Abstract
The effect of nicotine on conditioned inhibition was examined using a serial feature negative discrimination task. Nicotine (0.35mg/kg) or vehicle was administered before each of 16 training sessions. On some trials in each session, a tone was presented and followed by food reward. On other trials, the tone was preceded by a visual stimulus and not reinforced. Nicotine-treated rats exhibited greater discrimination between the two trial types as evidenced by less frequent responding during non-reinforced trials, and learned the discrimination in fewer sessions than vehicle-treated rats. In contrast, there were no group differences in responding during the reinforced trials.
Keywords: inhibition, cholinergic, nicotinic receptor, associative learning
1. Introduction
A wealth of research has established that stimulation of nicotinic acetylcholine receptors can enhance cognitive function (Levin, 2002). Administration of nicotine or nicotinic receptor agonists improves attention in normal humans (Levin et al., 1998) as well as rodents (McGaughy et al., 1999) while administration of nicotinic antagonists impairs attention (Grottick and Higgins, 2000). Likewise, nicotinic receptor agonists improve, and antagonists disrupt working memory (Levin, 2002; Ohno et al., 1993). Interestingly, the effect of nicotine is specific for certain aspects of cognitive function; for example, nicotinic compounds do not affect reference memory (Ohno et al., 1993).
In comparison, there is relatively little data on the effects of nicotine on inhibitory behavior. In a serial feature negative discrimination task, for example, subjects learn to respond when a stimulus, X, is presented alone, but withhold responding when X is preceded by a second stimulus, Y. Successful discrimination has been shown to depend on an intact hippocampus (Holland et al., 1999), and the ability to withhold responses is impaired in various disorders, including Attention-Deficit/Hyperactivity Disorder (ADHD; Barkley, 1997) and schizophrenia (Braff, 1993). Persons with these disorders use nicotine-containing produces at a much higher rate than the general population or other psychiatric populations (Ripoll et al., 2004), suggesting that nicotine may be used to self-medicate and alleviate cognitive impairment (Kumari and Postma, 2005). Whereas nicotine has been shown repeatedly to ameliorate attention deficits and other impairments associated with psychopathology (Ripoll et al., 2004; Levin et al., 1996), few studies have examined the effects on nicotine on inhibitory behavior, underscoring the need for research on the involvement of nicotinic cholinergic systems in inhibitory behavior.
2. Materials and Methods
This study examined the effects of nicotine on a serial feature negative discrimination in rats. Thirty-two male Long-Evans rats were obtained from Harlan Laboratories, Indianapolis, IN, USA and housed in pairs for a five day acclimation period with free access to food (Purina standard rat chow; Nestlé Purina, St. Louis, MO, USA) and water and maintained on a 14/10-h light-dark cycle. Rats were handled and weighed daily for three days post-acclimation and then individually housed. Over the course of the subsequent 7 days, body weights were gradually reduced to 85% of baseline. All rats were ~3 months old at the start of behavioral training. Animals were monitored and cared for throughout the experiment in compliance with the principles of laboratory animal care, including the Association for Assessment and Accreditation of Laboratory Animal Care guidelines, the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, and the Dartmouth College Institutional Animal Care and Use Committee.
Each day -(-) nicotine hydrogen tartrate (Sigma Aldrich Co., St Louis, MO, USA) was dissolved in sterile 1X phosphate buffered saline (vehicle) to a final concentration of 0.5 mg/ml and adjusted to pH 7.0 with 2N NaOH. Nicotine (or vehicle) was administered subcutaneously at a volume of 2.0 ml/kg, resulting in the equivalent of 0.35mg/kg free-base nicotine. This dose of nicotine was chosen based on numerous previous studies that have examined the effects of nicotine on associative learning (e.g., Olausson et al., 2004; Rochford et al., 1996). Rats were weighed before each conditioning session and placed in a plastic transporter used to transport subjects to and from the colony room. Nicotine or vehicle was administered 10 min before each conditioning session. Following the injection, rats remained in the transporter before being placed in the behavioral chambers.
Training took place in standard operant conditioning chambers (24 cm x 30.5 cm x 29 cm; Med Associates, St. Albans, VT, USA) constructed of aluminum front and back walls, clear acrylic sides and top, and grid floors. The conditioning chambers were enclosed in sound-attenuating cabinets (62 cm x 56 cm x 56 cm) equipped with an exhaust fan for air flow and background noise (~65 dB). A dimly illuminated food cup was recessed in the center of one end wall and a 6-W jeweled panel light, which served as the visual stimulus, was located 5 cm above that opening. A speaker was mounted next to the light and delivered the auditory stimulus (a 78dB, 1500 Hz tone). A photobeam was located across the entry of the food cup to monitor placement of the snout into the food cup to retrieve food pellets.
Rats were first trained to eat from the food cup during a single 64-minute session. Two 45-mg food pellets (Noyes, New Brunswick, NJ), which served as the unconditioned stimulus throughout the experiment, were randomly delivered sixteen times with an average inter-trial interval (ITI) of 4 min. Subsequent daily conditioning sessions lasted for 68 min and included sixteen trials of two types. Rats received four trials per session consisting of a 5-s presentation of the tone followed immediately by delivery of two food pellets. For the other twelve trials, the panel light was presented for 5 s, followed by a 5-s empty period, and then the tone was presented for 5 s. No food was delivered after the tone on these trials. The two trial types occurred randomly during the session and the order of trials differed on each day (ITIs averaged 4 min).
The number of breaks in the photobeam located across the entry of the food cup was monitored by the computer and provided a measure of conditioned food-cup behavior (i.e., nose-pokes) during presentation of the tone. The number of nose-pokes recorded was averaged for each trial type and training session. Previous studies indicate that several training sessions are needed for intact rats to learn the conditional discrimination, after which different rates and levels of conditioned responding are exhibited on reinforced and non-reinforced trials (Holland et al., 1999). It was hypothesized that rats receiving nicotine might discriminate between the two trial types sooner than control rats and/or exhibit a greater difference in responding during the two trial types, resulting in learning curves with different slopes in nicotine-treated and vehicle-treated rats. Thus, to assess group differences in conditioned responding we first estimated learning rates for each subject and each trial type by using linear regression to fit the data over the logarithm of trials. The logarithm was used because it provided the best fit to the individual data over trials. The regression equation was: responding = B0 + B1*log (trial), where the beta coefficient B1 served as the estimate of learning rate.
3. Results
One subject in the nicotine-treated group exhibited little conditioning to the tone and was excluded from the study. Fifteen rats remained in the group treated with nicotine and 16 rats were included in the vehicle group. The primary data of interest was nose-poke behavior into the food cup during presentation of the tone on each of the two trial types (Figure 1). The average slope of the learning curve as indicated by the beta coefficients for each group on each trial type is illustrated in Figure 2. Rats treated with nicotine discriminated between the reinforced and non-reinforced trials better than vehicle-treated rats. This was confirmed by conducting an analysis of variance (ANOVA) of the beta coefficients using Group (vehicle, nicotine) as the between-subjects variable and Trial type (reinforced, non-reinforced) as the within-subjects variable (alpha level of 0.05). This revealed a main effect of Group [F (1, 29) = 5.44, P < 0.05] and a significant Trial by Group interaction [F (1, 29) = 19.9, P < 0.001]. The mean beta coefficient for rats in the nicotine group was significantly different from that of control rats on the non-reinforced trials [t(29) = −4.26, P < 0.001]; in contrast, there were no significant group differences on the reinforced trials (P > 0.8). Rats treated with nicotine also discriminated between the two trial types sooner than rats treated with vehicle. Nicotine-treated rats began exhibiting significantly more nose-poke behavior during presentation of the tone on reinforced versus non-reinforced trials on the 6th training session [t (14) = 2.3, P < 0.04] while vehicle-treated rats needed 8 training sessions to start discriminating between the two trials types [(t (15) = 3.5, P < 0.003].
Figure 1.
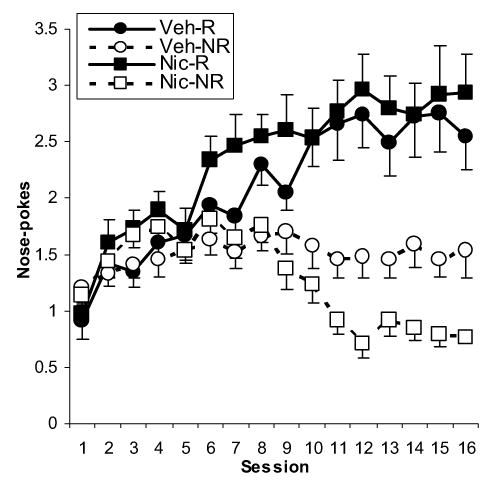
Mean number of food cup entries exhibited by vehicle-treated rats and nicotine-treated rats on each trial type across training days. R=reinforced trials, NR=non-reinforced trials. Data are means +/− S.E.M.
Figure 2.
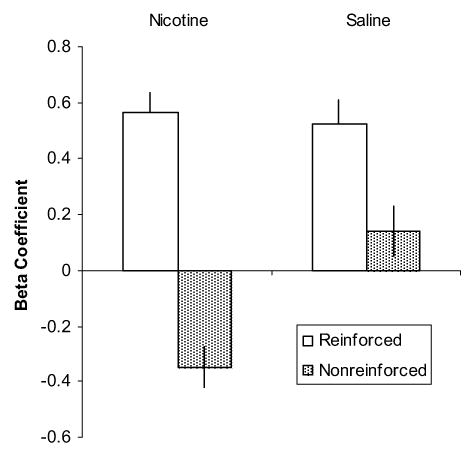
Mean beta coefficient values from the logarithmic curve estimation for each subject per trial type. Data are means +/− S.E.M.
In a subset of rats (8 in each group), training was continued for an additional seven days without drug injection to determine if the effect of nicotine on responding during non-reinforced trials would be maintained in the absence of nicotine. Indeed, responding to the tone on non-reinforced trials remained lower in rats previously treated with nicotine compared to controls. The mean ± SEM nose-pokes across the drug-free sessions were 1.30 ± 0.25 and 0.89 ± 0.19 for the control and nicotine-treated groups, respectively.
4. Discussion
Taken together, these findings indicate that nicotine treatment enhances conditioned inhibition as evidenced by improved discrimination between the two trial types. Nicotine-treated rats responded less during non-reinforced trials than vehicle-treated rats and also successfully discriminated between the two trial types in fewer training sessions. Notably, conditioned responding during the reinforced trials was comparable in both groups; thus, any difference in the degree of discrimination was not likely due to nicotine-induced changes in motivation or general activity. This is further supported by the finding that control and nicotine-treated rats exhibited comparable levels of nose-poke behavior during the 5-sec observation period immediately following food delivery on reinforced trials (1.96 ± 0.26 and 2.1 ± 0.17 nose-pokes for the control and nicotine groups, respectively).
Using a task very similar to the one used here, Holland and colleagues (1999) demonstrated that hippocampal lesions have an effect that is opposite to that of nicotine on conditioned inhibition. Hippocampal-lesioned rats responded more than control rats during presentation of the tone on non-reinforced trials, while there was little effect on food cup behavior during reinforced trials. Interestingly, there is a relatively high concentration of nicotinic receptors in hippocampus (Tribollet et al., 2004). Along with the present findings, these data suggest that nicotinic cholinergic modulation of hippocampal function may have an important role in conditioned inhibitory processes. This notion is consistent with previous findings that hippocampal nicotinic acetylcholine receptors play a key role in sensory gating, a commonly studied model of sensory inhibition (Luntz-Leybman et al., 1992).
The role of hippocampal nicotinic acetylcholine receptors has been explored extensively in other aspects of cognitive function. Hippocampal infusion of the centrally-acting nicotinic antagonist mecamylamine impairs working memory in rats (Ohno et al., 1993). Likewise, infusion of either an α7 or an α4β2-nicotinic receptor antagonist into the ventral hippocampus produces working memory deficits (Felix and Levin, 1997). Nicotinic acetylcholine receptors are also implicated in attentional function; for example, nicotinic receptor stimulation improves sustained attention in rats (Stolerman et al., 2000). Nicotine administration has also been shown to enhance latent inhibition in both humans and rodents indicating that nicotinic receptors can modulate the ability to decrease attention to uninformative stimuli (Rochford et al., 1996). Similarly, removal of cholinergic input to the hippocampus impairs the ability to reduce attention to irrelevant cues (Baxter et al., 1997). One proposed function of the hippocampus is to exclude uninformative stimuli from the focus of attention in order to protect memory from interference (Chan et al., 2001). This theoretical framework accounts for alterations in working memory, the ability to decrease attention, and inhibition following hippocampal damage or nicotinic receptor modulation and supports the notion that hippocampal nicotinic receptors may play a central role in these functions.
Stimulation of nicotinic acetylcholine receptors located on dopaminergic neurons results in increased activation of central dopaminergic systems and enhancement of dopaminergic-mediated functions (Rapier et al., 1990). Indeed, several brainstem cholinergic systems provide direct input to dopaminergic cell groups in the substantia nigra and ventral tegmental area (Wainer and Mesulam, 1990) and recent studies confirm a direct synaptic connection between cholinergic terminals and dopaminergic cell bodies in the ventral tegmental area (Garzon et al., 1999). Nicotine has been shown to increase the release of dopamine in both striatal and mesolimbic dopaminergic pathways (Rapier et al., 1990). The mesolimbic dopamine system originates in the ventral tegmental area and provides dopaminergic innervation of the limbic system, including the hippocampus, and regulates reward and motivational processes (Viggiano et al., 2002). Thus nicotine may affect conditioned inhibition by interacting with dopaminergic systems.
The present findings may have implications for understanding the propensity for certain clinical populations to use and abuse nicotine-containing products. Persons with schizophrenia or ADHD, for example, smoke cigarettes at a much higher rate than other populations (Ripoll et al., 2004). Impairments in behavioral inhibition are central to these disorders, reflecting an inability to inhibit learned responses and leading to deficits in behavioral control and executive functioning (Barkley, 1997). ADHD diagnosis is associated with impairment on go/no-go tasks (Vaidya et al., 1998) that share procedurally similarities to the serial feature negative discrimination task. Persons with ADHD also exhibit slower than normal reaction times on a stop signal task in which subjects must withhold a behavioral response to a visual stimulus when it is preceded by an auditory cue. Recently, nicotine treatment has been shown to normalize inhibitory behavior in persons with ADHD (Potter and Newhouse, 2004) and nicotine treatment also improves sensory gating in persons with schizophrenia (Thaker, 2002).
In summary, the present findings indicate that nicotine treatment enhances conditioned inhibition in rats. Insight into the effects of nicotine on inhibition is particularly important in light of current research on the involvement of nicotinic acetylcholine receptors in disorders that are characterized by deficits in inhibition. Recent studies provide evidence of a polymorphism in the α4 subunit gene of the nicotine acetylcholine receptor in persons with ADHD (Todd et al., 2003). Likewise, nicotinic receptor alterations have been linked to schizophrenia (Ripoll et al., 2004) and α7 nicotinic receptor agonists are being investigated as potential treatments. Additional studies using rodent models of inhibition may be useful to shed light on the involvement of specific nicotinic receptor subtypes in modulating inhibitory behavior and identify specific brain cholinergic systems that mediate the effects of nicotine.
Acknowledgments
We thank Drs. Robert N. Leaton and Thomas J. Gould for valuable discussion of the experimental design and data, and Dr. George Wolford for assistance with the statistical analyses.
Footnotes
Research Support This research was supported by NIHM/NIDA Grant R21 MH069670 and NSF Grant IBN 0441934.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psych Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Holland PC, Gallagher M. Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J Neurosci. 1997;17:5230–5236. doi: 10.1523/JNEUROSCI.17-13-05230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- Garzon M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol. 1999;410:197–210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Holland PC, Lamoureux JA, Han J, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobeh Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton S, Meck W, Rose JE. Nicotine effects on adults with Attention-Deficit/Hyperactivity Disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology. 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S. Blockade of hippocampal nicotine receptors impairs working memory but not reference memory in rats. Pharmacol Biochem Behav. 1993;45:89–93. doi: 10.1016/0091-3057(93)90091-7. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. The effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder (ADHD) Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacott S. Nicotinic modulation of [3H]dopamine release from striatal synaptosomes: pharmacological characterization. J Neurochem. 1990;54:937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- Ripoll N, Bronnec M, Bourin M. Nicotinic receptors and schizophrenia. Curr Med Res Opin. 2004;20:1057–1074. doi: 10.1185/030079904125004060. [DOI] [PubMed] [Google Scholar]
- Rochford J, Sen AP, Quirion R. Effect of nicotine and nicotinic receptor agonists on latent inhibition in the rat. J Pharmacol Exp Ther. 1996;277:1267–1275. [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Current progress in schizophrenia research: sensory gating deficit in schizophrenia: is the nicotinic alpha-7 receptor implicated? J Nerv Ment Dis. 2002;190:550–551. doi: 10.1097/01.NMD.0000027590.27232.ED. [DOI] [PubMed] [Google Scholar]
- Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Mol Psychiatry. 2003;8:103–108. doi: 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Grammatikopoulos G, Sadile AG. A morphometric evidence for a hyperfunctioning mesolimbic system in an animal model of ADHD. Behav Brain Res. 2002;130:181–189. doi: 10.1016/s0166-4328(01)00423-5. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Mesulam M-M. Ascending cholinergic pathways in the rat brain. In: Steriade S, Biesold D, editors. Brain Cholinergic Systems. Oxford University Press; New York, NY: 1990. pp. 65–119. [Google Scholar]


