presence of 20 μM IPTG and assayed for β-galactosidase activity. Assays were performed three times in duplicate on separate occasions; shown are the average values from all trials with standard deviations.
To determine whether these β′ substitutions affect the interaction between the β′ SNCRID and the σ70 NCR, we again used the two-hybrid assay. After introducing mutations encoding substitutions E148K, E162K, E170K, E171K, and E175K into plasmid pBRα-β′ 55–261, we transformed FW102 F′OL2–62 cells with pACλCI-σ70 94–448 together with pBRα-β′ 55–261 and the mutant variants. Each of the β′ substitutions reduced or nearly abolished the stimulatory effect of the λCI-σ70 fusion protein on lacZ transcription (Figure 6B). Western blotting confirmed that these substitutions did not affect α–β′ 55–261 protein levels (data not shown). Thus, these β′ substitutions, like those on the putative partner surface of the σ70 NCR, weaken the σ70 NCR/β′ SNCRID interaction and enhance λQ function in vivo.
To confirm that the effects of the β′ substitutions on transcription from PR′, like those of the σ70 NCR substitutions, are specific to the antitermination function of λQ, we examined transcription from PR′ in vitro in the presence of λQ. RNAP core enzymes containing β′-His6(WT), β′-His6(E148K), β′-His6(E171K), and β′-His6(E175K) were purified (see Materials and methods) and used to reconstitute holoenzymes with σ70 L402F. The β′ substitutions E148K, E171K, and E175K increased terminator readthrough in the presence of λQ protein to 13, 10, and 24%, respectively, compared with a 5.5% readthrough for RNAP containing WT β′ (data not shown).
To determine whether the effects of the β′ substitutions on λQ function were caused by enhanced pausing, we performed single-round in vitro transcription time-course assays with Eσ70 L402F, E[β′ E148K]σ70 L402F, and E[β′ E175K]σ70 L402F (Figure 7A and Supplementary Figure S1). Both these β′ substitutions increased pause capture significantly, with values of 19 and 71% for E[β′ E148K]σ70 L402F and E[β′ E175K]σ70 L402F, respectively, compared with 5.4% for Eσ70 L402F (Figure 7B). Thus, substitutions in β′ that weaken the σ70 NCR/β′ SNCRID interaction, like those in the σ70 NCR, enhance early elongation pausing. These results provide strong support for the hypothesis that the σ70 NCR/β′ SNCRID interaction inhibits early elongation pausing at λPR′.
Figure 7.
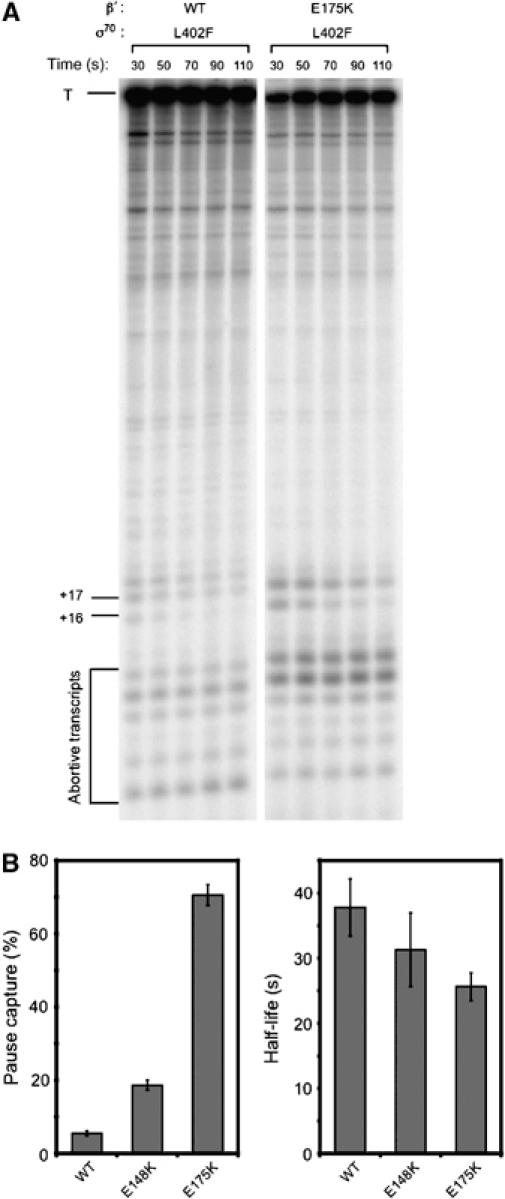
Substitutions in the β′ SNCRID enhance pausing at λPR′. (A) Single-round in vitro transcription time-course assays using a PR′ template and RNAP containing the indicated β′ and σ70 proteins. +16 and +17, 16- and 17-nt RNA species, respectively, produced from the λPR′ template; T, 194-nt terminated transcript produced from the λPR′ template. (B) Effects of substitutions on pause capture and pause half-life (calculated from plots shown in Supplementary Figure S1). Error bars represent standard deviations from at least three separate experiments. All data were obtained and analyzed as described for Figure 4.
Weakening the interaction between the σ70 NCR and the β′ SNCRID increases abortive RNA synthesis
While examining the effects of the σ70 NCR and β′ SNCRID substitutions on early elongation pausing by means of in vitro transcription assays, we noticed that the RNAP holoenzymes containing σ70 L402F with one of the suppressor substitutions in either σ70 or β′ synthesized significantly more 11-, 12-, and 13-nt abortive RNAs than Eσ70 L402F (Figures 4A and 7A). Note that the synthesis of these abortive transcripts was reduced by substitution L402F, consistent with previous observations with this and other σ70 substitutions that weaken the interaction between σ2 and the β′ coiled-coil (Ko et al, 1998; Chan and Gross, 2001). To quantify the effects of the σ70 NCR and β′ SNCRID substitutions on abortive RNA synthesis, we performed single-round in vitro transcription reactions under conditions (see Materials and methods) that permitted us to calculate abortive probabilities (defined as the number of transcripts of a given size divided by the total number of transcripts equal or greater in size) (Hsu, 1996) for the detectable abortive RNA products (8, 9, 11, 12, and 13 nt in length). Figure 8A and B shows that the σ70 L402F substitution reduced the abortive probabilities at +11, +12, and +13 (but not at +8 and +9), consistent with the results of previous studies of abortive initiation on the N25 anti promoter (Chan and Gross, 2001). The σ70 suppressor substitutions (assayed in the context of σ70 L402F) significantly increased the abortive probabilities at +11, +12, and +13, but not at +8 and +9 (Figure 8A and B). Similarly, the β′ suppressor substitutions (β′ E148K and β′ E175K) increased the abortive probabilities at +11, +12, and +13 without affecting the abortive probabilities at +8 and +9 (Figure 8C and D). Therefore, we conclude that weakening the interaction between the σ70 NCR and β′ SNCRID increases the likelihood that an initial transcribing complex will abort and release 11-, 12-, or 13-nt transcripts rather than escape into productive elongation. Conversely, we found that strengthening the σ70 NCR/β′ SNCRID interaction resulted in decreased abortive probabilities (Supplementary Figure S2D). The σ70 NCR/β′ SNCRID interaction thus functionally counteracts the σ2/β′ coiled-coil interaction, disruption of which facilitates promoter escape (Chan and Gross, 2001).
Figure 8.
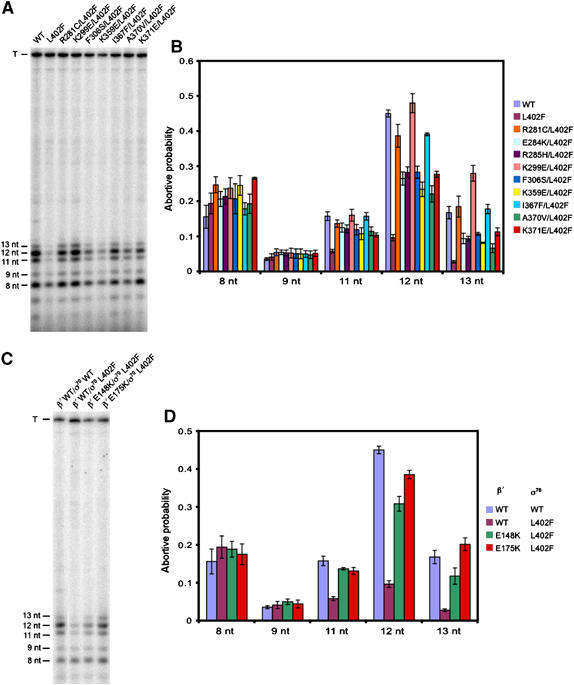
Substitutions that weaken the interaction between the σ70 NCR and β′ SNCRID increase abortive transcript synthesis. (A, C) Single-round in vitro transcription assays using a PR′ template and RNAP holoenzymes containing the indicated σ70 and β′ ((C) only) proteins. All RNAP holoenzymes in (A) contain wild-type β′. Shown are the 8-, 9-, 11-, 12-, and 13-nt abortive transcripts and the 194-nt terminated transcript (T) produced from the PR′ template. (B, D) Effects of σ70 and β′ substitutions on abortive probabilities at +8, +9, +11, +12, and +13. Abortive probability values, which describe the probability that RNAP will release an abortive RNA rather than extend at least one more nucleotide, were calculated for each position by dividing the number of moles of each RNA species by the total number of moles of all RNA species of equal or greater size (e.g. 11-nt/(11-nt+12-nt+13-nt+T)). Error bars represent standard deviations from at least three separate experiments.
Weakening the interaction between the σ70 NCR and the β′ SNCRID does not specifically affect open complex stability
As well as decreasing abortive transcript synthesis, the L402F substitution destabilizes open complexes (Ko et al, 1998), suggesting a relationship between open complex stability and abortive yield (Roberts and Roberts, 1996; Hsu, 2002). To address the possibility that disrupting the σ70 NCR/β′ SNCRID interaction inhibits promoter escape (increases abortive yields) by increasing open complex stability, we measured the kinetics of open complex dissociation for RNAP reconstituted with WT σ70, σ70 L402F, and the doubly substituted σ70 proteins (see Supplementary Results). We observed no correlation (positive or negative) between the effects of our σ70 NCR substitutions on open complex stability, on the one hand, and abortive yields, on the other (Supplementary Figure S4). We therefore conclude that the interaction between the σ70 NCR and the β′ SNCRID affects abortive transcript synthesis through a mechanism that does not involve open complex stability.
Discussion
Through a genetic screen for σ70 mutations that affect σ70-dependent promoter-proximal pausing, we have identified a previously uncharacterized interaction between the σ70 NCR and an N-terminal domain of β′ that we call the β′ SNCRID. Our genetic data suggest that this interaction is mediated in part by positively charged residues in the σ70 NCR and negatively charged residues in the β′ SNCRID, and mutant-suppressor analysis identified oppositely charged residues that likely approach one another closely at the σ70 NCR/β′ SNCRID interface (Supplementary Figure S5). Interaction between the σ70 NCR and the β′ SNCRID is consistent with FRET-based structural models of the E. coli RNAP holoenzyme and open complex (Mekler et al, 2002). We found that the σ70 NCR/β′ SNCRID interaction functions to facilitate promoter escape and inhibit σ70-dependent promoter-proximal pausing, as substitutions that weakened the interaction increased abortive transcript synthesis, and also increased pausing, whereas substitutions that strengthened the interaction had the opposite effects. The interaction between the σ70 NCR and the β′ SNCRID functionally counteracts the interaction between σ2 and the β′ coiled-coil in that the latter inhibits promoter escape (Chan and Gross, 2001) and promotes pausing (Ko et al, 1998).
Mechanistic significance of σ70 NCR/β′ SNCRID interaction
The correlation between the promoter escape and pausing phenotypes caused by altering the strength of the σ70 NCR/β′ SNCRID interaction underscores the relationship between these two processes. During both transcription initiation and early elongation pausing, σ2 is bound to the nontemplate strand of a −10 (or −10-like) element (Ring et al, 1996; Marr and Roberts, 1997; Young et al, 2001). According to current models, the abortive phase of transcription involves the synthesis and release of short RNA products while RNAP maintains its contacts with the promoter (with the −10 element, in particular) (Roberts and Roberts, 1996; Hsu, 2002). For this to occur—that is, for transcript elongation to occur without forward translocation of the enzyme with respect to the core promoter elements—it has been proposed that excess template DNA must transiently be accommodated in the main channel of the enzyme in a process referred to as DNA ‘scrunching', a proposal that has recently received direct experimental support (Kapanidis et al, 2006; Revyakin et al, 2006). The strain that accumulates during this DNA scrunching process can be relieved either by the release of an abortive RNA product or by breakage of the promoter contacts (i.e. promoter escape).
Events similar to those that occur during abortive initiation are thought to occur during early elongation pausing at PR′ (Marr and Roberts, 2000). Specifically, the location of the −10-like pause element (12 bp downstream from the PR′ −10 element) suggests that σ2 initially engages the −10-like element when the nascent transcript is ∼12 nt in length. The nascent RNA is then extended while σ2 remains bound to the −10-like element (requiring DNA scrunching) to produce either a 16- or 17-nt pause product. At this point, the interaction between σ2 and the pause element evidently hinders further extension and the accumulated strain can be relieved in one of two ways. Either the contacts between σ2 and the −10-like element are broken and pause capture does not occur, or RNAP enters a backtracked state in which the catalytic center of the enzyme slides back relative to the 3′ end of the nascent RNA transcript (returning to the +12 position; Marr and Roberts, 2000) and pause capture occurs. For RNAP to escape the pause, transcript cleavage (or forward translocation) must occur to regenerate a 3′ end (or reposition the existing 3′ end) at the enzyme's catalytic center (Marr and Roberts, 2000). According to this model, the events that cause RNAP to release longer abortive RNA products during the early stages of transcription are analogous to events that cause RNAP to pause during early elongation.
The analogy between the events that occur during the abortive phase of transcription and the events that occur during early elongation pausing suggests a possible explanation for the effect of the σ70 NCR/β′ SNCRID interaction on the two processes. In particular, we propose that the σ70 NCR/β′ SNCRID interaction might destabilize the interaction of σ2 with nontemplate strand DNA, specifically under conditions of DNA scrunching, thus facilitating promoter escape and hindering pause capture. In support of this proposal is our finding that disrupting the σ70 NCR/β′ SNCRID interaction did not have a specific effect on open complex half-life; an increase in open complex half-life would have been expected if the σ70 NCR/β′ SNCRID interaction functioned to destabilize the σ2/nontemplate strand interaction under all conditions.
Function of the σ NCR in other bacteria
Although not strictly required for transcription in vitro (Kumar et al, 1995), we found that removal of the σ70 NCR severely compromised cell growth (see also Kumar et al, 1995), suggesting that it plays an important role in transcription from at least some promoters. Moreover, the σ70 NCR (despite its name) resembles the nonconserved regions of primary σ factors from other Gram-negative proteobacteria including Pseudomonas aeruginosa, Vibrio cholerae, Haemophilus influenzae, and Caulobacter crescentus. Interestingly, the majority of substitutions that we isolated in our genetic screen affect residue positions that are among the most conserved within the family of σ70-type NCRs. For example, while the NCRs of the primary σ factors in P. aeruginosa and V. cholerae are only 37 and 56% identical, respectively, to the E. coli σ70 NCR (residues 127–373), the residues at 13 of the 15 positions identified in our screen were conserved across all three species. We suggest, therefore, that the σ70 NCR/β′ SNCRID interaction is likely to be conserved as well.
Although high-resolution structures are available for RNAP holoenzymes from Thermus aquaticus (Taq) and Thermus thermophilus (Murakami et al, 2002; Vassylyev et al, 2002), they are not informative with regard to the E. coli σ70 NCR/β′ SNCRID interaction because the corresponding regions of Thermus σA and β′ are greatly diverged from their counterparts in E. coli. In particular, β′ from the Thermus species contains a large (293 residue) inserted domain (the β′ NCD) in the region corresponding to the β′ SNCRID and conversely, the Thermus σA NCR is significantly smaller than the σ70 NCR (72 versus 247 residues) (Iyer et al, 2004). Nevertheless, the σA NCR is positioned to interact with a portion of the β′ NCD in high-resolution structural models of the Thermus RNAP holoenzymes (Chlenov et al, 2005). Consistent with this structural information, the bacterial two-hybrid assay permits detection of an interaction between the Taq σA NCR and the Taq β′ NCD (M Leibman and A Hochschild, unpublished data). Experiments are underway to determine whether this interaction is functionally analogous to the σ70 NCR/β′ SNCRID interaction in E. coli.
Functional antagonism between the σ2/β′ coiled-coil and the σ70 NCR/β′ SNCRID interactions
The σ2/β′ coiled-coil interaction and the σ70 NCR/β′ SNCRID interaction have opposing effects on both promoter escape and early elongation pausing, raising the question of how this facilitates the transcription process. Previous work has demonstrated that the σ2/β′ coiled-coil interaction is critical during promoter open complex formation, being required for promoter melting and contributing importantly to open complex stability (Young et al, 2001, 2004). However, by the same token, the σ2/β′ coiled-coil interaction, which is required for σ2 to establish sequence-specific contact with nontemplate strand DNA, limits promoter escape (and also escape from σ70-dependent promoter-proximal pause sites) (Ko et al, 1998; Chan and Gross, 2001). Moreover, evidence suggests that the β′ coiled-coil interacts more strongly with region 2 of σ70 than with region 2 of σ38 (which lacks the NCR) (S Garrity, A Yuan, and A Hochschild, unpublished data). Thus, we speculate that the σ70 form of the RNAP holoenzyme may require an opposing interaction to facilitate its escape from the promoter into productive elongation (see Figure 9). Our suggestion that the σ70 NCR/β′ SNCRID interaction functions to destabilize (directly or indirectly) the interaction of σ2 with nontemplate strand DNA specifically under conditions of DNA scrunching explains how the σ70 NCR/β′ SNCRID interaction might facilitate promoter escape in a manner that does not compromise open complex formation. We speculate further that the σ70 NCR/β′ SNCRID interaction may provide a target for regulatory factors that could modulate either promoter escape or escape from an early elongation pause.
Figure 9.
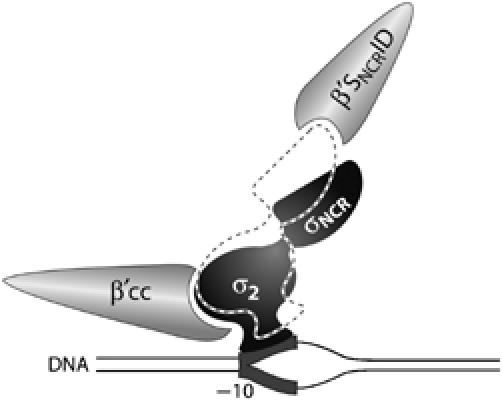
Model depicting opposing σ2/β′ coiled-coil and σ70 NCR/β′ SNCRID interactions. According to this model, optimal interaction between σ2 and the β′ coiled-coil (β′cc) promotes interaction between σ2 and the nontemplate strand of the −10 element (σ domains shown in black). The functionally antagonistic interaction between the σ70 NCR and β′ SNCRID distorts the interaction between σ2 and the β′cc, facilitating the release of σ2 from the DNA (σ domains shown as dashed outline).
Materials and methods
Expression vectors
Plasmid pBRσ70 contains the complete coding sequence of the σ70 subunit of E. coli RNAP under the control of a weak constitutive synthetic promoter with the sequence TTTACAACATGAAGTAACTTCTCGCATTATGTCTCGA. Plasmid pBRβ′ contains the complete coding sequence of the β′ subunit of E. coli RNAP under the control of the same weak constitutive synthetic promoter. Additionally, pBRβ′ contains the following restriction sites introduced as silent mutations into the β′ coding sequence: NotI at codons 257–259, XhoI at codons 872–873, and BamHI at codons 1182–1183. Plasmid pBRα (Dove et al, 1997) encodes the α subunit of E. coli RNAP under the control of tandem lpp and lacUV5 promoters. Plasmids pBRα-β′ 55–261 and pBRα-β′ 262–309 are pBRα derivatives that encode residues 1–248 of the α subunit of E. coli RNAP fused to residues 55–261 and 262–309, respectively, of the β′ subunit of E. coli RNAP under the control of tandem lpp and lacUV5 promoters. Plasmid pACλCI (Dove et al, 1997) encodes λCI under the control of the lacUV5 promoter. Plasmid pACλCI-σ70 94–448 is a pACλCI derivative that encodes λCI (residues 1–236) fused to residues 94–448 of the σ70 subunit of E. coli RNAP. Plasmid pACλQ (Nickels et al, 2002) encodes λQ under the control of the lacUV5 promoter. Plasmid pACΔQ (Nickels et al, 2002) encodes no functional λQ.
Strains and test promoters
Reporter strain BN147 contains sequence extending from −109 to +232 of λPR′ fused to a lacZ reporter gene. This reporter fusion is present on an F′ episome and has been described (Nickels et al, 2002). Reporter strain ML35 is identical to BN147 except that it contains the λPR′ mutation A(+5)T (Ring and Roberts, 1994). We constructed a derivative of strain FW102 (Whipple, 1998) that has a mutation encoding the L402F substitution at the chromosomal rpoD locus linked to a kanamycin resistance gene. The resulting strain, ML6, was mated with donor strains BN147 and ML35 to create the rpoD(L402F) reporter strains ML9 (λPR′) and ML37 (λPR′ A[+5]T), respectively. Strain ML102, a derivative of strain BL21(DE3), carries the L402F mutation at the chromosomal rpoD locus linked to a kanamycin resistance gene.
Libraries and screening
Expression libraries were generated by error-prone PCR of the complete coding sequence of σ70 (in three segments) and of the sequence encoding residues 1–258 of β′ in pBRσ70 and pBRβ′, respectively. Libraries were cotransformed with pACλQ into reporter strain ML37 and plated on indicator medium containing X-gal (60 μg/ml), IPTG (10 μM), and tPEG (250 μM). The λPR′ A(+5)T mutation (Ring and Roberts, 1994) in strain ML37 enhanced the difference in λQ-dependent lacZ activity between cells containing plasmid-encoded and chromosomally encoded σ70 L402F, and cells containing plasmid-encoded σ70 WT in addition to chromosomally encoded σ70 L402F. Library clones were screened for those that resulted in increased lacZ activity relative to unmutagenized pBRσ70(L402F) or pBRβ′. Identified mutations were recloned into pBRσ70 or pBRβ′ before cotransforming with either pACλQ or pACΔQ into reporter strain ML9 for β-galactosidase assays.
β-Galactosidase assays
Cells were grown in LB supplemented with the appropriate antibiotics at the following concentrations: carbenicillin (100 μg/ml), tetracycline (10 μg/ml), chloramphenicol (25 μg/ml), and kanamycin (50 μg/ml). IPTG was used at the indicated concentrations. SDS–CHCl3-permeabilized cells were assayed as described (Dove and Hochschild, 2004).
Proteins
His6-tagged σ70 proteins were purified as described after overproduction from plasmid pLNH12-His (Panaghie et al, 2000) in BL21DE3 cells. RNAP core enzymes were purified as described (Vrentas et al, 2005) from strain ML102 cells containing plasmid pVS10 (Artsimovitch et al, 2003), which directs the synthesis of high levels of α, β, β′-His6, and ω. The presence of the L402F mutation at the chromosomal rpoD locus in ML102 cells ensured that the core enzyme preparations were not contaminated with WT σ70. Holoenzyme was formed by incubating the core enzyme with a 5- to 10-fold excess of the appropriate σ70 protein. λQ and NusA proteins were gifts from J Roberts.
In vitro transcription
The transcription assays were performed with holoenzymes prepared as described above except for the assay shown in the left-hand panel of Figure 4A, which was performed using holoenzyme reconstituted from commercially obtained E. coli RNAP core (Epicentre) and purified σ70 proteins. For analysis of promoter-proximal pausing under single-round conditions, open complexes were formed by incubating 40 nM RNAP with 5 nM λPR′ or placUV5 template for 10 min at 37°C in transcription buffer (20 mM Tris–HCl, pH 8.0, 0.1 mM EDTA, 10 mM DTT, 50 mM KCl, and 100 μg/ml BSA) plus either 200 μM each of ATP, GTP and CTP and 50 μM [α-32P]UTP (4 mCi/ml) to generate internally labeled RNA or 200 μM each of GTP, CTP and UTP and 50 μM [γ-32P]ATP (1 mCi/ml) to generate end-labeled RNA. Transcription was initiated by addition of 4 mM MgCl2 and 5 μg/ml rifampicin, and the reactions were incubated at 37°C. At the indicated times after the addition of the MgCl2–rifampicin mixture, aliquots of each reaction were removed and quenched in five volumes of stop buffer (600 mM Tris–HCl, pH 8.0, and 12 mM EDTA) supplemented with tRNA. Samples were then extracted with phenol/chloroform (1:1), precipitated with ethanol, resuspended in 4.5 μl loading buffer (95% (v/v) formamide, 20 mM EDTA, 0.05% (w/v) bromophenol blue, 0.05% (w/v) xylene cyanol), and electrophoresed on 15% (w/v) polyacrylamide sequencing gels. Bands were visualized by phosphorimager.
For analysis of abortive transcript synthesis under single-round conditions, open complexes were formed by incubating 40 nM RNAP with 5 nM λPR′ template for 10 min at 37°C in transcription buffer. Reactions were initiated by the simultaneous addition of 50 μg/ml heparin plus 100 μM each of UTP, GTP and CTP and 50 μM [γ-32P]ATP at 1 mCi/ml. Reactions were incubated for 20 min at 37°C before they were quenched with five volumes of stop buffer. Samples were then prepared for electrophoresis and electrophoresed as described above.
For all in vitro transcription assays with λPR′, linear template DNA, which was generated by the PCR from plasmid pFW11-PR′-lacZ (Nickels et al, 2002), contained PR′ sequence extending from −109 to +232. For in vitro transcription assays with placUV5, linear template DNA was generated by the PCR from a pFW11-derived plasmid carrying placUV5 sequence extending from −60 to +36 (Nickels et al, 2004).
Supplementary Material
Supplementary Figure S1
Acknowledgments
We thank S Dove and B Nickels for their comments on the manuscript, S Garrity for assistance in preparing Figure 3 and C Vrentas, T Gaal, W Ross, and R Saecker for helpful suggestions. We also thank J Roberts for purified NusA and λQ proteins, V Svetlov and I Artsimovitch for RNAP expression vectors, C Gross for σ70 shut-off strain CAG20153, and S Garrity for plasmids pBRα-β′ 55–261, pBRα-β′ 262–309, and pACλCI-σ70 94–448. This work was supported by NIH grant GM44025 to AH.
References
- Arthur TM, Burgess RR (1998) Localization of a σ70 binding site on the N terminus of the Escherichia coli RNA polymerase β′ subunit. J Biol Chem 273: 31381–31387 [DOI] [PubMed] [Google Scholar]
- Artsimovitch I, Landick R (2000) Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci USA 97: 7090–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsimovitch I, Svetlov V, Murakami KS, Landick R (2003) Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. J Biol Chem 278: 12344–12355 [DOI] [PubMed] [Google Scholar]
- Bown J, Barne K, Minchin S, Busby S (1997) Extended−10 promoters. Nucleic Acids Mol Biol 11: 41–52 [Google Scholar]
- Chan CL, Gross CA (2001) The anti-initial transcribed sequence, a portable sequence that impedes promoter escape, requires σ70 for function. J Biol Chem 276: 38201–38209 [DOI] [PubMed] [Google Scholar]
- Chlenov M, Masuda S, Murakami KS, Nikiforov V, Darst SA, Mustaev A (2005) Structure and function of lineage-specific sequence insertions in the bacterial RNA polymerase β′ subunit. J Mol Biol 353: 138–154 [DOI] [PubMed] [Google Scholar]
- deHaseth PL, Zupancic ML, Record TM Jr (1998) RNA polymerase–promoter interactions: the comings and goings of RNA polymerase. J Bacteriol 180: 3019–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighan P, Hochschild A (2007) The bacteriophage λ Q anti-terminator protein regulates late gene expression as a stable component of the transcription elongation complex. Mol Microbiol 63: 911–920 [DOI] [PubMed] [Google Scholar]
- Dove SL, Hochschild A (2004) A bacterial two-hybrid system based on transcription activation. Methods Mol Biol 261: 231–246 [DOI] [PubMed] [Google Scholar]
- Dove SL, Joung JK, Hochschild A (1997) Activation of prokaryotic transcription through arbitrary protein–protein contacts. Nature 386: 627–630 [DOI] [PubMed] [Google Scholar]
- Ebright RH (2000) RNA polymerase: structural similarities between bacterial RNA polymerase and eukaryotic RNA polymerase II. J Mol Biol 304: 687–698 [DOI] [PubMed] [Google Scholar]
- Feklistov A, Barinova N, Sevostyanova A, Heyduk E, Bass I, Vvedenskaya I, Kuznedelov K, Merkiene E, Stavrovskaya E, Klimasauskas S, Nikiforov V, Heyduk T, Severinov K, Kulbachinskiy A (2006) A basal promoter element recognized by free RNA polymerase σ subunit determines promoter recognition by RNA polymerase holoenzyme. Mol Cell 23: 97–107 [DOI] [PubMed] [Google Scholar]
- Geszvain K, Gruber TM, Mooney RA, Gross CA, Landick R (2004) A hydrophobic patch on the flap-tip helix of E. coli RNA polymerase mediates σ70 region 4 function. J Mol Biol 343: 569–587 [DOI] [PubMed] [Google Scholar]
- Grayhack EJ, Yang XJ, Lau LF, Roberts JW (1985) Phage lambda gene-Q antiterminator recognizes RNA-polymerase near the promoter and accelerates it through a pause site. Cell 42: 259–269 [DOI] [PubMed] [Google Scholar]
- Gross CA, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B (1998) Thefunctional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Symp Quant Biol 63: 141–155 [DOI] [PubMed] [Google Scholar]
- Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL (2006) rRNA promoter regulation by nonoptimal binding of σ region 1.2: an additional recognition element for RNA polymerase. Cell 125: 1069–1082 [DOI] [PubMed] [Google Scholar]
- Hsu LM (1996) Quantitative parameters for promoter clearance. Methods Enzymol 273: 59–71 [DOI] [PubMed] [Google Scholar]
- Hsu LM (2002) Promoter clearance and escape in prokaryotes. Biochim Biophys Acta 1577: 191–207 [DOI] [PubMed] [Google Scholar]
- Iyer LM, Koonin EV, Aravind L (2004) Evolution of bacterial RNA polymerase: implications for large-scale bacterial phylogeny, domain accretion, and horizontal gene transfer. Gene 335: 73–88 [DOI] [PubMed] [Google Scholar]
- Kapanidis AN, Margeat E, Ho SO, Kortkhonjia E, Weiss S, Ebright RH (2006) Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science 314: 1144–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DC, Marr MT, Guo TS, Roberts JW (1998) A surface of Escherichia coli σ70 required for promoter function and antitermination by phage λ Q protein. Genes Dev 12: 3276–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Williamson HS, Fujita N, Ishihama A, Hayward RS (1995) A partially functional 245-amino-acid internal deletion derivative of Escherichia coli σ70. J Bacteriol 177: 5193–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K (2002) A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science 295: 855–857 [DOI] [PubMed] [Google Scholar]
- Lonetto M, Gribskov M, Gross CA (1992) The σ70 family: sequence conservation and evolutionary relationships. J Bacteriol 174: 3843–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Severinova E, Darst SA (1996) Crystal structure of a σ70 fragment from E. coli RNA polymerase. Cell 87: 127–136 [DOI] [PubMed] [Google Scholar]
- Marr MT, Roberts JW (1997) Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science 276: 1258–1260 [DOI] [PubMed] [Google Scholar]
- Marr MT, Roberts JW (2000) Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol Cell 6: 1275–1285 [DOI] [PubMed] [Google Scholar]
- Mekler V, Kortkhonjia E, Mukhopadhyay J, Knight J, Revyakin A, Kapanidis AN, Niu W, Ebright YW, Levy R, Ebright RH (2002) Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase–promoter open complex. Cell 108: 599–614 [DOI] [PubMed] [Google Scholar]
- Mooney RA, Darst SA, Landick R (2005) Sigma and RNA polymerase: an on-again, off-again relationship? Mol Cell 20: 335–345 [DOI] [PubMed] [Google Scholar]
- Murakami KS, Darst SA (2003) Bacterial RNA polymerases: the whole story. Curr Opin Struct Biol 13: 31–39 [DOI] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA (2002) Structural basis of transcription initiation: T. aquaticus RNA polymerase holoenzyme at 4 A resolution. Science 296: 1280–1284 [DOI] [PubMed] [Google Scholar]
- Nickels BE, Garrity SJ, Mekler V, Minakhin L, Severinov K, Ebright RH, Hochschild A (2005) The interaction between σ70 and the β flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc Natl Acad Sci USA 102: 4488–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels BE, Mukhopadhyay J, Garrity SJ, Ebright RH, Hochschild A (2004) The σ70 subunit of RNA polymerase mediates a promoter-proximal pause at the lac promoter. Nat Struct Mol Biol 11: 544–550 [DOI] [PubMed] [Google Scholar]
- Nickels BE, Roberts CW, Sun H, Roberts JW, Hochschild A (2002) The σ70 subunit of RNA polymerase is contacted by the λQ antiterminator during early elongation. Mol Cell 10: 611–622 [DOI] [PubMed] [Google Scholar]
- Panaghie G, Aiyar SE, Bobb KL, Hayward RS, deHaseth PL (2000) Aromatic amino acids in region 2.3 of Escherichia coli sigma 70 participate collectively in the formation of an RNA polymerase–promoter open complex. J Mol Biol 299: 1217–1230 [DOI] [PubMed] [Google Scholar]
- Revyakin A, Liu C, Ebright RH, Strick TR (2006) Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science 314: 1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring BZ, Roberts JW (1994) Function of a nontranscribed DNA strand site in transcription elongation. Cell 78: 317–324 [DOI] [PubMed] [Google Scholar]
- Ring BZ, Yarnell WS, Roberts JW (1996) Function of E. coli RNA polymerase σ factor σ70 in promoter-proximal pausing. Cell 86: 485–493 [DOI] [PubMed] [Google Scholar]
- Roberts CW, Roberts JW (1996) Base-specific recognition of the nontemplate strand of promoter DNA by E. coli RNA polymerase. Cell 86: 495–501 [DOI] [PubMed] [Google Scholar]
- Roberts JW, Yarnell W, Bartlett E, Guo J, Marr M, Ko DC, Sun H, Roberts CW (1998) Antitermination by bacteriophage λ Q protein. Cold Spring Harbor Symp Quant Biol 63: 319–325 [DOI] [PubMed] [Google Scholar]
- Sharp MM, Chan CL, Lu CZ, Marr MT, Nechaev S, Merritt EW, Severinov K, Roberts JW, Gross CA (1999) The interface of σ with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev 13: 3015–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6. Å resolution. Nature 417: 712–719 [DOI] [PubMed] [Google Scholar]
- Vrentas CE, Gaal T, Ross W, Ebright RH, Gourse RL (2005) Response of RNA polymerase to ppGpp: requirement for the ω subunit and relief of this requirement by DksA. Genes Dev 19: 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple FW (1998) Genetic analysis of prokaryotic and eukaryotic DNA-binding proteins in Escherichia coli. Nucleic Acids Res 26: 3700–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell WS, Roberts JW (1992) The phage λ gene Q transcription antiterminator binds DNA in the late gene promoter as it modifies RNA-polymerase. Cell 69: 1181–1189 [DOI] [PubMed] [Google Scholar]
- Young BA, Anthony LC, Gruber TM, Arthur TM, Heyduk E, Lu CZ, Sharp MM, Heyduk T, Burgess RR, Gross CA (2001) A coiled-coil from the RNA polymerase β′ subunit allosterically induces selective nontemplate strand binding by σ70. Cell 105: 935–944 [DOI] [PubMed] [Google Scholar]
- Young BA, Gruber TM, Gross CA (2004) Minimal machinery of RNA polymerase holoenzyme sufficient for promoter melting. Science 303: 1382–1384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1


